Abstract
Background
Our recent paper, based on a pilot cohort of 119 women, showed that serum progesterone <35 nmol/L was prognostic of spontaneous miscarriage by 16 weeks in women with threatened miscarriage in early pregnancy. Using a larger cohort of women from the same setting (validation cohort), we aim to assess the validity of serum progesterone <35 nmol/L with the outcome of spontaneous miscarriage by 16 weeks.
Methods
In a prospective cohort study, 360 pregnant women presenting with threatened miscarriage between gestation weeks 6–10 at a tertiary hospital emergency unit for women in Singapore were recruited for this study. The main outcome measure measured is spontaneous miscarriage prior to week 16 of gestation. Area under the ROC curve (AUC) and test characteristics (sensitivity, specificity, positive and negative predictive value) at a serum progesterone cutpoint of <35 nmol/L for predicting high and low risk of spontaneous miscarriage by 16 weeks were compared between the Pilot and Validation cohorts.
Results
Test characteristics and AUC values using serum progesterone <35 nmol/L in the validation cohort were not significantly different from those in the Pilot cohort, demonstrating excellent accuracy and reproducibility of the proposed serum progesterone cut-off level.
Conclusions
The cut-off value for serum progesterone (35 nmol/L) demonstrated clinical relevance and allow clinicians to stratify patients into high and low risk groups for spontaneous miscarriage.
Keywords: Progesterone, Cut-off level, Predictive, Miscarriage
Background
Threatened miscarriage—defined as an ongoing pregnancy associated with vaginal bleeding, with or without abdominal pain [1]—is the most common gynecological emergency, occurring in 15–20% of ongoing pregnancies [2], with 25% progressing to spontaneous miscarriage [3, 4]. Many clinical resources are utilized in attempting a definitive diagnosis of spontaneous miscarriage among women with threatened miscarriage, as not all are at equal risk of miscarriage. Individualizing outpatient management based on an initial risk evaluation would be a boon to clinical care and suggest any additional therapy.
While maternal factors like older age (>33 years) and lower body mass index (BMI) <20 kg/m2 have been found to contribute to spontaneous miscarriage [5], recent focus has been on identifying serum biological markers prognostic of spontaneous miscarriage. A recent meta-analysis by Pillai et al of prospective studies investigating biomarkers to determine pregnancy outcome for women presenting with threatened miscarriage have showed conflicting results with the need for larger studies and further validation [6]. Other studies have looked at various biomarkers such as serum beta HCG, estradiol, PAPP-A, inhibin, CA 125 as well as progesterone. In addition, other studies have also looked at using different combination of maternal demographics, serum biomarkers and ultrasound features to predict pregnancy viability that shows promise but require the implementation of a mathematical model and algorithm. This could prove to be unwieldy in a busy clinical service due to multiple variables that need to be collected in order to use the model effectively [7–10].
Presently, the most promising is serum progesterone. A recent prospective cohort study of women with no signs of threatened miscarriage from 4 to 12 weeks of gestation reported risk of miscarriage to be significantly higher among women with low serum progesterone (<38.3 nmol/L or 12 ng/ml) [5]. Progesterone levels were 48% lower in women experiencing threatened miscarriage with subsequent spontaneous miscarriage compared to women who delivered at term. [9, 11] In our group’s paper, serum progesterone levels were significantly lower in women presenting with threatened miscarriage who subsequently experienced spontaneous miscarriage by 16 weeks gestation compared to those who did not miscarry. A cut-off serum progesterone level of 35 nmol/L was proposed to prognosticate low and high risk for spontaneous miscarriage after presenting with threatened miscarriage in early pregnancy [12]. Notwithstanding these findings, serum progesterone is currently not used for miscarriage risk assessment in a clinical setting. One explanation may be insufficient evidence regarding appropriate or ‘optimal’ serum progesterone cut-off levels for risk stratification of spontaneous miscarriage. Thus, a validated serum progesterone cut-off as a prognostic risk assessment will allow clinicians the means to better stratify patients into low- and high-risk populations.
