Abstract
Purpose:
To compare the efficacy and side effects of loteprednol versus fluorometholone after myopic photorefractive keratectomy (PRK).
Methods:
One hundred and twenty four eyes of 62 patients who underwent PRK were enrolled in this study. One eye of each subject was randomized to receive loteprednol 0.5% and the fellow eye was given fluorometholone 0.1%. Patients were followed up for three months.
Results:
There was no significant difference in uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, corneal haze, intraocular pressure (IOP), and ocular discomfort and redness between groups at the final visit. At 3 months postoperatively, 20/25 or better UDVA was achieved in 95% of the loteprednol group and 92% of the fluorometholone group (P > 0.05). There was neither visually significant corneal haze nor ocular hypertension (IOP rise > 10 mmHg or IOP > 21 mmHg) in any group.
Conclusion:
The efficacy and side effects of loteprednol 0.5% and fluorometholone 0.1% after myopic PRK are comparable.
Keywords: Corneal Haze, Fluorometholone, Intraocular Pressure, Loteprednol, Photorefractive Keratectomy
INTRODUCTION
Photorefractive keratectomy (PRK) is a noninvasive refractive procedure gaining popularity among ophthalmologists as it eliminates flap related complications. However, corneal haze and myopic regression remain the most common complications after PRK.[1] Topical corticosteroids have a significant role in decreasing the incidence of these complications after PRK.[2] Alongside the benefits, these drugs have some major side effects including elevation in IOP and delayed wound healing which are more prominent in cases of refractive surgery. Some patients experience higher degree of IOP elevation even with lower doses or shorter durations of treatment with topical corticosteroids. In fact, corticosteroid induced ocular hypertension and glaucomatous optic neuropathy remain major drawbacks of topical corticosteroid therapy. While early generation corticosteroids, such as dexamethasone and prednisolone, are more likely to result in clinically significant increases in IOP, newer generation corticosteroids such as loteprednol etabonate offer similar anti-inflammatory efficacy with less risk of elevation in IOP as compared to older corticosteroids.[3,4] Their safety and efficacy in the management of dry eye,[5] allergic ocular diseases[6] and post-surgical inflammation[7] has been shown in many studies; however, few randomized, controlled studies have evaluated the new generation of topical corticosteroids after PRK. The present clinical trial compares efficacy and side effects of loteprednol 0.5% versus fluorometholone 0.1% suspensions following PRK.
METHODS
In this prospective clinical trial, patients who underwent PRK randomly received loteprednol in one eye and fluorometholone in the fellow eye and the pre- and postoperative outcomes were compared within 3 months. This study was performed at Torfeh Eye Hospital from November 2014 to February 2016. The study was approved by the Ethics Committee of the Ophthalmic Research Center affiliated to Shahid Beheshti University of Medical Sciences, Tehran, Iran. Before operation, informed consent was obtained. This randomized clinical trial was registered at http://www.irct.ir (IRCT2016081629386N1).
Inclusion criteria were age of more than 18 years, stable refraction for at least 12 months with myopia between −8.0 to −1.0 diopters (D) and astigmatism less than 4.0 D with normal eye examination (except refractive error), and corrected distance visual acuity (CDVA) of 20/20.
Patients who were unable to return for follow-up or needed to increase the topical steroid dosage for any reason, patients who did not follow the instructions, patients with anisometropia (more than 1 D) or significant preoperative central corneal thickness difference between the two eyes (more than 20 µm), those who received mitomycin-C (MMC) just in one eye, and patients with postoperative complications such as keratitis were excluded from the study. Pregnant women and patients with the history of systemic or autoimmune diseases and those receiving immunosuppressive agents were also not included.
Surgical Technique
Photorefractive keratectomy was performed in the following manner: the epithelium was removed after exposure to 20% alcohol solution for 20 seconds. Laser ablation was performed using the Wavelight Allegretto EX500Hz excimer laser machine (Alcon, Forth Worth, TX, USA). Treatments profiles were wavefront-optimized PRK with an optical zone of 6.5 mm and a total ablation zone of 9 mm. All patients received a pledget soaked in MMC 0.02% for 20 seconds after the laser ablation and then irrigating with 50 ml balanced salt solution. This was followed by one drop of chloramphenicol 0.3%, a topical nonsteroidal anti-inflammatory drug, and a soft bandage contact lens (Purevision 2, Bausch and Lomb, Rochester, NY, USA).
