Abstract
Purpose:
To develop clinical practice guidelines (CPGs) for prevention, diagnosis, treatment and follow-up of ocular injuries caused by exposure to mustard gas.
Methods:
The clinical questions were designed by the guideline team. Websites and databases including National Guidelines Clearinghouse, National Institute for Clinical Excellence, Cochrane, and PubMed were searched to find related CPGs and explore possible answers to the clinical questions. Since there were no relevant CPGs in the literature, related articles in Persian and English languages were extracted. Each article along with its level of evidence was summarized. Additionally, hand search was performed by looking the reference list of each article. Consequently, recommendations were developed considering the clinical benefits and side effects of each therapeutic modality. The recommendations were re-evaluated in terms of customization criteria. All recommendations along with the related evidence were scored from 1 to 9 by experts from all medical universities of Iran. The level of agreement among the experts was evaluated by analyzing the given scores.
Results:
The agreement was achieved for all recommendations. The experts suggested a number of minor modifications which were applied to the recommendations. Finally, CPGs were developed with 98 recommendations under three major domains including prevention of injury, diagnosis and management of the acute and delayed-onset mustard gas ocular injuries.
Conclusion:
Considering the lack of CPGs for the prevention, diagnosis, and management of mustard gas-induced keratitis, these recommendations would be useful to prevent the serious ocular complications of mustard gas and standardize eye care services to the affected individuals.
Keywords: Clinical Practice Guidelines, Eye Injury, Iran, Mustard Gas
INTRODUCTION
Mustard gas (MG) is a cytotoxic chemical agent with the ability to form large blisters. It is available in sulfur mustard and nitrogen mustard forms;[1] both of them are used as a chemotherapy agent in the treatment of some dermatological diseases.[2] While nitrogen mustard is more toxic, sulfur mustard (SM) is more common as a chemical weapon since it can remain active for a longer period of time.[3,4,5,6,7]
SM was used as a chemical weapon for the first time in 1917, on a battlefield in Ypres, Belgium, during World War I.[8,9,10,11] The majority of MG victims were inflicted during wars. In recent years, the highest rate of MG use as a chemical warfare was in the Iraq-Iran war (1980–1988).[12,13]
MG causes severe inflammation in several tissues such as skin, eyes and respiratory tract.[2,14,15] Because of the wet and mucosal surface of the cornea and conjunctiva and the high volume of interactions and metabolic activities of corneal epithelial cells, eye is the most sensitive tissue to SM.[16,17,18,19] The delayed-onset MG injuries have been reported one to forty years after the initial exposure. That is why about thirty years after the end of the Iraq-Iran war; we are still faced with the incidence of new cases of delayed-onset MG ocular lesions.[4,20,21,22,23,24,25]
In the chronic and delayed injuries, patients usually complain of photophobia, foreign body sensation and dry eye. Ophthalmic examination shows microscopic corneal abrasion, ischemic limbal area and sometimes aberrant blood vessels in the corneal periphery (pannus).[4,21,26,27,28,29]
Decreased corneal sensation, recurrent corneal abrasions and damage to the limbal vessels lead to irregular corneal surface combined with stromal thinning.[21,30,31] Limbal damage leads to loss of the limbal stem cells and mucin-secreting goblet cells resulting in reduced tear secretion, which is the most common complaint of patients in the chronic and delayed-onset injuries.[32] These complications appear in almost 0.5 percent of the affected individuals who have been severely damaged during their first exposure and usually lead to progressive and permanent visual impairment and even blindness.[3,22]
At this time, there is no cure for SM chronic and delayed keratitis caused by and medications are mostly used as a palliative treatment.[33,34] On the other hand, there are still new cases of delayed-onset keratitis due to MG exposure after many years have passed since the exposure and there is a possibility of using MG weapons in the future. Therefore, the evidence-based clinical practice guidelines (CPGs) for prevention, diagnosis, treatment and follow-up of early and late eye injuries due to MG were developed by the Knowledge Management Unit (KMU) of the Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences under the supervision of the Office for Healthcare Standards, Deputy of Curative Affairs, Iran Ministry of Health and Medical Education.
METHODS
The CPGs developing team included corneal fellowships, general ophthalmologists, the Head of the Center for Injured Veterans in the Iranian Foundation of Martyrs and Veterans Affairs, a PhD by research candidate (a Master of Science degree holder in optometry), the Head of the Office for Healthcare Standards, Deputy of Curative Affairs, Iran Ministry of Health and Medical Education and a Master of Science degree holder in biostatistics.
Extracting the Current Clinical Guidelines
The Guidelines International Network (G-I-N), National Institute for Clinical Excellence (NICE), National Guidelines Clearinghouse (NGC), Scottish Intercollegiate Guidelines Network (SIGN), New Zealand Guidelines Group, National Health and Medical Research Council (NHMRC), Cochrane, Bandolier, CADTH, Trip Database, PubMed, Google Scholar, SID, Medlib and Magiran were explored to extract the relevant clinical guidelines. There were no similar CPGs in the literature. To complete the evidence, related archived articles in the Iranian Foundation of Martyrs and Veterans Affairs were also evaluated.
Designing the Clinical Questions and Extracting the Evidences
Clinical questions in the field of ocular injuries due to MG exposure were designed and the components of each clinical question {population (patients), intervention (exposure), comparison and outcome: PICO} were entered in Table 1.
Table 1.
Analysis of recommendations
Since no related CPGs was found, the evidence in English and Persian languages were extracted from the above-mentioned databases and websites using PICO to answer the questions and develop clinical recommendations.
Analyzing the Evidences
After reviewing and apprising each of the evidences, they were summarized in Table 2. The level of evidence was determined using Table 3.
Table 2.
Analysis of the evidences
Table 3.
Level of evidence
Developing the CPGs Recommendations Considering Clinical Benefits and Adaptability
The CPGs developing team composed clinical recommendations by implementing the details of Table 2 and considering the clinical advantages including benefits, side effects, effect size and costs and entered them in Table 4.
Table 4.
Clinical benefits of the recommendations
Then, the adaptability of each recommendation was evaluated in Table 5 based on three criteria: 1- applicability (access to proper equipment, skills at using them, and their affordability for patient), 2- acceptability (patient's preferences, cultural considerations and patient's acceptability of the therapeutic protocol), 3-external validity (similarity between patient's characteristics/disease type and their interference with studied evidences).
Table 5.
Adaptability of the recommendations (external validity)
Consensus (External Review)
The recommendations along with Tables 1, 2, 4 and 5 were sent to all faculty members who were experts in this field from Baqiyatallah University of Medical Sciences, Shahid Beheshti University of Medical Sciences, Ahvaz Jundishapur University of Medical Sciences, Mashhad University of Medical Sciences, Shahed University and Shiraz University of Medical Sciences. The faculty members were asked to score the recommendations from 1 to 9 considering the clinical advantages and the adaptability criteria. They were also asked to introduce any evidence that was not included in the tables and might alter the content of the recommendations.