The current study employed a large, prospective cohort (Validation cohort) with the aim of validating the serum progesterone cut-off value of 35 nmol/L based on the results of an earlier, smaller study at the same institution (Pilot cohort). We compared areas under ROC curves (AUCs) as well as sensitivity, specificity, positive and negative predictive values between the Pilot and Validation cohorts using 35 nmol/L as the risk prognosis cut-off for spontaneous miscarriage at or before 16 weeks of gestation in women presenting with threatened miscarriage in weeks 6–10 of pregnancy.
Methods
A total of 360 pregnant women, aged 21 years and above, presenting at the KK Women’s and Children’s Hospital (KKH) 24-h Women’s Clinic from September 2013 to June 2015 were recruited in the Validation cohort. Inclusion criteria were a single intrauterine pregnancy between gestation weeks 6 to 10 (confirmed and dated by ultrasonography), with pregnancy-related per vagina bleeding. Women with previous episodes of per vagina bleeding or those treated with progesterone for previous per vagina bleeding in the current pregnancy, or women diagnosed with inevitable miscarriage, missed miscarriage, blighted ovum or planned termination of pregnancy were excluded (Fig. 1).
Fig. 1.
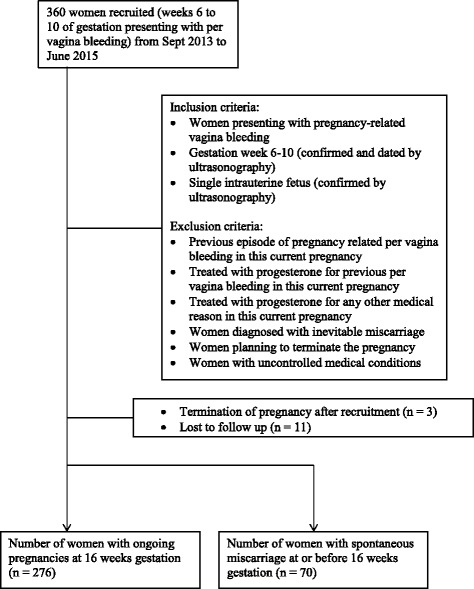
Sample selection flow chart
Maternal blood samples were taken to measure serum progesterone level at presentation. Blood was collected in plain tubes and centrifuged for 10 min at 3000 g within 2 h of collection. Serum progesterone level was measured in the KKH clinical laboratory using a commercial ARCHITECT progesterone kit (Abbott, Ireland).
Covariates for the analysis were maternal demographics, health, obstetric and lifestyle factors collected by an investigator administered questionnaire in either English or Chinese (Table 1).
Table 1.
Comparison of serum progesterone levels and maternal characteristics at baseline by pregnancy status at 16 weeks gestation for Pilot and Validation cohorts
| Maternal characteristics | Ongoing pregnancy at 16 weeks gestation | Spontaneous miscarriage at or before 16 weeks gestation | ||||
|---|---|---|---|---|---|---|
| Pilot Cohort (N = 89) |
Validation Cohort (N = 276) | p value | Pilot Cohort (N = 30) |
Validation Cohort (N = 70) | p value | |
| Serum biological markers | ||||||
| Progesterone, mean ± SD (nmol/L) | 59.8 ± 23.5 | 60.3 ± 22.7 | 0.848 | 26.4 ± 17.1 | 31.4 ± 20.1 | 0.237 |
| Demographics | ||||||
| Age, mean ± SD (years) | 29.3 ± 4.83 | 30.3 ± 4.10 | 0.0769 | 31.3 ± 4.73 | 31.6 ± 5.22 | 0.774 |
| Age of spouse, mean ± SD (years) | 32.9 ± 5.43 | 33.1 ± 5.96 | 0.772 | 33.9 ± 5.10 | 34.6 ± 6.38 | 0.628 |
| Race | ||||||
| Chinese (%) | 53.9 | 52.0 | 0.271 | 53.3 | 38.6 | 0.396 |
| Malay (%) | 19.1 | 25.5 | 16.7 | 31.4 | ||
| Indian (%) | 16.9 | 10.2 | 16.7 | 14.3 | ||
| Others (%) | 10.1 | 12.4 | 13.3 | 15.7 | ||
