Abstract
Purpose:
To examine the safety of a single intravitreal injection of autologous bone Marrow Mesenchymal stem cells (MSCs) in patients with advanced retinitis pigmentosa (RP).
Methods:
A prospective, phase I, nonrandomized, open-label study was conducted on 3 eyes of 3 volunteers with advanced RP. Visual acuity, slit-lamp examination, fundus examination, optical coherence tomography, fundus auto-fluorescence, fluorescein angiography and multifocal electroretinography were performed before and after an intravitreal injection of approximately one-million MSCs. The patients were followed for one year. Further evaluation of MSCs was performed by injection of these cells into the mouse vitreous cavity.
Results:
No, adverse events were observed in eyes of 2 out of 3 patients after transplantation of MSCs. These patients reported improvements in perception of the light after two weeks, which lasted for 3 months. However, severe fibrous tissue proliferation was observed in the vitreous cavity and retrolental space of the third patient's eye, which led to tractional retinal detachment (TRD), iris neovascularization and formation of mature cataract. Injection of this patient's MSCs into the vitreous cavity of mice also resulted in fibrosis; however, intravitreal injections of the two other patients' cells into the mouse vitreous did not generate any fibrous tissue.
Conclusion:
Intravitreal injection of autologous bone marrow MSCs into patients' eyes with advanced RP does not meet safety standards. Major side effects of this therapy can include fibrosis and TRD. We propose thorough evaluation of MSCs prior to transplantation by intravitreal injection in the laboratory animals.\
Keywords: Intravitreal Injection, Mesenchymal Stem Cells, Retinitis Pigmentosa
INTRODUCTION
Recent preclinical studies, and clinical trials suggest bone marrow-derived cells for the treatment of retinal degenerative diseases.[1,2,3,4,5,6,7,8,9,10,11] This improvement in retinal function can be attained through different mechanisms such as the paracrine effect and RPE repair.[12] The safety and efficacy of intravitreal injection of bone marrow mesenchymal stem cells (MSCs) in restoring retinal function in patients with retinitis pigmentosa (RP), however, has not been explored.
In this study, we investigated the safety and feasibility of intravitreally transplanted autologous bone marrow MSCs in patients with advanced RP over a one-year period. Herein, we report the early onset of severe fibrosis after cell transplantation in one patient's eye. In two other patients, temporary improvement in visual function was observed.
METHODS
This phase I, prospective open-label study was performed to evaluate the safety of autologous bone marrow MSC transplantation in subjects with advanced RP. The safety outcome was defined as any deleterious modifications on clinical and paraclinical parameters. The secondary objective was to assess potential efficacy through improvements in multifocal electroretinography (mfERG), optical coherence tomography (OCT), enhanced depth imaging OCT (EDI-OCT), fundus auto-fluorescence (FAF) imaging and fluorescein angiography (FA).
The study was approved by the Ethics Committee of the Ophthalmic Research Center at Shahid Beheshti University of Medical Sciences, Tehran, Iran. Ethical guideline provisions from the Declaration of Helsinki were followed. Written consent for participation and for publication was obtained from the volunteers.
Three volunteer subjects with advanced RP aged 42–53 years were recruited. Visual acuity (VA) of all patients was poor light perception in both eyes with no projection. One patient had the history of cataract surgery; the two others had mild posterior subcapsular spoke-like cataracts. Slit lamp examination showed trace cells in the anterior vitreous. Fundus examination of all patients revealed diffuse pigment clumps as bone spicules, arterial narrowing and advanced waxy pallor of the optic disc. The left eye of each patient was included in this study.
Patients underwent multifocal electroretinography (mfERG, Roland Consult, Brandenburg, Germany) at baseline and at months 3, 6 and 12 after the injection. Optical coherence tomography (OCT) (Spectralis, Heidelberg Engineering, Heidelberg, Germany) was performed to assess the macular and peripapillary nerve fiber layer thicknesses. Enhanced depth imaging- OCT was performed to evaluate the central choroidal thickness. Fundus auto-fluorescence (FAF) imaging was performed by SLO (Model HRA/HRA 2; Heidelberg Engineering, Dossenheim, Germany) at baseline and at months 1, 3, 6 and 12. Fluorescein angiography (FA) was accomplished using SLO (Model HRA/HRA 2; Heidelberg Engineering, Dossenheim, Germany) at baseline and at month 3 after intravitreal injection of MSCs.