Evaluation of the Level of Agreement and Formulation of Final Recommendations
The scores were analyzed by the RAM model to determine the levels of agreement on each recommendation.[35]
The agreement was achieved for all recommendations. Some of the experts suggested a number of minor modifications. After applying the modifications, 98 final recommendations were developed in three main categories: Prevention (Code A), ocular injuries in the acute phase of MG exposure (Code B), and ocular injuries in the chronic and delayed-onset phase of MG exposure (Code C), which will be presented in results section. Evidence levels (EL) have been presented for each recommendation. Appendix (195KB, pdf) shows the summary of each section recommendations.
SUMMARY OF RECOMMENDATIONS
RESULTS
Code A (Prevention)
A-1- When there is the possibility of using MG, it is recommended to prevent ocular injuries by considering the following points:
A-1-1- It is recommended that all personnel carry necessary standard protective equipment including protective mask and google, protective clothing and some potable water.[15,36,37,38,39]
EL: III – Consensus
A-1-2- It is necessary to teach all people who are exposed to in danger of MG exposure how to use the above-mentioned equipment and how to wash their eyes in a fast and steady manner.
EL: Consensus
A-1-3- If there is a possibility of MG exposure, it is recommended to evacuate all people who their presence is not necessary in the field.[4]
EL: IV, Consensus
A-2- In case of contacts with MG, it is recommended that the following procedures be performed immediately to reduce the eye injuries:
A- 2-1- All patients with symptoms of MG injury should immediately rinse their eyes with bathing water or normal saline for 10 to 15 minutes even if no ocular symptoms are present.[40]
EL: IV, Consensus
A-2-2- If the clean water is not accessible, it is recommended to put a wet towel over the eyes, mouth and nose.
EL: Consensus
A-2-3-All people with any sign of toxicity should be evacuated from the field and all their clothing and equipment be removed. Then, their whole body should be washed for 10 to 15 minutes with plenty of water while their eyes are closed.
EL: Consensus
A-2-4- After washing the whole body, it is recommended to bathe the eyes again with bathing water or normal saline for 5 minutes.
EL: Consensus
A-2-5- It is recommended that all people who have been present in the field but have no signs of toxicity should remove all their clothing and equipment and wash their whole body for 10 to 15 minutes with plenty of bathing water while their eyes are closed immediately after leaving the contaminated place.[3,41]
EL: IV
A-2-6- For those people in civilian areas (residential, factories, agricultural lands, etc.), who are asymptomatic and have been exposed to MG without using protective equipment, it is recommended to wash their eyes for 10 to 15 minutes using a normal saline solution, sodium bicarbonate solution 1.5%, Dichloramine-T 0.5%, sodium/magnesium sulfate, acide/zinc borique or with copious amounts of potable water.[41,42,43]
EL: IV
A-2-7- All health care personnel who have been in contact with exposed individuals should take off all their clothes as soon as possible after the end of their mission and wash their whole body especially their eyes with bathing water.
EL: Consensus
A-2-8- It is recommended to wash the eyes without using any kind of shampoo even diluted baby shampoo.
EL: Consensus
A-2-9- It is not recommended to put any eye patch or bandage after washing the eyes.[43,44,45]
EL: IV
A-2-10- It is recommended that local anesthetic eye drops (tetracaine or proparacaine eye drops) or any other topical pain medications not be used without a prescription and medical supervision.[46]
EL: IV
A-2-11- If artificial tears are available, it is recommended that the exposed subjects use them frequently.
EL: Consensus
A-2-12- It is recommended that the health care personnel anesthetize the affected individuals' eyes with the topical eye drops, insert a eyelid speculum and wash them for 10 to 15 minutes using normal saline or potable water.
EL: Consensus
A-2-13- If ocular pain is present, systemic analgesics should be prescribed under the supervision of medical personnel.
EL: Consensus
A-2-14- For the prevention of secondary infection, antibiotic eye drops are recommended (chloramphenicol or ciprofloxacin) every 6 hours for one week under the supervision of medical staff.[39]
EL: IV
A-2-15-It is recommended to wash all washable equipment in the health care center being involved in treating exposed subjects at the end of the day. The equipment which is not washable should be cleaned.
EL: Consensus
A-2-16- All objects in contact with MG should be placed in sturdy and durable polyethylene containers with a thickness of 6-mil and disposed of, to prevent the spread of MG contamination.[47]
EL: Consensus
A-2-17- Stability of MG is higher in cold regions, while in hot regions its stability is reduced. But due to rapid evaporation in hot regions, the concentration will increase. It is recommended that all people who are exposed to the MG, leave the infected areas as soon as possible.
EL: Consensus
Code B (Acute Phase of Ocular Injuries due to MG Exposure)
B-1- According to the internal consensus, the signs and symptoms of acute eye injuries were divided into three forms of mild, moderate and severe [Figure 1]
Figure 1.
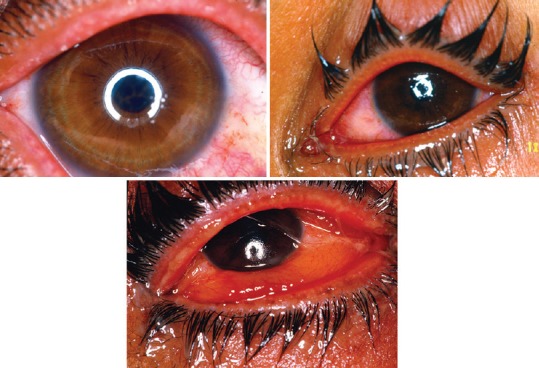
Ocular injuries due to mustard gas exposure in acute phase. Reproduced from “Javadi MA, Feizi S. Mustard gas ocular injuries in chemical warfare victims. 1st ed. Tehran: FARHANG FARDA; 2014”.