| Marital status | ||||||
| Married | 92.1 | 94.6 | 0.444 | 90.0 | 97.1 | 0.158 |
| Single | 7.87 | 5.45 | 10.0 | 2.86 | ||
| Highest educational level | ||||||
| University degree (%) | 42.7 | 39.3 | 0.179 | 46.7 | 42.9 | 0.724 |
| ITE or polytechnic (%) | 30.3 | 40.4 | 36.7 | 32.9 | ||
| Primary and secondary (%) | 27.0 | 20.4 | 16.7 | 24.3 | ||
| Health, obstetric and lifestyle factors | ||||||
| Planned Pregnancy (%) | 59.6 | 58.2 | 0.902 | 63.3 | 44.3 | 0.126 |
| Gestation age at recruitment, mean (wks) | 7.49 ± 1.43 | 7.48 ± 1.47 | 0.968 | 6.89 ± 1.30 | 6.63 ± 1.06 | 0.311 |
| Fetal pole present (%) | 93.3 | 100 | 0.0002 | 50.0 | 100 | <0.0001 |
| Fetal heart present (%) | 87.6 | 95.6 | 0.011 | 30.0 | 70.0 | 0.0003 |
| Number of children | ||||||
| None (%) | 55.1 | 45.5 | 0.143 | 43.3 | 32.9 | 0.367 |
| 1 or more (%) | 44.9 | 54.6 | 56.7 | 67.1 | ||
| Previous miscarriage (%) | 20.2 | 24.0 | 0.563 | 20.0 | 22.9 | 1.000 |
| BMI, mean ± SD (kg/m2) | 23.7 ± 5.09 | 22.9 ± 4.29 | 0.204 | 22.1 ± 3.81 | 23.6 ± 4.81 | 0.0903 |
| Medical comorbidities | ||||||
| Diabetes mellitus (%) | 0 | 0.360 | 1.000 | 0 | 0 | 1.000 |
| Hypertension (%) | 0 | 0 | 1.000 | 0 | 0 | 1.000 |
| Thyroid disease (%) | 1.12 | 1.09 | 1.000 | 0 | 1.43 | 1.000 |
| Gynecological disease (%) | 12.4 | 12.0 | 1.000 | 0 | 10 | 0.0991 |
| Smoking during pregnancy (%) | 3.37 | 5.45 | 0.579 | 0 | 5.71 | 0.313 |
| Exposed second hand smoke at home (%) | 40.5 | 44.7 | 0.539 | 30 | 38.6 | 0.498 |
| Alcohol during pregnancy (%) | 1.12 | 0.72 | 1.000 | 0 | 0 | 1.000 |
| Nausea during pregnancy (%) | 70.8 | 77.5 | 0.203 | 40 | 55.7 | 0.192 |
Entries in boldface were used to show that these variables were significantly related to different or associated with spontaneous miscarriage
SD standard deviation, CRL crown-rump length, BMI maternal body mass index
Outcome measures and follow-up
The primary outcome measured was spontaneous miscarriage, defined by self-reported uterine evacuation after inevitable or incomplete miscarriage, or complete miscarriage with an empty uterus, by the 16th week of gestation. All participants were contacted at the 16th week of pregnancy to verify pregnancy status.
Statistical Methods
The progesterone cut-off of <35 nmol/L for high risk of miscarriage before the 16th week of pregnancy was determined and developed using the Pilot study cohort as reported in Ku et al. [12] The <35 nmol/L serum progesterone cut-off form the basis for our validation study.
Baseline maternal demographics and pregnancy characteristics were statistically compared between the Pilot and the Validation cohorts with respect to two patient subgroups: (i) patients who experienced spontaneous miscarriage at 16 weeks of gestation and (ii) patients with ongoing pregnancy at 16 weeks gestation. The 2-sample t-test was used to compare continuous baseline variables and Fisher’s exact test to compare categorical variables.