Isolation of Bone Marrow Cells and Mesenchymal Stem Cell Culture
Bone marrow aspiration was performed in operation room at Royan Institute for Stem Cell Biology and Technology affiliated to Academic Center for Education, Culture and Research (ACECR), Tehran, Iran. Subjects were sedated prior to the procedure and monitored by an anesthesiologist. After local anesthesia using 2% lidocaine, approximately 50 ml bone marrow was aspirated from the anterior and posterior iliac crest.
Cell separation and culture procedures were performed in a good manufacturing practice facility using standardized procedures. The mononuclear cell fraction was separated from the bone marrow by centrifugation under Ficoll-Hypaque gradient (GE Healthcare Life Sciences, Little Chalfont, UK) at 2000 revolutions per minute (rpm) without a break, at room temperature, for 30 minutes. After three washes in saline solution, cells were plated in culture. The cells were cultured in minimum essential Eagle's medium with alpha modifications supplemented with 10% gamma irradiated fetal bovine serum (FBS, HyClone, Logan, UT, USA) and 1X L-glutamine (Gibco, Thermo Fisher Scientific Inc., Waltham, USA). One-half of the medium was exchanged every 3 days. Once the cells achieved 80 to 90% confluence, they were dissociated with 0.25% trypsin/0.53 mM ethylenedinitrilotetraacetic acid (Gibco, Thermo Fisher Scientific Inc., Waltham, USA). and resuspended in saline solution containing 20% human serum albumin. MSC suspensions (1 × 106 cells/0.1 ml) were transferred into 1 ml syringes for local injection in subjects. Before transplantation, the cells were characterized by viability, flow cytometry analysis, and tested for sterility. The viability of cells was over 95%.
Flow Cytometry
Single-cell suspensions were stained with phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated anti-human CD90 (clone 5E10, BD 555595), CD105 clone 266, BD 560839), CD73 (clone AD2, BD 550257), CD44 (clone C26, BD 555478), CD45/CD34 (BD 341071), Cd 11b (clone p1H12, BD 550338), antibodies or the isotype-matched controls IgG2b (clone GC198, Millipore MABC006F), IgG1 (Dako X0932), IgG1, and k isotype control (clone MOPC-21, BD 551436). Suspensions were subsequently analyzed on a BD FACS Calibur flow cytometry system (BD Biosciences, San Jose, CA, USA) with software, version 2.5.1 (BD Biosciences). For each marker and isotype, 20000 events were acquired.
Cell Transplantation and Subject Follow-up
After instillation of an anesthetic eye drop (Tetracaine, Sina Darou Laboratories Co., Tehran, Iran), preparation and drape was performed in a sterile standard manner. A lid speculum was placed to keep the eye open. Each patient received an intravitreal injection of MSCs (106 per 0.1 ml) trans pars plana, 3.75 and 3.25 mm behind the limbus in the phakic and pseudophakic eyes, respectively, with a 27-gauge needle. After the intravitreal injection, anterior chamber paracentesis was done to reduce the intraocular pressure (IOP). Evaluation was performed during the 12-month follow-up period.
In vivo Animal Studies
Adult male C57/BL6 mice and B6Nude mice (20–25 g) were housed under light- and temperature-controlled conditions. All experiments were conducted in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Royan Institute.
Procured MSCs obtained from the RP patients, were injected into the mice vitreous cavities and the corresponding eyes were subjected to routine histopathologic and immunohistochemical studies. For immunofluorescence analysis, the sections were permeabilized with 0.3% Triton X-100 in phosphate-buffered saline (PBS) and blocked with normal secondary host serum. The sections were stained overnight with primary antibodies against specific anti-human Thy1 (Millipore, CBL415) and glial fibrillary acidic protein (GFAP; Sigma-Aldrich G3893). The stained sections were examined with a fluorescent microscope (Olympus, IX71, Japan) that had a DP72 digital camera following treatment with secondary antibodies: Goat anti-mouse Alexa-fluor 568 (Molecular Probes) or goat anti-mouse FITC (Sigma-Aldrich). Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI). A number of sections were stained with hematoxylin and eosin (H and E).
RESULTS
Clinical Assessments
MSCs used for transplantation were positive at passage one for CD90 (>90%), CD105 (>90%), CD73 (>90%) and CD44 (>60%). These MSCs showed low expression of CD11b (<30%) and slight double expression of CD45/34 (<10%) [Figure 1].
Figure 1.