B-1-1- Patients with the following signs and symptoms of acute ocular injuries should be regarded as the mild form:[39,40,48]
Symptoms
Foreign body sensation in eyes, tearing, photophobia, blepharospasm
Signs
Eyelids hyperemia, vascular dilation and hyperemia of the conjunctiva, lack of corneal involvement
EL: IV- Consensus
B-1-2- Patients with the following signs and symptoms of acute ocular injuries should be regarded as the moderate form:[25,39,48]
Symptoms
Same as mild injury, plus any of the following symptoms:
Dry eye sensation, eye pain
Signs
Same as mild injury, plus any of the following signs:
Conjunctival edema, corneal epithelial edema, corneal epithelial erosion, superficial punctate keratopathy (more in the lid fissure area)
EL: IV- Consensus
B-1-3- Patients with the following signs and symptoms of acute ocular injuries should be regarded as the severe form:[25,39,40,48,49]
Symptoms
Same as mild and moderate injuries, plus any of the following symptoms:
Severe ocular pain, swelling, redness, sores and spasms of the eyelids, reduced vision
Signs
Same as mild and moderate injuries, plus any of the following signs:
Inflammation, edema and in some cases, secondary infection of the conjunctiva, ischemia and necrosis of the conjunctiva, limbal ischemia and necrosis, corneal epithelial irregularity and defect, corneal stromal edema, possible corneal infection, inflammation of the anterior chamber (uveitis), perforation of the cornea
EL: IV- Consensus
Important Recommendations
It should be noted that the symptoms of acute ocular injuries may range from the least to the most severe forms:
Eyelids: From swelling and redness to blepharospasm
Conjunctiva: From redness and foreign body sensation to severe chemosis
Cornea: From foreign body sensation to severe visual reduction and pain
It should also be noted that the signs of acute ocular injuries may be seen from the least to the most severe forms:
Eyelids: From hyperemia to ulcer and necrosis
Conjunctiva: From hyperemia to necrosis
Limbus: From hyperemia to necrosis
Cornea: From normal to corneal perforation
B-2- Diagnostic recommendations for the acute ocular injuries (mild, moderate and severe forms)
B-2-1- It is recommended that all personnel use MG detectors as soon as the use of chemical weapons is speculated to make a definitive diagnosis and rule out possible differential diagnoses.[49,50,51,52]
EL: IV- Consensus
B- 2-2- Since there is no laboratory test to definitively diagnose the acute phase, it is recommended that the diagnosis of this phase be determined by precise evaluation of the clinical signs and symptoms.[49,50,51,52]
EL: IV- Consensus
B-3- Treatment recommendations for the acute phase ocular injuries (mild, moderate and severe forms)
B-3-1- Mild form:
B-3-1-1- The eyes should be washed with abundant potable water immediately after MG exposure. In case of no access to potable water, any type of water (such as ponds, streams) can be used.
EL: Consensus
B-3-1-2- Topical antibiotics eye drops should be prescribed (e.g., chloramphenicol or ciprofloxacin eye drops every 6 hours to prevent bacterial infection).[7,38,52,53,54]
EL: IV- Consensus
B-3-1-3- Corticosteroids eye drops should be administered every 8 to 12 hours for a week.[38,52]
EL: IV- Consensus
B-3-1- 4- Artificial tears and lubricants should be administered every 6 hours.[7,38,52,53,54]
EL: IV- Consensus
B-3-1- 5- Wearing sunglasses
B- 3-2- Moderate form (starred ones are similar to the mild form):
B-3-2-1- Washing eyes with abundant potable water immediately after gas exposure and in case of no access to potable water, any bathing water (such as ponds, streams) should be used.*
EL: IV- Consensus
B-3-2-2- Topical antibiotics eye drops should be prescribed (e.g., ophthalmic chloramphenicol or ciprofloxacin* every 6 hours to prevent bacterial infection).[7,38,52,53,54]
EL: IV- Consensus
B-3-2-3- Corticosteroids eye drops* should be administered every 6 to 8 hours for a week.[38,52]
EL: IV- Consensus
B-3-2-4- Artificial tears with no preservatives (ocular lubricants)* should be administered every 4 to 6 hours.[7,38,52,53,54]
EL: IV- Consensus
B-3-2-5- Oral analgesics should be used under medical supervision if pain is present.
EL: Consensus
B-3-2-6- Wearing sunglasses*
B-3-3- Severe form (starred ones are similar to the mild and moderate forms):
B-3-3-1- Eyes should be washed with abundant potable water immediately after gas exposure and in case of lack of access to potable water, any bathing water (such as ponds, streams) should be performed.*
EL: IV- Consensus
B-3-3-2- Topical antibiotics eye drops should be prescribed every 6 hours for a week to prevent bacterial infection.*
EL: Consensus
B-3-3-3- In the absence of conjunctival and corneal infections, corticosteroids eye drops* should be administrated (one drop every 4 to 6 hours for a week and then reduce the dose as needed).[38]
EL: IV- consensus
B-3-3-4- Artificial tears with no preservatives (ocular lubricant)* should be administered every 2 to 4 hours.[7,38,52,53,54]
EL: IV- consensus
B-3-3-5- Bandage contact lens (in the presence of a large defect in the corneal epithelium and absence of dry eye and severe ischemia and corneal or conjunctival infection).[38]
EL: IV
B-3-3-6- Oral Doxycycline capsule (100 mg every 12 hours for 2 weeks and then as required).[52]
EL: IV
B-3-3-7- Oral analgesics should be used under medical supervision if pain is present*
EL: Consensus
B-3-3-8- Wearing sunglasses*
B-3-3-9- If there is any suspicion of secondary bacterial or fungal conjunctivitis, conjunctival specimen should be evaluated and then broad-spectrum antibiotics (e.g., ciprofloxacin eye drop) should be prescribed every one hour. Thereafter, the type and dosage of antibiotics should be adjusted based on the patient's response and the antibiogram results.
EL: Consensus
B-3-3-10- If any signs and symptoms of corneal infection are present, evaluating an immediate corneal specimen is recommended and depending on the severity of keratitis, fortified broad-spectrum antibiotics like gentamicin (14 mg per mL) eye drop, cefazolin (50 mg per ml) or vancomycin eye drop and ceftazidime eye drop every 5 to 15 minutes for an hour and then every half an hour for 24 hours should be started. Best antibiotic medication and its dosage should be adjusted depending on the patient's response and the antibiogram results.
EL: Consensus
B-3-3-11- If there is no response to treatment and perforation of the cornea happens, the following actions should be considered:
B-3-3-11-1- If the perforation diameter is equal to or less than 2 mm, in the absence of corneal infection and iris prolapse, applying cyanoacrylate glue and a bandage contact lens along with antibiotic eye drops and intravenous antibiotics are recommended.
EL: Consensus
B-3-3-11-2- If the perforation diameter is more than 2 mm or in the presence of iris prolapse, penetrating keratoplasty (PK) is recommended to protect the eye.
EL: Consensus
B-4- Follow-up recommendations for the acute ocular injuries (mild, moderate and severe forms)
B-4-1- Mild form: It is recommended that the next visit be performed one week after the diagnosis unless symptoms are intensified.
EL: Consensus
B-4-2- Moderate form: Daily visit is recommended until corneal symptoms are subsided and then weekly visit up to improvement of the conjunctiva and the eyelids.[38]
EL: IV -Consensus
B- 4-3- Severe form: It is recommended that the patients be hospitalized. Otherwise daily visit should be scheduled until complete healing of the corneal lesions are observed.[38]
EL: IV – Consensus
B-4-3-1- In case of infection or perforation of the cornea, the patient must be admitted to a hospital for treatment.