After operation, loteprednol 0.5% eye drop (Lotemax; Bausch & Lomb, Rochester, NY, USA) was randomly used in one eye and fluorometholone 0.1% eye drop (FML; Allergan, Irvine, CA, USA) was administered in the other eye four times a day for one month. Eye drops were tapered gradually (drugs continued 3 times daily for the second month and 2 times daily for the third month). The treatment protocol was clearly explained to the patients by a clinician who was masked to the study. At each follow-up visit, patients were asked how they were using their eye drops.
Visual acuity (VA) was measured by Snellen chart and converted to LogMAR notation for statistical analysis. Intraocular pressure (IOP) was measured over the central cornea by Goldmann applanation tonometry (GAT) before and at each postoperative visit. Average of 2 measurements per eye was considered for analysis. If 2 measurements differed more than 2 mmHg, the third one was obtained and the average of the closest measurements was considered for statistical analysis.
No adjustment for corneal thickness was done for correction of the measured IOP postoperatively. Steroid induced ocular hypertension was defined as IOP higher than 21 mmHg or a 10 mmHg rise in IOP compared to baseline.
Follow-up examinations were performed on days 1, 3, and 5 then at months 1, 2 and 3 postoperatively by the same physician who was masked to the randomization protocol. Closer follow-up examinations (every 1 to 2 weeks) were scheduled in patients who developed ocular hypertension.
Preoperative characteristics including sex, age, manifest spherical equivalent (SE), central corneal thickness (CCT), and postoperative outcomes including IOP (as the primary outcome), uncorrected (UDVA) and corrected distance visual acuity (CDVA), corneal haze, side effects (eye redness, eye discomfort), and postoperative ocular pain (as the secondary outcomes) were compared between the two groups during the first 3 months after surgery. Post PRK corneal haze was defined as being visually significant if it was associated with decreased visual acuity or subjective complaints by the patient or moderate to severe corneal haze easily visible with direct slit illumination with or without iris details obscuration.[8]
In order to present data, we used mean, standard deviation, median and range, frequency and percent. The sample size was calculated based on IOP changes. To assess the improvement within the groups, linear mixed model and adjustment for the multiple comparisons performed by the Bonferroni method were applied. We used paired t-test in order to evaluate the difference between the two groups. All statistical analyses were performed by SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp., USA). P value less than 0.05 was considered as statistically significant.
RESULTS
Sixty-two patients including 26 male and 36 female subjects with 3 month follow-up were evaluated in this study. Patients' mean age was 28.1 ± 5.6 (range, 19 to 43) years. Mean SE was − 3.49 ± 1.49 (range, -7.25 to -0.75) D at baseline, which reduced to − 0.04 ± 0.24 (range, -0.75 to 0.63) D after PRK (P < 0.01). The absolute value of mean SE correction was 3.29 ± 1.38 (range, 0.38 to 7.00) D. Mean preoperative corneal thickness was 542 ± 30 (range, 482 to 610) µm. All patients had CDVA of 20/20 before surgery. Baseline characteristics of the patients in each group are shown in Table 1. There was no significant difference in any parameter between the two groups at baseline. Mean duration of post-surgical ocular pain was 2 ± 1.60 (range, 0 to 7) days. Patients' complaints regarding ocular discomfort or eye redness after instillation of eye drops were not significantly different between the two groups.
Table 1.
Preoperative characteristics of the patients’ eyes
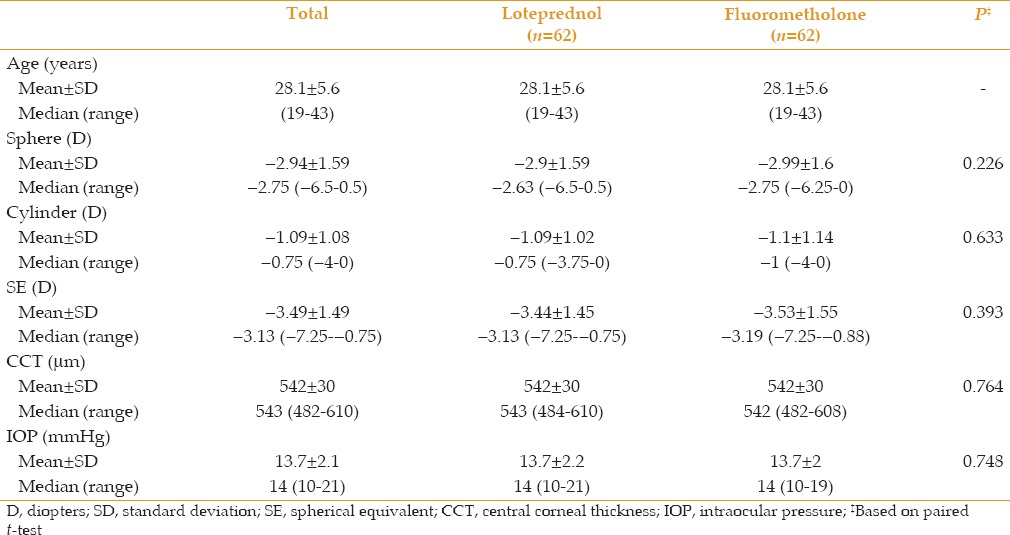
Mean UDVA was 0.014 ± 0.031 LogMAR in the fluorometholone group and 0.009 ± 0.022 LogMAR in the loteprednol group 3 months postoperatively. Mean postoperative CDVA was 0.004 ± 0.013 LogMAR and 0.001 ± 0.006 LogMAR in the fluorometholone and loteprednol groups, respectively [Table 2]. Mean SE and its change during follow-up is shown in Table 3. At 3 months postoperatively, UDVA of 20/25 or better was achieved in 59 eyes (95%) of the loteprednol group and 57 eyes (92%) of the fluorometholone group (P = 0.31). All patients had UDVA of 20/30 or better. There was no significant difference in visual outcomes between groups. No visually significant corneal haze was seen 3 months postoperatively.