Logistic regression equations were fitted to the respective Pilot and Validation cohort data sets. The outcome was the binary response ‘Yes/No’ corresponding to occurrence/non-occurrence of spontaneous miscarriage. The single predictor was progesterone level (nmol/L). Parameter estimates were obtained for the intercept (b 0) and progesterone coefficient (b 1). ROC analysis was performed using progesterone concentration as a continuous variable. The Youden criterion, which consists of identifying the value of progesterone that maximizes the sum of sensitivity and specificity, was applied to identify the ‘optimum’ progesterone cut-off for each cohort. ROC curves and AUCs for the Pilot and Validation cohorts were computed and compared statistically the using the approach of Hanley and McNeil for comparing independent ROC curves derived from different samples. 95% confidence intervals on the difference between the Pilot and Validation AUCs were obtained.
Using the progesterone cut-off of <35 nmol/L, test parameters of sensitivity, specificity, PPV and NPV were estimated and compared between the Pilot and Validation cohorts using Fisher’s exact test. Exact Clopper-Pearson 95% confidence intervals were calculated on test parameter estimates. Differences in Pilot and Validation parameter estimates were obtained and 95% confidence intervals on differences were calculated using a normal approximation approach. Likelihood ratios corresponding to positive (LR+) and negative (LR-) test outcomes were calculated. Agreement of actual versus predicted Validation cohort miscarriages based on the <35 nmol/L cut-off was assessed using McNemar’s test. Corresponding confidence intervals on the difference were calculated using the Wald z method for differences of correlated proportions. Analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Of the 360 women recruited in the Validation cohort, 11 were lost to follow up and 3 withdrew from the study due to induced termination of pregnancy. Of the 346 women included in the analysis, 70 (20.2%) experienced spontaneous miscarriage prior to week 16. Of the 119 women in the Pilot cohort, 30 (25.2%) experienced spontaneous miscarriage prior to week 16 of gestation. The difference (95% Confidence Interval (CI)) in incidence proportions between the Pilot and Validation cohorts was 0.05 (-0.03, 0.14) and not statistically significant (p = 0.301).
Serum progesterone levels (nmol/L) did not differ significantly between Pilot (P) and Validation (V) cohorts for women with ongoing pregnancies at 16 weeks (P-59.8, V-60.3; p = 0.848) or women experiencing spontaneous miscarriage prior to week 16 (P-26.4, V-31.4; p = 0.237). In women with ongoing pregnancy at week 16, the only maternal demographic and clinical variables exhibiting a statistically significant difference between the two cohorts were presence of fetal pole (%) (P-93.3, V-100; p = 0.0002) and fetal heart (%) (P-87.6, V-95.6; p = 0.011). The same held true in women with spontaneous miscarriage prior to week 16, with significant differences in presence of fetal pole (P-50.0, V-100; p <0.0001) and fetal heart (P-30.0, V-70.0; p = 0.0003) (Table 1).
Logistic regression equations parameter estimates for the intercept (b 0) and progesterone coefficient (b 1) were (b 0, b 1) = (2.8439, -0.0971) for the Pilot cohort and (2.1063, -0.0782) for the Validation cohort. Receiver Operating Characteristic (ROC) analysis was performed on the Validation cohort data with progesterone level as a continuous variable.
Validation of serum progesterone for prognosticating risk of spontaneous miscarriage
AUC (95% CI) for the Pilot and Validation cohort serum progesterone ROC curves was 0.89 (0.81, 0.97) and 0.83 (0.77, 9.89), respectively. The difference (95% CI) was 0.06 (-0.04, 0.15) and not statistically significant (p = 0.267) (Fig. 2). In applying the Youden index criterion to the ROC curve, the progesterone concentration in the Validation cohort that maximized sensitivity + specificity was 35 nmol/L, which was consistent with the Pilot cohort result. For the Validation cohort (<35 nmol/L cut-off), test sensitivity = 65.7%, specificity = 92.0%, Positive Predictive Value (PPV) = 67.7%, Negative Predictive Value (NPV) = 91.3%, LR + = 8.21 and LR- = 0.37 for spontaneous miscarriage by week 16. There were no significant differences between the Pilot and Validation cohorts among any test performance parameters (Table 2).
Fig. 2.
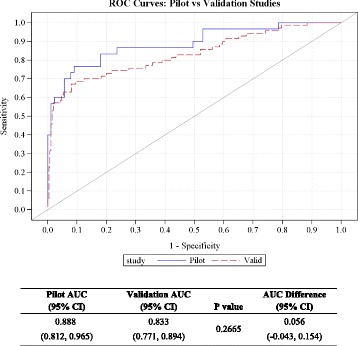
Comparison of ROC curves for serum progesterone from Pilot and Validation cohorts at < 35 nmol/L cut-off
Table 2.