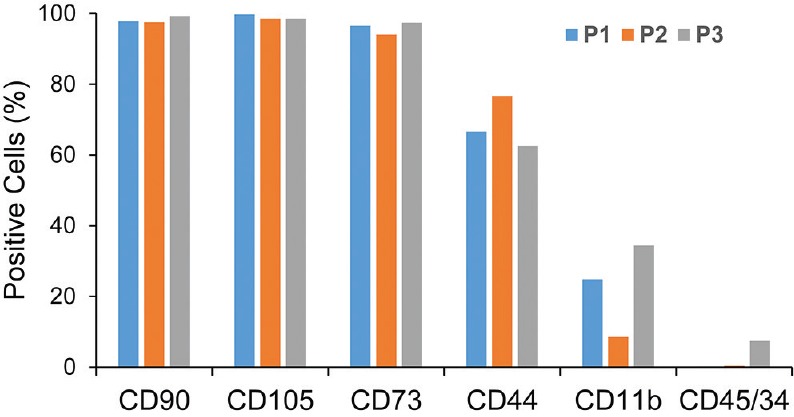
Flow cytometry of bone marrow-mesenchymal stem cells (MSCs) of patients with retinitis pigmentosa (RP) at primary culture. P, patient.
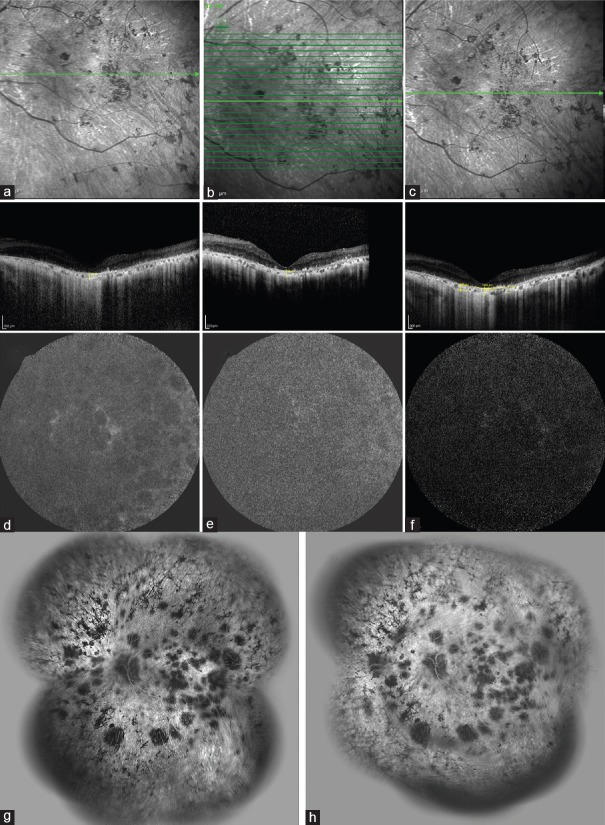
One day after intravitreal transplantation of 106 MSCs per 0.1 ml, ocular examination revealed no signs of any significant intraocular inflammation or increase in IOP. During the postoperative course, we observed no signs of adverse events in patients P1 and P2 at two weeks after intravitreal injection of the MSCs [Figure 2a–c]. These patients reported visual improvement 2 weeks after intravitreal injection of the MSCs which persisted up to 3 months. Multifocal ERG performed at months 3, 6 and 12 revealed no significant change compared to the baseline mfERG. Baseline OCT showed severe thinning of the macular center, marked atrophy of the outer retina, photoreceptor loss, severe reduction of the peripapillary nerve fiber layer and central choroidal thickness. A comparison of OCT findings before and at different time points after the MSCs injection did not show any significant differences in terms of central macular thickness (CMT), peripapillary nerve fiber layer thickness and central choroidal thickness (CCT) [Figure 2a–c]. Baseline FAF showed diffuse hypoautofluorescence with no change after the injection [Figure 2d–f]. FA revealed window defects and abnormal visibility of the choriocapillaris secondary to severe RPE atrophy in addition to extensive areas of hypofluorescence due to loss of the choriocapillaris. There was no significant leakage observed before and 3 months after the injection [Figure 2g and h].
Figure 2.