Table 2.
Visual outcomes at month 3 postoperatively
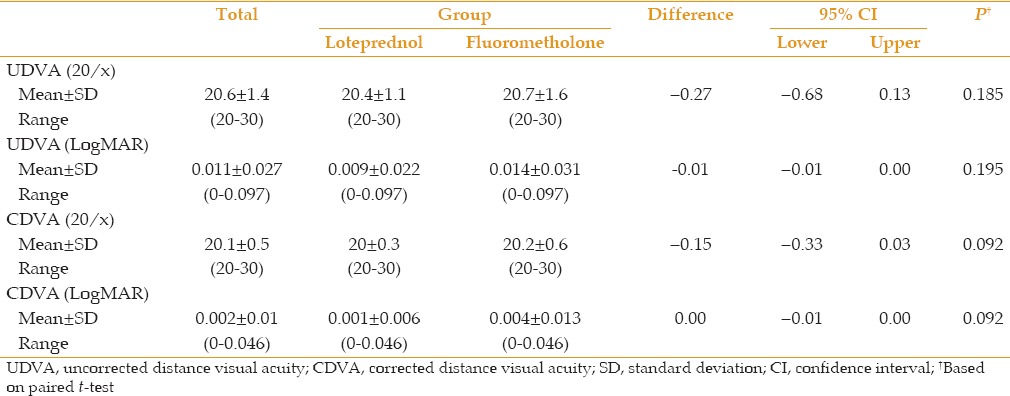
Table 3.
Comparison of mean spherical equivalent (SE) and its change with baseline values in each group and between groups
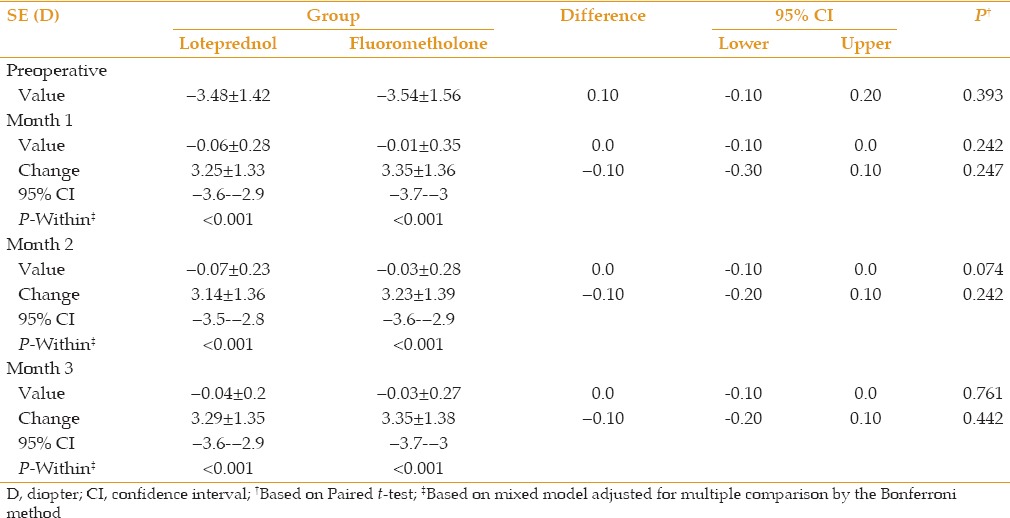
Mean baseline IOP was 13.7 ± 2.2 mmHg and 13.7 ± 2.0 mmHg in the loteprednol and fluorometholone groups, respectively (P > 0.05). At the first two months after operation, no significant change in IOP was seen; however, at month 3, a significantly decreasing trend was observed in each group (P < 0.05) with no statistically significant difference between the two groups [Table 4]. Steroid induced ocular hypertension (IOP rise >10 mmHg or IOP >21 mmHg) was not observed in any group; however, IOP increased more than 5 mmHg in three (5%) eyes treated with loteprednol. IOP declined with discontinuation of the drug after 3 months. There was no statistically significant difference between the two drugs regarding IOP elevation.