Comparison of sensitivity, specificity, PPV, and NPV between Pilot and Validation cohorts for progesterone at cut-off < 35 nmol/L for high risk of miscarriage before 16th week of pregnancy
| Statistic (95% CI) Counts |
Study | P-value1 | Diff (95% CI) |
|
|---|---|---|---|---|
| Pilot | Valid | |||
| Sensitivity | 0.767 (0.577, 0.901) 23/30 |
0. 657 (0.534, 0.767) 46/70 |
0.349 | 0.110 (-0.093, 0.274) |
| Specificity | 0.888 (0.803, 0.945) 79/89 |
0. 920 (0.881, 0.949) 253/275 |
0.389 | -0.032 (-0.119, 0.031) |
| PPV | 0.697 a
(0.513, 0.844) 23/33 |
0. 677 b
(0.552, 0.785) 46/68 |
1.000 | 0.021 (-0.177, 0.196) |
| NPV | 0. 919 a
(0.839, 0.967) 79/86 |
0. 913 b
(0.874, 0.944) 253/277 |
1.000 | 0.005 (-0.077, 0.062) |
| LR+ | 6.82 | 8.21 | ||
| LR- | 0.26 | 0.37 | ||
1 Fisher’s exact test, a Prevalence = 0.25; b Prevalence = 0.20
Agreement of actual versus predicted miscarriages by 16 weeks in the validation cohort
Using the <35 nmol/L serum progesterone cut-off, among the 346 women in the validation cohort, results were True Positive = 46, True Negative = 254, False Positive = 24 and False Negative = 22 translating to accuracy (95% CI) of 300/346 = 0.87 (0.83, 0.90). The null hypothesis of equality of the proportion of miscarriages predicted by the model (0.197 = 68/346) versus the actual proportion (0.202 = 70/346) was non-significant by McNemar’s test (p = 0.768) for the difference (95% CI) of -0.006 (-0.044, 0.033).
Discussion
There is significant interest in developing clinically useful models for risk prognosis of pregnancy complications and outcomes, and to stratify pregnant women as low or high risk. The advantages of serum progesterone as a marker for spontaneous miscarriage in women with threatened miscarriage are three-fold: (1) high reliability for reassuring women at low risk of miscarriage (NPV > 90%), (2) provides clinical guidance for unnecessary progestogen treatments or bed rest, and (3) may prompt mobilization of resources supporting an expectant mother’s psychological wellbeing.
Main findings
Our study was targeted at women presenting with threatened miscarriage in early pregnancy in order to validate a previously suggested serum progesterone cut-off level of <35 nmol/L for predicting risk of spontaneous miscarriage in this high-risk population. In the past, a reliable marker or model for clinical prognosis of pregnancy outcome in women presenting with threatened miscarriage in early pregnancy has not been available. In this study, we have successfully validated the serum progesterone cut-off value of <35 nmol/L (11 ng/ml)—originally identified in a previous study [12], as a clinically useful predictor of miscarriage prior to week 16 of pregnancy in a temporally different population from the same centre. Using this cut-off, individual patients can be quickly stratified as being at low risk or high risk of spontaneous miscarriage.
Strengths and interpretation
Early pregnancy is maintained via mediation by hormones and endocrine-immune interactions [13]. Progesterone is increasingly recognized as a critical hormone during implantation where it plays an important role in sustaining decidualization, controlling uterine contractility and promoting maternal immune tolerance to the fetal semi-allograft. [14] Thus, high level of serum progesterone may be protective against early pregnancy loss. In contrast, low level of serum progesterone could contribute to an increased risk of subsequent spontaneous miscarriage, especially in women who presents with threatened miscarriage in the first trimester of pregnancy. However, very few studies have reported a specific cut-off progesterone level with high predictive value for spontaneous miscarriage [11, 15–17].