EL: Consensus
Code C (Chronic and Delayed-onset Ocular Injuries due to MG Exposure)
C-1- According to the internal consensus, the signs and symptoms of chronic and delayed-onset ocular injuries were divided into three forms; mild, moderate and severe [Figure 2]
Figure 2.
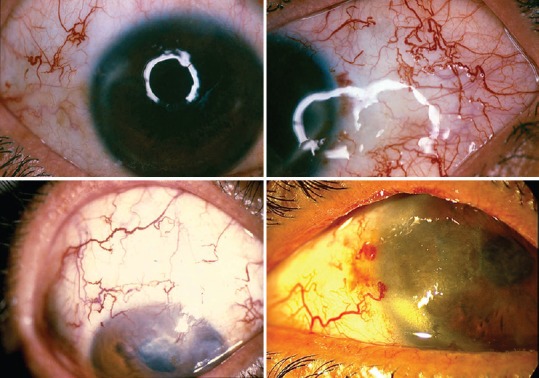
Chronic and delayed--onset ocular injuries due to mustard gas exposure. Reproduced from “Javadi MA, Feizi S. Mustard gas ocular injuries in chemical warfare victims. 1st ed. Tehran: FARHANG FARDA; 2014”.
C-1-1- Patients with the following signs and symptoms of the chronic and delayed ocular injuries should be regarded as the mild form:[18,22,27,37,39,40,48,55,56,57,58,59,60]
Symptoms
Photophobia, burning, foreign body sensation in eyes, dry eye, tearing, and slight redness of the eye
EL: II- Consensus
Signs
Meibomian gland dysfunction, chronic blepharitis, reduced thickness of the tear meniscus layer, telangiectasia of the conjunctival blood vessels, comma shape vascular tortuosity in the palpebral fissure area (nasal and temporal), subconjunctival fibrosis, subconjunctival hemorrhage, scarring of the conjunctiva, punctate epithelial erosions
EL: II – Consensus
C-1-2- Patients with the following signs and symptoms of the chronic and delayed ocular injuries should be regarded as the moderate form:
Symptoms
Same as mild injury, together with any of the following symptoms:
Reduced vision, marked red eye, itchy eyes, ocular pain
EL: II – Consensus
Signs
Same as mild injury, together with any of the following signs:
Corneal irregular astigmatism, periods of relapse and remission, mild to moderate limbal ischemia, irregular cornea, thinning of the corneal periphery, corneal opacity as well as lipid and amyloid material and deposition in the corneal periphery, peripheral corneal vascularization, peripheral stromal scars of the cornea, peripheral intra-corneal hemorrhage, transparency of the corneal center, decreased corneal sensation
EL: II – Consensus
C-1-3- Patients with the following signs and symptoms of the chronic and delayed ocular injuries should be regarded as the severe form:[18,22,37,39,40,48,55,60,61,62]
Symptom
Same as mild and moderate injuries, in addition to any of the following symptom:
Severe photophobia, severe vision loss, severe pain
EL: II- Consensus
Signs
Same as mild and moderate injuries, in addition to any of the following signs:
Severe limbal ischemia, limbal cell deficiency, thinning and opacity of the central and peripheral parts of the cornea, corneal opacity as well as lipid and amyloid deposition in the cornea, central and peripheral corneal vascularization, band keratopathy and scars in the central and peripheral corneal stroma, central and peripheral intra-corneal hemorrhage, corneal conjunctivalization, corneal descemetocele, corneal ulcer, corneal melting and perforation, history of limbal and corneal surgeries
EL: II – Consensus
Further Recommendations
Although our experience over the past 30 years suggests no direct relationship between mustard gas exposure and development of glaucoma, cataract, vitreoretinal and optic nerve disorders in the chronic phase, it is recommended that all patients with a history of intraocular surgery or those who are receiving long-term steroid therapy, be evaluated for these ocular diseases.
EL: Consensus
C-2- Diagnostic recommendations for the chronic and delayed-onset ocular injuries (mild, moderate and severe forms)
C-2-1- Chronic inflammation, overriding of the conjunctival epithelium to the cornea, decreased conjunctival goblet cells and epithelial thinning can be observed using an optical microscope. Electron microscopy shows destruction of the corneal basement membrane and cytoplasmic vacuolization but fluorescence microscopy findings are nonspecific. Routine biopsy is not recommended for detection of MG keratopathy unless during the surgical treatment.[38,63]
EL: III- Consensus
C-2- 2- Confocal microscopy findings include decreased thickness of the cornea, thinning and irregularity of the epithelium and basal layer as well as reduced number of stromal keratocytes, presence of spindle shape keratocytes, hyper-reflective deposits in the stroma and reduced endothelial cells. Endothelial changes are not significant.[38,64,65]
EL: II
C-2-3- In all patients with MG keratopathy with clinical signs, limbal cell defects can be detected by impression cytology during the chronic phase. Sometimes, these findings do not match with the clinical symptoms. Therefore, impression cytology is not usually recommended unless the clinical diagnosis is not sufficient.[50,66]
EL: III- Consensus
C-2-4- Laboratory methods for measuring white blood cells, polymorphonuclear leukocytes, the level of hydrogen peroxide, inflammatory cytokines (interleukin -1Ra, interleukin1beta, interleukin 1 alpha, tumor necrosis factor), serum levels of syllable period-selective, red blood cells, platelets, C-reactive protein, Rheumatoid factor, matrix metallopeptidase 9, serum C3, C4, natural killer cells, Immunoglobulin M, Immunoglobulin F and Immunoglobulin E have been evaluated in several studies for diagnosis of ocular disorders in chronic phase, but none are specific for MG exposure and are not usually recommended.[40,67,68,69,70,71,72,73]
EL: II- Consensus
Serum levels of cytokines in people who have been exposed to MG is less than the control group even 20 years after exposure. But since this is a common finding in many chronic diseases, this test is not usually recommended.[68]
EL: II- Consensus
Generally, these tests are not recommended in cases where the clinical diagnosis of MG exposure is certain. But they can be useful in cases where the clinical signs are not sufficient for diagnosis.
C-3- Treatment recommendations for the chronic and delayed-onset ocular injuries (mild, moderate and severe forms)
C-3-1- Mild form:
C-3-1-1- Wearing appropriate sunglasses especially for people who live in dry and sunny environments.
EL: Consensus
C-3-1-2- Supplying more moisture in the living environment or to live in the humid areas.