Enhanced depth imaging-optical coherence tomography (EDI-OCT) (a-c), auto-fluorescence (FAF) (d-f), and fluorescein angiography (FA) (g-h) images of the left eye of patient P2. (a) Prior to the cell injection, there was a severe reduction in the central macular and choroidal thicknesses, diffuse thinning of the inner and outer nuclear layers, and diffuse disruption of the external limiting membrane, ellipsoid zone and inter-digitation zone. Three (b) and 6 (c) months after cell injection there were no significant changes observed compared to baseline. FAF image shows significant hypoautofluorescence due to diffuse retinal pigment epithelium (RPE) loss (d). No significant changes were visible at months 3 (e) and 12 (f) after cell injection. FA image shows areas of hyperfluorescence due to the window defect caused by RPE atrophy and hypofluorescent areas due to the loss of choriocapillaris. No apparent leakage or changes were visible before (g) or at 3 months (h) after intravitreal injection of mesenchymal stem cells (MSCs).
In contrast, fundus examination of patient P3 showed extensive pre-retinal and vitreal fibrosis two weeks after the intravitreal injection of MSCs [Figure 3a–c]. The fibrous tissue increased after one month and led to tractional retinal detachment and further vision loss to no light perception (NLP). At 3-month follow-up, ciliary injection, cyclitic membrane manifesting as a retrolental fibrovascular tissue, shallow anterior chamber, ocular hypotony and nearly total tractional retinal detachment were seen [Figure 3d]. Patient experienced pain and discomfort in the injected eye. A treatment regimen including betamethasone and homatropine eye drops was initiated. At the 1-year follow-up, ocular examination revealed vision of NLP, mature cataract, extensive iris neovascularization, ocular hypotony and shallow anterior chamber [Figure 3e].
Figure 3.
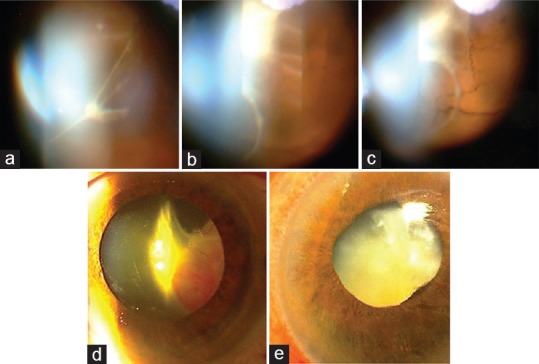
Slit lamp photographs of the left eye of patient P3. (a) Vitreal and pre-retinal fibrous membrane formation was observed 2 weeks after intravitreal injection of mesenchymal stem cells (MSCs). (b and c) The fibrous tissue severity increased after one month and led to total tractional retinal detachment. (d) Retrolental fibrovascular tissue was visible three months after intravitreal cell injection. (e) Six months after cell injection, mature cataract, ciliary injection, shallow anterior chamber and neovascularization of the iris were present.
Safety Outcome of MSCs of Patient P3
We sought to determine if patient P3 MSCs could form a fibrotic layer in the animal retina as they did in the patient's retina and compare them with the MSCs of patients P1 and P2. Intravitreal injection of MSCs of all three patients was performed in the mice eyes (n = 17) and the injected eyes were monitored repeatedly with the retina morphometry. On day 60, depth-selective histology images of the retina represented a fibrotic layer after injection of patient P3 MSCs [Figure 4a]. However, the injection of patients P1 and P2 MSCs into the vitreous cavity of the mice eyes did not result in the formation of any fibrotic layers [Figure 4a].
Figure 4.
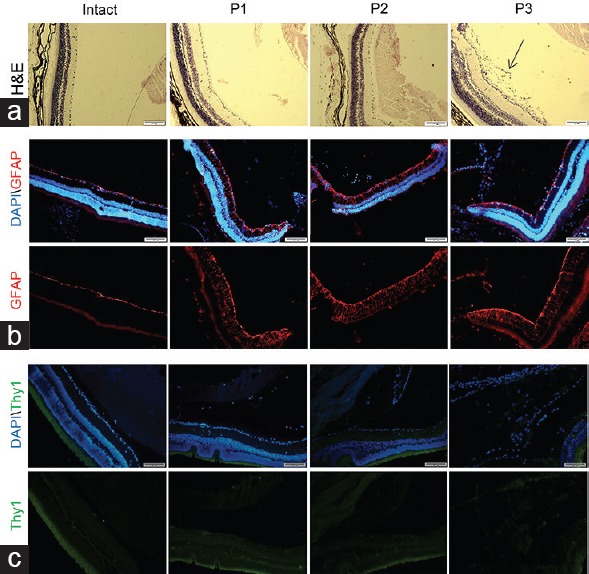
Evaluation of the mouse retina 60 days after injection of patients’ mesenchymal stem cells (MSCs). (a) Histology of the retina after staining with H and E. The fibrotic layer and marked pre-retinal membrane in the vitreous cavity behind the lens capsule were observed after transplantation of the MSCs of patient P3. Immunohistofluorescence staining for human GFAP (b) and Thy1 (c). The images of the retina sections did not depict any significant expression of human GFAP in three animals and also no expression of human Thy1 protein, which indicated that the generated layer possibly was not fibrosis or gliosis from a human origin. Nuclei visualized with DAPI staining (blue). The green stain in C was related to auto.fluorescence of the retinal pigment epithelium. H and E; Hematoxylin and eosin; GFAP, Glial fibrillary acidic protein; DAPI, 4’,6-diamidino-2-phenylindole.