The serum progesterone cutoff level of <35 nmol/L originally proposed by Ku et al [12] provides important information that can be used to provide individualized and patient-specific risk assessment for spontaneous miscarriage. This provides prognostic data available at entry to care. Our validated proposed cut off level for serum progesterone allows clinicians to quickly assess individual patient risk by calculating a probability based on presence or absence of various factors. This threshold level for serum progesterone can be applied in a clinical setting, accurately identifying high-risk patients who may require more therapeutic intervention or heightened surveillance. In addition, this subpopulation of patients may potentially contribute to further pharmacological intervention studies whereby its therapeutic effectiveness and efficacy can be determined. Although measurements of serum progesterone levels are not recommended for routine clinical use at this time, it can be determined that serum progesterone is a highly specific predictive biomarker for spontaneous miscarriage, with a high negative predictive value, allowing us to reassure anxious patients with a low risk of miscarriage. Finally, we are able to demonstrate that our serum progesterone cut off value of 35 nmol/L was highly reproducible in a temporally different patient population, thus supporting the validity of the model.
Limitation
However, our study has a few limitations. This proposed serum progesterone cut-off value was shown to be applicable in women who present with threatened miscarriage in early pregnancy. It is uncertain if the predictive values will be applicable in a low-risk population, but the sensitivity and the specificity should remain constant, as these parameters are independent of disease prevalence. External validation is required before widespread implementation into clinical practice is possible.
Conclusion
We present a proposed cut off serum progesterone value of <35 nmol/L as a validated threshold level to predict spontaneous miscarriage, which demonstrates both excellent accuracy and acceptable reproducibility. This threshold level allows clinicians to perform risk stratification relating to risk of spontaneous miscarriage, allowing for better prognostication and guiding therapeutic interventions. This model is both clinically applicable and easily implemented, as well as provides research opportunities for future miscarriage intervention studies.
Acknowledgements
We wish to thank all the families who participated in our research and all the dedicated staff from all participating departments.
Funding
This study is funded by the SingHealth Foundation Research Grant Call research fund.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available as further research and analysis are being performed on the datasets for future publications but are available from the corresponding author on reasonable request.
Authors’ contributions
SML developed the research design, analysis strategy, conducted patient recruitment and follow-up and was the main co-first author of the manuscript. CWK developed the research design, analysis strategy, conducted patient recruitment and follow-up and was the main co-first author of the manuscript. JCA Jr performed to the statistical analyses and contributed to the interpretation of results and presentation, and provided editorial guidance. RM contributed to the development of research design and analysis strategy, interpretation of results and writing of the manuscript. NST contributed to experimental design and analysis strategy, interpretation of results and presentation, and provided editorial guidance. TØ contributed to the development of research design and analysis strategy, interpretation of results and writing of the manuscript. TCT contributed to the development of research design, analysis strategy, provided editorial support and is the principal investigator of the SingHealth Foundation Research Grant Call research fund. All authors have reviewed and approved the final version of the paper.
Competing interests
The authors have no conflicts of interest.
Consent for publication
Not applicable
Ethics approval and consent to participate
The institutional review board at SingHealth (CIRB ref: 2013/320/D) approved the study. All patients have given verbal and written consent to be included in this study.
Abbreviations
- AUC
Area under the ROC curve (AUC)
- BMI
Body mass index
- CI
Confidence interval
- KKH
KK women’s and children’s hospital
- LR
Likelihood ratio
- NPV
Negative predictive value
- P
Pilot
- PPV
Positive predictive value
- ROC
Receiver operating characteristic
- V
Validation
References
- 1.Cunningham FG. Williams Obstetrics 24th edition. USA: McGraw-Hill Education. Medical; 2014.