EL: Consensus
C-3-1-3- Artificial tears and ocular lubricants with or without preservatives (1 to 4 times a day).[22,27,38]
EL: II – Consensus
C-3-1-4- Topical treatment for blepharitis (warm compresses, shampoo scrubs, topical antibiotics). In cases resistant to topical therapy, systemic antibiotics (tetracycline, azithromycin, and doxycycline) are recommended.[74]
EL: II- Consensus
C-3-1-5- Temporary punctual occlusion[22,27,29,30,31]
EL: II- Consensus
C- 3-2- Moderate form (starred items are similar to the mild form):
C-3-2-1- Appropriate sunglasses should be worn especially for people who live in dry and sunny environments.*
EL: Consensus
C-3-2-2- Supplying more moisture in the living environment or to live in the humid areas.*
EL: Consensus
C-3-2-3- Prescribing artificial tears and lubricants eye drop with no preservatives (1 to 4 times a day).[22,27,38]*
EL: II- Consensus
C-3-2-4- Topical treatment of blepharitis (warm compresses, shampoo scrubs, topical antibiotics). In cases resistant to topical therapy, systemic antibiotics (tetracycline, azithromycin, and doxycycline) are recommended.[74]*
EL: II – Consensus
C-3-2-5- Temporary* or permanent punctual occlusion[22,27,29,30,31]
EL: II – Consensus
C-3-2-6- Tarsorrhaphy in case of severe dry eye[22,27]
EL: II
C-3-2-7- Prescribing the corticosteroids eye drops with control of possible complications or topical cyclosporine A (0.05%) (Twice a day)[22,27,29,30,38,75]
EL: II
C-3-2-8- In cases with significant peripheral corneal thinning who present with noticeable symptoms such as redness, tearing, decreased visual acuity and risk of corneal perforation, kratolimbal allograft surgery is recommended.[31]
EL: II
C-3-2-9- In cases where corneal pathology is present in the periphery (thinness and moderate to severe ischemia of the conjunctiva), conjunctival advancement surgery is not recommended.[38]
EL: IV
C-3-3- Severe form (starred items are similar to the mild or moderate forms):
C-3-3- 1- medical treatments:
C-3-3-1-1- Wearing sunglasses*
EL: Consensus
C-3-3-1- 2- Supplying more moisture in the living environment or living in the humid areas*
EL: Consensus
C-3-3-1-3- Prescribing artificial tears and lubricant eye drops with no preservatives (1 to 4 times a day)[22,27,38]
EL: II- Consensus
C-3-3-1- 4- Topical treatment of blepharitis (warm compresses, shampoo scrubs, topical antibiotics). In cases resistant to topical therapy, systemic antibiotics (tetracycline, azithromycin, and doxycycline) are recommended.[74]*
EL: II – Consensus
C-3-3-1-5- Temporary or permanent punctual occlusion[22,27,29,30,31]*
EL: II
C-3-3-1-6- Prescribing topical corticosteroids with control of possible complications or topical cyclosporine A (0.05%) (Twice a day).[22,27,29,30,31]*
EL: II
C-3-3- 2- Surgical treatments:
C-3-3-2-1- Tarsorrhaphy in case of severe dry eye[22,27]*
EL: II
C-3-3-2-2- If epithelial defect is resistant to medical treatment, it is recommended to perform amniotic membrane transplantation in addition to tarsorrhaphy.[22,31]
EL: II
C-3-3-2-3- In case of ischemia and thinning of the cornea and the sclera adjacent to it, kratolimbal allograft is recommended.[31,74,76]
EL: II
C-3-3-2-4- If stem cell transplantation is needed, keratolimbal method is preferable to using stem cells from first-degree relatives.[27]
EL: II
C-3-3-2-5- In case of ischemia coupled with thinning of the cornea, sclera and limbus combined with central corneal epithelial cells deficit, combined surgery of amniotic membrane transplantation and limbal stem cell transplantation is recommended.[31]
EL: II
C-3-3-2-6- In cases of epithelial defect resistant to medical treatment it is recommended to administer bandage contact lens while doing other treatments.
EL: Consensus
C-3-3-2-7- If the opacity has affected the central cornea and normal endothelial cells are present, lamellar transplantation is preferred.[30]
EL: II
C-3-3-2-8- Deep anterior corneal transplantation with big bubble technique is not recommended in these patients and the conventional method is preferred.[27,31]
EL: II
C-3-3-2-9- If lamellar graft of the cornea and limbal stem cell transplantation is needed, combined surgery is preferable to the sequential surgery.[31]
EL: II
C-3-3-2-10- In case of corneal perforation and endothelial cells damage, it is recommended to perform PK.[30]
EL: II
C-3-3-2-11- If both PKP and stem cell transplantation are indicated, it is advisable to perform stem cell transplantation first followed by PKP after a few months (combined surgery is not recommended).[29]
EL: III
C-4- Follow-up recommendations related in the chronic and delayed-onset ocular injuries (in mild, moderate and severe forms)
C-4-1- Mild form:
C-4-1-1- If symptoms have not changed in the course of the disease, it is recommended that patients be examined every year.
EL: Consensus
C-4-1-2- If a change in symptoms or signs is noticed by the patient or the physician, it is recommended to perform eye examinations at appropriate intervals.
EL: Consensus
C-4-2- Moderate form (starred ones are similar to the mild form):
C-4-2-1- If medical treatment is adequate for the patient and the patient's condition is stable, it is recommended the patient be followed every 6 months.
EL: Consensus
C-4-2-2- If a change in symptoms or signs is observed by the patient or the physician, it is recommended to perform eye examinations at appropriate intervals.*
EL: Consensus
C-4-2-3- Patients who undergo keratolimbal surgery should be followed according to the known protocol for keratolimbal surgery (visit on the first and third days, first week, second week, first, second and third months and then every 3 months in the first year and then every 6 months). In case of patient's complaints or any unusual finding in ocular examination, patient should be followed at appropriate intervals.
EL: Consensus
C-4-2-4- It is recommended that medical therapy, especially systemic steroids and immunosuppressive medications be prescribed in collaboration with a nephrologist or an oncologist.
EL: Consensus
C-4-3- Severe form (starred ones are similar to the mild and moderate forms):
C-4-3-1- If the patient's condition is stable; it is recommended the patient be followed every 3 months.
EL: Consensus
C-4-3-2- If a change in symptoms or signs is observed by the patient or the physician, it is recommended to perform eye examinations at appropriate intervals.*
EL: Consensus
C-4-3-3- Patients who have a history of surgery should be followed based on the type of surgery.
EL: Consensus
DISCUSSION
Considering severe ocular injures due to MG exposure, Iran's unique experience in this field and the lack of similar CPGs in the world, this CPGs were developed in KMU, Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences under the supervision of the Iranian Ministry of Health and Medical Education with a total of 98 recommendations.
Due to the importance of appropriate diagnosis and treatment of the affected patients and the lack of standard procedures for patient management, all experts in this field who had practically managed and followed chemically injured patients for many years were invited to participate as a member of the CPGs developing team or an external reviewer (consensus).
An agreement score was achieved for all recommendations developed by the CPGs developing team in the external review. Then, these recommendations were considered as final recommendations after minor editing suggested by the experts.