Table 4.
Comparison of mean intraocular pressure (IOP) and its change with baseline values in each group and between groups
DISCUSSION
The role of corticosteroids in prevention of corneal haze after PRK has become more remarkable recently; however, their side effects are not negligible. Introduction of newer corticosteroids such as loteprednol etabonate with anti-inflammatory effects similar to older corticosteroids with less rise in IOP is changing the trend in clinical practice. Loteprednol etabonate, a retro-metabolically designed corticosteroid, is quickly changed into inactive metabolites by nonspecific esterases found in the cornea.[9] The faster metabolism of loteprednol is believed to lead to a lower side effect profile compared to other steroids, thus exerting a smaller effect on the IOP.[10]
Mifflin et al[11] retrospectively compared fluorometholone with loteprednol etabonate after myopic PRK, according to their protocol, patients in the loteprednol 0.5% and fluorometholone 0.1% groups were treated first with prednisolone acetate 1% followed by either loteprednol 0.5% or fluorometholone 0.1% eye drops. In the current study, however, patients were treated only with either fluorometholone or loteprednol during a 3-month course. In our study, the visual outcome was comparable between groups and more than 92% of patients in each group achieved UDVA of 20/25 or better. However, in the study by Miffin et al the final UDVA was statistically better in the loteprednol group compared to the fluorometholone group. The visual outcomes in our study was similar to other studies where wavefront optimized platform of WaveLight Allegretto system was used.[12,13] Thanathanee et al[14] also showed that there was no difference in UDVA when either dexamethasone or loteprednol etabonate was used after PRK.
No visually significant corneal haze was seen during a 3-month follow-up in the present study. However, longer follow-up time and larger population is needed for more accurate judgment using intraoperative MMC especially for high refractive errors, followed by topical steroids during the postoperative period has dramatically decreased the incidence of corneal haze after PRK.[15] As the duration of applying MMC and mean SE was similar for both eyes of the same patient in this study, assessment of the isolated possible effect of these two drugs on prevention of corneal haze could be more accurate compared to the previous studies.
In the present study, there was no significant difference in mean IOP between groups at months 1, 2, and 3. At the first two months postoperatively, no significant change was seen, but at month 3, a comparable and decreasing trend was observed in each group. This might be due to tapering off the topical steroids at this time point. Intraocular pressure increased more than 5 mmHg in three (5%) eyes treated with loteprednol; two eyes at month 1 and one eye at month 3. This did not occur in any eye in the fluorometholone group, but this difference was not statistically significant. In another study, this rise occurred in 6% of patients treated with loteprednol versus 19% in the dexamethasone group.[14] Moreover, steroid induced ocular hypertension (IOP rise > 10 mmHg) was not seen in any case in our study. In contrast, ocular hypertension developed in 8% of patients who were treated with bethamethasone and fluorometholone regimen in another study.[16] As there are various drug regimens all over the world, the comparison is very difficult; however, many reports show that newer generation of corticosteroids such as loteprednol etabonate, induce less rise in IOP.
Mean days of ocular pain after PRK was equal among the two groups. This aspect was not reported previously. Ocular discomfort, burning sensation and eye redness are common side effects of topical medications. Our patients did not report any of these problems using these topical steroids.
The advantage of this study was the fellow eye comparison design of the study with restricted inclusion and exclusion criteria which decreased the confounding effects of intervening variables such as patients' compliance and adherence to drugs, diurnal variations in IOP, and physiologic and metabolic variations among patients. The limitations of the current study were short duration of follow-up, small sample size, and the measurement of just visually significant corneal haze.
In conclusion, the present study showed that there was no difference in efficacy and adverse effects of loteprednol etabonate versus fluorometholone suspensions after myopic PRK. Further studies with longer time and larger sample size are required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors appreciate Dr. Mohammad Faghihi, Dr. Saeed Karimi and Dr. Homayoun Nikkhah's help and support. We also thank the optometry team, the secretory of refractive surgery department, and all patients who participated in this study.