- 2.Jouppila P. Clinical consequences after ultrasonic diagnosis of intrauterine hematoma in threatened abortion. J Clin Ultrasound. 1985;13(2):107–11. doi: 10.1002/jcu.1870130205. [DOI] [PubMed] [Google Scholar]
- 3.Basama FM, Crosfill F. The outcome of pregnancies in 182 women with threatened miscarriage. Arch Gynecol Obstet. 2004;270(2):86–90. doi: 10.1007/s00404-003-0475-z. [DOI] [PubMed] [Google Scholar]
- 4.Kouk LJ, Neo GH, Malhotra R, Allen JC, Beh ST, Tan TC, et al. A prospective study of risk factors for first trimester miscarriage in Asian women with threatened miscarriage. Singapore Med J. 2013;54(8):425–31. doi: 10.11622/smedj.2013148. [DOI] [PubMed] [Google Scholar]
- 5.Arck PC, Rucke M, Rose M, Szekeres-Bartho J, Douglas AJ, Pritsch M, et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed Online. 2008;17(1):101–13. doi: 10.1016/S1472-6483(10)60300-8. [DOI] [PubMed] [Google Scholar]
- 6.Pillai RN, Konje JC, Tincello DG, Potdar N. Role of serum biomarkers in the prediction of outcome in women with threatened miscarriage: a systematic review and diagnostic accuracy meta-analysis. Hum Reprod Update. 2016;22(2):228–39. doi: 10.1093/humupd/dmv054. [DOI] [PubMed] [Google Scholar]
- 7.Al Mohamady M, Fattah GA, Elkattan E, Bayoumy R, Hamed DA. Correlation of serum CA-125 and progesterone levels with ultrasound markers in the prediction of pregnancy outcome in threatened miscarriage. Int J Fertil Steril. 2016;9(4):506–11. doi: 10.22074/ijfs.2015.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdallah Y, Daemen A, Kirk E, Pexsters A, Naji O, Stalder C, et al. Limitations of current definitions of miscarriage using mean gestational sac diameter and crown-rump length measurements: a multicenter observational study. Ultrasound Obstet Gynecol. 2011;38(5):497–502. doi: 10.1002/uog.10109. [DOI] [PubMed] [Google Scholar]
- 9.Johns J, Muttukrishna S, Lygnos M, Groome N, Jauniaux E. Maternal serum hormone concentrations for prediction of adverse outcome in threatened miscarriage. Reprod Biomed Online. 2007;15(4):413–21. doi: 10.1016/S1472-6483(10)60367-7. [DOI] [PubMed] [Google Scholar]
- 10.Guha S, Van Belle V, Bottomley C, Preisler J, Vathanan V, Sayasneh A, et al. External validation of models and simple scoring systems to predict miscarriage in intrauterine pregnancies of uncertain viability. Hum Reprod. 2013;28(11):2905–11. doi: 10.1093/humrep/det342. [DOI] [PubMed] [Google Scholar]
- 11.al-Sebai MA, Kingsland CR, Diver M, Hipkin L, McFadyen IR. The role of a single progesterone measurement in the diagnosis of early pregnancy failure and the prognosis of fetal viability. Br J Obstet Gynaecol. 1995;102(5):364–9. doi: 10.1111/j.1471-0528.1995.tb11286.x. [DOI] [PubMed] [Google Scholar]
- 12.Ku CW, Allen JC, Jr., Malhotra R, Chong HC, Tan NS, Ostbye T, et al. How can we better predict the risk of spontaneous miscarriage among women experiencing threatened miscarriage? Gynecol Endocrinol. 2015;31(8):1–5. [DOI] [PubMed]
- 13.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345(19):1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 14.Arck P, Hansen PJ, Mulac Jericevic B, Piccinni MP, Szekeres-Bartho J. Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am J Reprod Immunol. 2007;58(3):268–79. doi: 10.1111/j.1600-0897.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 15.Duan L, Yan D, Zeng W, Yang X, Wei Q. Predictive power progesterone combined with beta human chorionic gonadotropin measurements in the outcome of threatened miscarriage. Arch Gynecol Obstet. 2011;283(3):431–5. doi: 10.1007/s00404-010-1367-7. [DOI] [PubMed] [Google Scholar]
- 16.Abdelazim IA, Elezz AA, Elsherbiny M. Relation between single serum progesterone assay and viability of the first trimester pregnancy. Springerplus. 2012;1(1):80. doi: 10.1186/2193-1801-1-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanita O, Hanisah AH. Potential use of single measurement of serum progesterone in detecting early pregnancy failure. Malays J Pathol. 2012;34(1):41–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available as further research and analysis are being performed on the datasets for future publications but are available from the corresponding author on reasonable request.