Due to critical war conditions, high level evidence such as meta-analyses, systematic reviews, and clinical trials on the management of acute MG injury is limited in the literature and most of the data is not based on strong methodology. Moreover, most of the evidence related to ocular injures due to MG exposure in chronic and delayed onset injuries is also confined. Published review articles are not systematic reviews and are written by experts in the field as narrative reviews.
Since most evidence of ocular injuries due to MG exposure has been critically reviewed in this project, we believe it will be helpful to perform well-designed research projects in the future.
In conclusion, the CPGs for the “prevention, diagnosis, treatment and follow-up of early and late eye injuries due to mustard gas” will promote the standardization of the prevention, diagnosis, treatment and follow-up of the affected patients at national and international levels.
Financial Support and Sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This research was supported by Janbazan Medical and Engineering Research Center, Tehran, Iran. We are immensely grateful to the faculty members of all universities for scoring the recommendations of this clinical practice guideline. We thank Mr. Saeed Rahmani for his valuable assistance. We also appreciate Ms. Soheila Khoshneshin's technical assistance.
REFERENCES
- 1.Smith KJ. The prevention and treatment of cutaneousinjury secondary to chemical warfare agents. Application of these finding to other dermatologic conditions and wound healing. Dermatol Clin. 1999;17:41–60. doi: 10.1016/s0733-8635(05)70069-3. [DOI] [PubMed] [Google Scholar]
- 2.Smith KJ, Skelton H. Chemical warfare agents. Their past and continuing threat and evolving therapies. Part I. Skin Med. 2003;2:215–221. doi: 10.1111/j.1540-9740.2003.02509.x. [DOI] [PubMed] [Google Scholar]
- 3.Safarinejad MR, Moosavi SA, Montazeri B. Ocular injuries caused by mustard gas: Diagnosis, treatment, and medical defense. Mil Med. 2001;166:67–70. [PubMed] [Google Scholar]
- 4.Solberg Y, Alcalay M, Belkin M. Ocular injury by mustard gas. Surv Ophthalmol. 1997;41:461–466. doi: 10.1016/s0039-6257(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 5.Watson AP, Griffin GD. Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program. Environ Health Perspect. 1992;98:259–280. doi: 10.1289/ehp.9298259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann I. A study of eighty-four cases of delayed mustard gas keratitis fitted with contact lenses. Br J Ophthalmol. 1944;28:441–447. doi: 10.1136/bjo.28.9.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aasted A, Darre E, Wulf HC. Mustard gas: Clinical, toxicological, and mutagenic aspects based on modern experience. Ann Plast Surg. 1987;19:330–333. [PubMed] [Google Scholar]
- 8.Takafuji ET, Kok AB. The chemical warfare threat and the military healthcare provider. In: Zajtchuk R, editor. Textbook of military medicine: Medical aspects of chemical and biological warfare: Part I. USA: Office of the surgeon general department of the army; 1997. pp. 101–128. [Google Scholar]
- 9.Blewett W. Tactical weapons: Is mustard still king. NBC Defense Technol Int. 1986;1:64–66. [Google Scholar]
- 10.Jenner J, Graham SJ. Treatment of sulphur mustard skin injury. Chem Biol Interact. 2013;206:491–495. doi: 10.1016/j.cbi.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Blodi FC. Mustard gas keratopathy. Int Ophthalmol Clin. 1971;11:1–13. [PubMed] [Google Scholar]
- 12.Dworkin J, Prescott M, Jamal R, Hardawan SA, Abdullah A, Galea S. The long-term psychosocial impact of a surprise chemical weapons attack on civilians in Halabja, Iraqi Kurdistan. J Nerv Ment Dis. 2008;196:772–775. doi: 10.1097/NMD.0b013e3181878b69. [DOI] [PubMed] [Google Scholar]
- 13.Namazi S, Niknahad H, Razmkhah H. Long-term complications of sulphur mustard poisoning in intoxicated Iranian veterans. J Med Toxicol. 2009;5:191–195. doi: 10.1007/BF03178265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panahi Y, Gholami N, Ghojazadeh M, Moslemi F, Naghavi-Behzad M, Azami-Aghdash S, et al. complications and carcinogenic effects of mustard gas-a systematic review and meta-analysis in Iran. Asian Pac J Cancer Prev. 2015;16:7567–7573. doi: 10.7314/apjcp.2015.16.17.7567. [DOI] [PubMed] [Google Scholar]
- 15.Saladi RN, Smith E, Persaud AN. Mustard: A potential agent of chemical warfare and terrorism. Clin Exp Dermatol. 2006;31:1–5. doi: 10.1111/j.1365-2230.2005.01945.x. [DOI] [PubMed] [Google Scholar]
- 16.Mandel M, Gibson WS. Clinical manifestations and treatment of gas poisoning. J Am Med Assoc. 1917;69:1970–1971. [Google Scholar]
- 17.Pickard HL. Ocular action of dichloroethyl sulfide (mustard gas) Am J Ophthalmol. 1919;3:136. [Google Scholar]
- 18.Etezad-Razavi M, Mahmoudi M, Hefazi M, Balali-Mood M. Delayed ocular complications of mustard gas poisoning and the relationship with respiratory and cutaneous complications. Clin Experiment Ophthalmol. 2006;34:342–346. doi: 10.1111/j.1442-9071.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 19.Balali-Mood M, Mousavi Sh, Balali-Mood B. Chronic health effects of sulphur mustard exposure with special reference to Iranian veterans. Emerg Health Threats J. 2008;1:e7. doi: 10.3134/ehtj.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duke-Elder S, MacFaul PA. Injuries. Part 2. Non-mechanical injuries. In: Duke-Elder S, editor. System of Ophthalmology. Vol. 14. London: Henry Kimpton; 1972. pp. 1133–1158. [Google Scholar]
- 21.Dahl H, Gluud B, Vangsted P, Norn M. Eye lesions induced by mustard gas. Acta Ophthalmol Suppl. 1985;173:30–31. doi: 10.1111/j.1755-3768.1985.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 22.Javadi MA, Yazdani S, Sajjadi H, Jadidi K, Karimian F, Einollahi B, et al. Chronic and delayed-onset mustard gas keratitis: Report of 48 patients and review of literature. Ophthalmology. 2005;112:617–625. doi: 10.1016/j.ophtha.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Iovieno A, Anand S, Dart JK. Late-onset peripheral ulcerative sclerokeratitis associated with alkali chemical burn. Am J Ophthalmol. 2014;158:1305–1309.e4. doi: 10.1016/j.ajo.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Rowell M, Kehe K, Balszuweit F, Thiermann H. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263:9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Pleyer U, Sherif Z, Baatz H, Hartmann C. Delayed mustard gas keratopathy: Clinical findings and confocal microscopy. Am J Ophthalmol. 1999;128:506–507. doi: 10.1016/s0002-9394(99)00178-6. [DOI] [PubMed] [Google Scholar]