REFERENCES
- 1.American Academy of Ophthalmology. Excimer laser photorefractive keratectomy (PRK) for myopia and astigmatism. Ophthalmology. 1999;106:422–437. [PubMed] [Google Scholar]
- 2.Vetrugno M, Maino A, Quaranta GM, Cardia L. The effect of early steroid treatment after PRK on clinical and refractive outcomes. Acta Ophthalmol Scand. 2001;79:23–27. doi: 10.1034/j.1600-0420.2001.079001023.x. [DOI] [PubMed] [Google Scholar]
- 3.Lane SS, Holland EJ. Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery. J Cataract Refract Surg. 2013;39:168–173. doi: 10.1016/j.jcrs.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: Are they all the same? Ophthalmol Ther. 2013;2:55–72. doi: 10.1007/s40123-013-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung HH, Ji YS, Sung MS, Kim KK, Yoon KC. Long-Term Outcome of Treatment with Topical Corticosteroids for Severe Dry Eye Associated with Sjögren's Syndrome. Chonnam Med J. 2015;51:26–32. doi: 10.4068/cmj.2015.51.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu LQ, Chen X, Lou H, Cheng JW, Wei RL. Loteprednol etabonate in the treatment of allergic conjunctivitis: A meta-analysis. Curr Med Res Opin. 2015;31:1509–1518. doi: 10.1185/03007995.2015.1058250. [DOI] [PubMed] [Google Scholar]
- 7.Stewart R, Horwitz B, Howes J, Novack GD, Hart K. Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5 for postoperative inflammation. J Cataract Refract Surg. 1998;24:1480–1489. doi: 10.1016/s0886-3350(98)80170-3. [DOI] [PubMed] [Google Scholar]
- 8.Lohmann CP, Gartry DS, Muir MK, Timberlake GT, Fitzke FW, Marshall J. Corneal haze after excimer laser refractive surgery: Objective measurements and functional implications. Eur J Ophthalmol. 1991;1:173–180. doi: 10.1177/112067219100100403. [DOI] [PubMed] [Google Scholar]
- 9.Noble S, Goa KL. Loteprednol etabonate: Clinical potential in the management of ocular inflammation. Bio Drugs. 1998;10:329–339. doi: 10.2165/00063030-199810040-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett JD, Horwitz B, Laibovitz R, Howes JF. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol Ther. 1993;9:157–165. doi: 10.1089/jop.1993.9.157. [DOI] [PubMed] [Google Scholar]
- 11.Mifflin MD, Leishman LL, Christiansen SM, Sikder S, Hsu M, Moshirfar M. Use of loteprednol for routine prophylaxis after photorefractive keratectomy. Clin Ophthalmol. 2012;6:653. doi: 10.2147/OPTH.S30282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassiri N, Safi S, Aghazade Amiri M, Sheibani K, Safi H, Panahi N, et al. Visual outcome and contrast sensitivity after photorefractive keratectomy in low to moderate myopia: Wavefront-optimized versus conventional methods. J Cataract Refract Surg. 2011;37:1858–1864. doi: 10.1016/j.jcrs.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Moshirfar M, Churgin DS, Betts BS, Hsu M, Sikder S, Neuffer M, et al. Prospective, randomized, fellow eye comparison of WaveLight® Allegretto Wave® eye-Q versus VisX CustomVueTM sTAr s4 irTM in photorefractive keratectomy: Analysis of visual outcomes and higher-order aberrations. Clin Ophthalmol. 2011;5:1185. doi: 10.2147/OPTH.S24319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanathanee O, Sriphon P, Anutarapongpan O, Athikulwongse R, Thongphiew P, Rangsin R, et al. A Randomized Controlled Trial Comparing Dexamethasone with Loteprednol Etabonate on Postoperative Photorefractive Keratectomy. J Ocul Pharmacol Ther. 2015;31:165–168. doi: 10.1089/jop.2014.0107. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi H, Taheri SM, Fotouhi A, Kheiltash A. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy in high myopia: A prospective clinical study. BMC ophthalmology. 2004;4:12. doi: 10.1186/1471-2415-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javadi MA, Mirbabaei-Ghafghazi F, Mirzade M, Yazdani S, Yaseri M. Steroid induced ocular hypertension following myopic photorefractive keratectomy. J Ophthalmic Vis Res. 2008;3:42. [PMC free article] [PubMed] [Google Scholar]