Subsequently, we investigated the retinal glial responses to transplanted cells and evaluated the pre-retinal/retrolental tissue for graft-induced reactive gliosis by measuring the glial fibrillary acidic protein (GFAP) expression on the animal retina. Immunohistofluorescence on day 60 post-transplantation of MSCs of all three patients showed expression of GFAP in the Müller cell processes throughout the retina. However, its expression was limited to the nerve fiber layer in the intact retina [Figure 4b]. Nonetheless, no positive labeling was observed within the pre-retinal/retrolental tissue with MSCs of patient P3. This finding suggested that pre-retinal/retrolental tissue was not a glial reaction [Figure 4b].
In order to determine whether the fibrotic layer observed in the mice retina originated from human transplanted cells, we used an antibody against human specific-Thy1 which did not have any cross-reaction with the mice's [Figure 4c]. The results showed that the pre-retinal/retrolental tissue did not have a human source. The cells of this layer were not positive for human specific marker TRA-1-85, a marker which is specific for human cells. All antibodies were tested on human cells as the positive control (data not shown).
Furthermore, we examined tumor formation in cells derived from the patients' MSCs by transplanting the cells into the retinas and the subcutaneous regions of B6Nude mice (n = 6). None of the animals exhibited tumor formation within 6 months after treatment.
DISCUSSION
Eye is one of the first organs to be targeted by regenerative medicine. Three phase I clinical trials using embryonic stem cell-derived retinal pigment epithelium (RPE) have already been reported[13,14,15] and several others using a variety of cell types including bone marrow- or umbilical cord-mesenchymal stem cells, fetal neural or retinal progenitor cells, and adult stem cells-derived RPE are in earlier stages of development.[16]
In the present study, three patients with advanced RP received intravitreal injection of the autologous MSCs. Two eyes showed no adverse effects and the patients expressed improvement in the quality of their sight after two weeks, which persisted up to 3 months after intravitreal MSCs injection. However, the third patient developed a severe and progressive adverse event 2 weeks after cell transplantation. Siqueira et al conducted a prospective phase I trial and reported that intravitreal injection of autologous bone marrow-derived mononuclear cells in human eyes with advanced RP or cone-rod dystrophy was associated with no detectable structural or functional impairment over a period of 10 months.[8] In a phase II trial, they demonstrated that autologous bone marrow-derived mononuclear cells had a positive effect on cystoid macular edema (CME) associated with RP.[9] Recently, these researchers reported that cell therapy with these cells improved the quality of life of patients with RP, although the improvement was lost over time.[10] An improvement in ischemic macular edema or ischemic and degenerative retinal conditions after intravitreal injection of autologous bone marrow-derived hematopoietic stem cells has also been reported.[11,17]
Although we could not do biopsy of the fibrous tissue formed in the eye of the patient P3, we performed an animal experiment and observed that transplantation of MSCs of patient P3 induced an extra layer that resembled fibrosis after injection into the mouse vitreous cavity. MSCs of the other two patients did not generate fibrosis in the animal vitreous. Recently, Tassoni et al also demonstrated that following intravitreal injection of bone marrow MSCs, the recipient retina underwent GFAP overexpression, extensive macrophage infiltration, and retinal folding and detachment.[17] We did not detect any human MSCs in the affected animals eyes after two months, hence we have concluded that this reaction was related to the inflammatory responses to donor bone marrow MSCs.[17] This reaction following transplantation is not limited to MSCs, as it occurs in response to many other donor cell types such as neural cells,[18] Muller stem cells,[19] and photoreceptors[20] being transplanted either into the vitreous cavity or in the subretinal space for regenerative and protective purposes.