- 27.Javadi MA, Jafarinasab MR, Feizi S, Karimian F, Negahban K. Management of mustard gas-induced limbal stem cell deficiency and keratitis. Ophthalmology. 2011;118:1272–1281. doi: 10.1016/j.ophtha.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Shiyovich A, Rosman Y, Krivoy A, Statlender L, Kassirer M, Shrot S. Harefuah. Long-term complications of sulfur mustard exposure: A therapeutic update. Harefuah. 2014;153:199–205, 237. [PubMed] [Google Scholar]
- 29.Javadi MA, Yazdani S, Kanavi MR, Mohammadpour M, Baradaran-Rafiee A, Jafarinasab MR, et al. Long-term outcomes of penetrating keratoplasty in chronic and delayed mustard gas keratitis. Cornea. 2007;26:1074–1078. doi: 10.1097/ICO.0b013e3181334752. [DOI] [PubMed] [Google Scholar]
- 30.Feizi S, Javadi MA, Jafarinasab MR, Karimian F. Penetrating keratoplasty versus lamellar keratoplasty for mustard gas–induced keratitis. Cornea. 2013;32:396–400. doi: 10.1097/ICO.0b013e3182609287. [DOI] [PubMed] [Google Scholar]
- 31.Jafarinasab MR, Feizi S, Javadi MA, Karimian F, Soroush MR. Lamellar keratoplasty and keratolimbal allograft for mustard gas keratitis. Am J Ophthalmol. 2011;152:925–932. doi: 10.1016/j.ajo.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 32.New Zealand Guidelines Group. Handbook For The Preparation of Explicit Evidence-Based Clinical Practice Guidelines: New Zealand Guidelines Group. 2001 [Google Scholar]
- 33.Balali-Mood M, Hefazi M. The clinical toxicology of sulfur mustard. Arch Iran Med. 2005;8:162–179. [Google Scholar]
- 34.Panahi Y, Naderi M, Zare MA, Poursaleh Z. Ocular Effects of Sulfur Mustard. Iran J Ophthalmol. 2013;25:90–106. [Google Scholar]
- 35.New Zealand Guidelines Group. Handbook for the preparation of explicit evidence-based clinical practice guidelines: New Zealand Guidelines Group. 2001 [Google Scholar]
- 36.Agin KH, Ghasemi-Bromand M. The study of relationship between pulmonary system disability and long term eye complications in Iranian victims exposed to sulfur mustard gas [in Persian] Ann Mil Health Sci Res. 2003;1:157–161. [Google Scholar]
- 37.Ghasemi H, Ghazanfari T, Yaraee R, Soroush MR, Ghassemi-Broumand M, Poorfarzam S, et al. Systemic and ocular complications of sulfur mustard: A panoramic review. Informa Health Care. 2009;28:14–23. [Google Scholar]
- 38.Baradaran-Rafii A, Eslani M, Tseng SC. Sulfur mustard-induced ocular surface disorders. Ocul Surf. 2011;9:163–178. doi: 10.1016/s1542-0124(11)70026-x. [DOI] [PubMed] [Google Scholar]
- 39.Graham JS, Schoneboom BA. Historical perspective on effects and treatment of sulfur mustard injuries. Chem Biol Interact. 2013;206:512–522. doi: 10.1016/j.cbi.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin Pharmacol Toxicol. 2006;99:273–282. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- 41.Balali-Mood M, Hefazi M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam Clin Pharmacol. 2005;19:297–315. doi: 10.1111/j.1472-8206.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 42.William F, Hughes Mustard gas injuries to the eyes. Arch Ophthalmol. 1942;27:582–601. [Google Scholar]
- 43.Berens C, Hartmann E. the effect of war gases and other chemicals on the eyes of the civilian population. Bull N Y Acad Med. 1943;19:356–367. [PMC free article] [PubMed] [Google Scholar]
- 44.De Courcy T. L. A case of mustard gas keratitis under constant observation for a period of twenty years. Br J Ophthalmol. 1943;27:54–60. doi: 10.1136/bjo.27.2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter S. Atkinson. Delayed mustard gas keratitis (Dichlorodiethyl Sulfide). A report of two cases. Trans Am Ophthalmol Soc. 1947;45:81–92. [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenwasser GO. Complications of topical ocular anesthetics. Int Ophthalmol Clin. 1989 Fall;29:153–158. doi: 10.1097/00004397-198902930-00005. [DOI] [PubMed] [Google Scholar]
- 47.Sulfur mustard: Blister agent. [Last accessed on 2015 Jul 23]. Available at: https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750008.html .
- 48.Ghasemi H, Owlia P, Jalali-Nadoushan MR, Pourfarzam S, Azimi G, Yarmohammadi ME, et al. A clinicopathological approach to sulfur mustard-induced organ complications: A major review. Cutan Ocul Toxicol. 2013;32:304–324. doi: 10.3109/15569527.2013.781615. [DOI] [PubMed] [Google Scholar]
- 49.McNutt P, Hamilton T, Nelson M, Adkins A, Swartz A, Lawrence R, et al. Pathogenesis of acute and delayed corneal lesions after ocular exposure to sulfur mustard vapor. Cornea. 2012;31:280–290. doi: 10.1097/ICO.0B013E31823D02CD. [DOI] [PubMed] [Google Scholar]
- 50.Kadar T, Dachir S, Cohen M, Gutman H, Cohen L, Brandeis R, et al. Prolonged impairment of corneal innervation after exposure to sulfur mustard and its relation to the development of delayed limbal stem cell deficiency. Cornea. 2013;32:e44–50. doi: 10.1097/ICO.0b013e318262e885. [DOI] [PubMed] [Google Scholar]
- 51.McNutt P, Tuznik K, Nelson M, Adkins A, Lyman M, Glotfelty E, et al. Structural, morphological, and functional correlates of corneal endothelial toxicity following corneal exposure to sulfur mustard vapor. Invest Ophthalmol Vis Sci. 2013;54:6735–6744. doi: 10.1167/iovs.13-12402. [DOI] [PubMed] [Google Scholar]
- 52.Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, et al. Ocular injuries following sulfur mustard exposure--pathological mechanism and potential therapy. Toxicology. 2009;263:59–69. doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Jampol LM, Axelrod A, Tessler H. Pathways of the eye's response to topical nitrogen mustard. Invest Ophthalmol. 1976;15:486–489. [PubMed] [Google Scholar]
- 54.Banin E, Morad Y, Berenshtein E, Obolensky A, Yahalom C, Goldich J, et al. Injury induced by chemical warfare agents: Characterization and treatment of ocular tissues exposed to nitrogen mustard. Invest Ophthalmol Vis Sci. 2003;44:2966–2972. doi: 10.1167/iovs.02-1164. [DOI] [PubMed] [Google Scholar]
- 55.Sedghipour MR, Shenasi A, Rahbani Nobar MB, Fouladi RF, Amini R. The ocular complications of mustard gas poisoning and their association with the respiratory system involvement: An experience in 112 Iranian veterans. Cutan Ocul Toxicol. 2012;31:48–52. doi: 10.3109/15569527.2011.613425. [DOI] [PubMed] [Google Scholar]
- 56.Ghasemi H, Ghazanfari T, Babaei M, Soroush MR, Yaraee R, Ghassemi-Broumand M, et al. Long-term ocular complications of sulfur mustard in the civilian victims of sardasht, Iran. Cutan Ocul Toxicol. 2008;27:317–326. doi: 10.1080/15569520802404382. [DOI] [PubMed] [Google Scholar]
- 57.Ghasemi-Broumand M, Aslani J, Emadi SN, Amiri Z. The prevalence of the late onset ocular, respiratory and cutaneous complications due to mustard gas exposure in chemical bombing victims of Sardasht residents [in Persian] Pajoohandeh J. 2006;11:9–15. [Google Scholar]
- 58.Ghasemi-Broumand MR, Amiri Z. Delayed ocular complications of mustard gas on 500 veterans [in Persian] J Rehabil. 2007;8:67–74. [Google Scholar]
- 59.Ghassemi-Broumand M, Agein Kh. Relationship between socio-demographic factors with intensity of the late complications of sulfur mustard induced diseases in 500 Iranian victims (after15 years) [in Persian] Ann Mil Health Sci Res. 2004;2:269–273. [Google Scholar]
- 60.Ghasemi H, Ghazanfari T, Ghassemi-Broumand M, Javadi MA, Babaei M, Soroush MR, et al. Long-term ocular consequences of sulfur mustard in seriously eye-injured war veterans. Cutan Ocul Toxicol. 2009;28:71–77. doi: 10.1080/15569520902913936. [DOI] [PubMed] [Google Scholar]
- 61.Mansour Razavi S, Salamati P, Saghafinia M, Abdollahi M. A review on delayed toxic effects of sulfur mustard in Iranian veterans. Daru. 2012;20:51. doi: 10.1186/2008-2231-20-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balali-Mood M, Hefazi M. A review on the delayed complications of sulphur [in Persian] J Birjand Univ Med Sci. 2005;12:5–15. [Google Scholar]
- 63.Jadidi Kh, Sadeghipour AR, Naderi M, Haghighi M, Rafizade P. Histopathological study of delayed mustard gas keratopathy [in Persian] J Mil Med. 2011;12:191–195. [Google Scholar]
- 64.Lagali N, Fagerholm P. Delayed Mustard Gas Keratitis: Clinical course and in vivo confocal microscopy findings. Cornea. 2009;28:458–462. doi: 10.1097/ICO.0b013e31818a7dd0. [DOI] [PubMed] [Google Scholar]
- 65.Jafarinasab MR, Zarei-Ghanavati S, Kanavi MR, Karimian F, Soroush MR, Javadi MA. Confocal microscopy in chronic and delayed mustard gas keratopathy. Cornea. 2010;29:889–894. doi: 10.1097/ICO.0b013e3181ca324c. [DOI] [PubMed] [Google Scholar]
- 66.Baradaran-Rafii A, Javadi MA, Rezaei Kanavi M, Eslani M, Jamali H, Karimian F. Limbal stem cell deficiency in chronic and delayed-onset mustard gas keratopathy. Ophthalmology. 2010;117:246–252. doi: 10.1016/j.ophtha.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 67.Jadidi K, Mir Kheshti N, Ghasami F, Alavi SA, Talebi Sh, Hadi Zadeh F, et al. The role of reactive Oxygen species in delayed ophthalmic disorders of Sulfur Mustard [in Persian] J Mil Med. 2005;7:9–14. [Google Scholar]
- 68.Yaraee R, Ghazanfari T, Ebtekar M, Ardestani SK, Rezaei A, et al. Alterations in serum levels of inflammatory cytokines (TNF, IL-1alpha, IL-1beta and IL-1Ra) 20 years after sulfur mustard exposure: Sardasht-Iran cohort study. Int Immunopharmacol. 2009;9:1466–1470. doi: 10.1016/j.intimp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Amiri S, Ghazanfari T, Yaraee R, Salimi H, Ebtekar M, Shams J, et al. Serum levels of GM-CSF 20 years after sulfur mustard exposure: Sardasht-Iran Cohort Study. Int Immunopharmacol. 2009;9:1499–1503. doi: 10.1016/j.intimp.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 70.Ghasemi H, Yaraee R, Hassan ZM, Faghihzadeh S, Soroush MR, Pourfarzam S, et al. Association of ophthalmic complications in patients with sulfur mustard induced mild ocular complications and serum soluble adhesion molecules: Sardasht–Iran Cohort Study. Int Immunopharmacol. 2013;17:980–985. doi: 10.1016/j.intimp.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Ghazanfari T, Yaraee R, Ghasemi H, Masdari Z, Vaez Mahdavi M, Mohammad Hasan Z. Relationship between serum levels of MMP-9 and late ocular complication in chemical veterans due to mustard gas exposure [in Persian] Iran J War Public Health. 2009;1:1–16. [Google Scholar]
- 72.Ghasemi H, Ghazanfari T, Yaraee R, Ghassemi-Broumand M, Soroush MR, Pourfarzam S, et al. Evaluation of relationship between the serum levels of inflammatory mediators and ocular injuries induced by sulfur mustard: Sardasht-Iran Cohort Study. Int Immunopharmacol. 2009;9:1494–1498. doi: 10.1016/j.intimp.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 73.Shams J, Ghazanfari T, Yaraee R, Vaez Mahdavi MR, Soroush MR, Hassan ZM, et al. Long-term hematological consequences of sulfur mustard on civilians of Sardasht 20 years after exposure. Toxin Rev. 2009;28:39–43. [Google Scholar]
- 74.Karimian F, Zarei-Ghanavati S, A BR, Jadidi K, Lotfi-Kian A. Microbiological evaluation of chronic blepharitis among Iranian veterans exposed to mustard gas: A case-controlled study. Cornea. 2011;30:620–623. doi: 10.1097/ICO.0b013e3181e16f7c. [DOI] [PubMed] [Google Scholar]
- 75.Jadidi K, Panahi Y, Ebrahimi A, Mafi M, Nejat F, Sahebkar A. Topical cyclosporine a for treatment of dry eye due to chronic mustard gas injury. J Ophthalmic Vis Res. 2014;9:417–422. doi: 10.4103/2008-322X.150803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Javadi MA, Baradaran-Rafii A. Living-related conjunctival-limbal allograft for chronic or delayed-onset mustard gas keratopathy. Cornea. 2009;28:51–57. doi: 10.1097/ICO.0b013e3181852673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUMMARY OF RECOMMENDATIONS