In summary, intravitreal transplantation of bone marrow MSCs for treatment of RP warrants animal studies in order to elucidate the possible adverse effects of this approach in the management of RP. As a solution, we propose evaluation of the cells in animals prior to their intravitreal injection in patients.
Financial Support and Sponsorship
This work was supported by grants from Royan Institute and the Ophthalmic Research Center at Shahid Beheshti University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137:503–519. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, Mandai M, Kamao H, Hashiguchi T, Shikamura M, Kawamata S, et al. Protective Effects of Human iPS-Derived Retinal Pigmented Epithelial Cells in Comparison with Human Mesenchymal Stromal Cells and Human Neural Stem Cells on the Degenerating Retina in rd1 mice. Stem Cells. 2015;33:1543–1553. doi: 10.1002/stem.1960. [DOI] [PubMed] [Google Scholar]
- 4.Jian Q, Li Y, Yin ZQ. Rat BMSCs initiate retinal endogenous repair through NGF/TrkA signaling. Exp Eye Res. 2015;132:34–47. doi: 10.1016/j.exer.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, et al. Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp Eye Res. 2014;118:135–144. doi: 10.1016/j.exer.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Jonas JB, Witzens-Harig M, Arseniev L, Ho AD. Intravitreal autologous bone marrow-derived mononuclear cell transplantation: A feasibility report. Acta Ophthalmol. 2008;86:225–226. doi: 10.1111/j.1600-0420.2007.00987.x. [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB, Witzens-Harig M, Arseniev L, Ho AD. Intravitreal autologous bone-marrow-derived mononuclear cell transplantation. Acta Ophthalmol. 2010;88:e131–e132. doi: 10.1111/j.1755-3768.2009.01564.x. [DOI] [PubMed] [Google Scholar]
- 8.Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: A phase I trial. Retina. 2011;31:1207–1214. doi: 10.1097/IAE.0b013e3181f9c242. [DOI] [PubMed] [Google Scholar]
- 9.Siqueira RC, Messias A, Voltarelli JC, Messias K, Arcieri RS, Jorge R. Resolution of macular oedema associated with retinitis pigmentosa after intravitreal use of autologous BM-derived hematopoietic stem cell transplantation. Bone Marrow Transplant. 2013;48:612–613. doi: 10.1038/bmt.2012.185. [DOI] [PubMed] [Google Scholar]
- 10.Siqueira RC, Messias A, Messias K, Arcieri RS, Ruiz MA, Souza NF, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial) Stem Cell Res Ther. 2015;6:29. doi: 10.1186/s13287-015-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, Jonnal R, et al. Intravitreal autologous bone marrow CD34 + cell therapy for ischemic and degenerative retinal disorders: Preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci. 2015;56:81–89. doi: 10.1167/iovs.14-15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siqueira RC, Voltarelli JC, Messias AM, Jorge R. Possible mechanisms of retinal function recovery with the use of cell therapy with bone marrow-derived stem cells. Arq Bras Oftalmol. 2010;73:474–479. doi: 10.1590/s0004-27492010000500019. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 15.Song WK, Park KM, Kim HJ, Lee JH3, Choi J4, Chong SY5, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: Preliminary results in Asian patients. Stem Cell Reports. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharti K, Rao M, Hull SC, Stroncek D, Brooks BP, Feigal E, et al. Developing cellular therapies for retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2014;55:1191–1202. doi: 10.1167/iovs.13-13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tassoni A, Gutteridge A, Barber AC, Osborne A, Martin KR. Molecular mechanisms mediating retinal reactive gliosis following bone marrow mesenchymal stem cell transplantation. Stem Cells. 2015;33:3006–3016. doi: 10.1002/stem.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinouchi R, Takeda M, Yang L, Wilhelmsson U, Lundkvist A, Pekny M, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nature Neurosci. 2003;6:863–868. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- 19.Singhal S, Lawrence JM, Bhatia B, Ellis JS, Kwan AS, Macneil A, et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Muller stem cells into degenerating retina. Stem Cells. 2008;26:1074–1082. doi: 10.1634/stemcells.2007-0898. [DOI] [PubMed] [Google Scholar]
- 20.Barber AC, Hippert C, Duran Y, West EL, Bainbridge JW, Warre-Cornish K, et al. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2013;110:354–359. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]