Abstract
The cornea is the most commonly transplanted tissue in the body. Although corneal grafts generally have high success rates, transplantation onto inflamed and vascularized host beds, or so-called high-risk corneal transplantation, has a high rate of graft rejection. The management of this high-risk corneal transplantation is challenging and involves numerous measures. One of the key measures to prevent graft rejection in these cases is the use of systemic immunosuppressive agents. In this article, we will review the systemic immunosuppressive agents most commonly used for high-risk corneal transplantation, which include corticosteroids, cysclosporine A, tacrolimus, mycophenolate mofetil, and rapamycin. Benefits, risks, and published data on the use of these medications for high-risk corneal transplantation will be detailed. We will also summarize novel immunoregulatory approaches that may be used to prevent graft rejection in high-risk corneal transplantation.
Keywords: Corneal Transplantation, Graft Rejection, High-risk Graft, Immunomodulation, Immunosuppression
INTRODUCTION
The first human corneal allograft was performed by Eduard Zirm in 1905; that is 49 years before the first successful solid organ (kidney) transplantation. The efficacy of this surgical procedure made it the most common form of human solid tissue transplantation with more than 100,000 procedures performed annually worldwide, and about 40,000 in the USA.[1] Due to corneal immune privilege, corneal transplantation has been associated with high success rates, including 90% survival after 1 year and 55% after 15 years when performed in avascular and non-inflamed host beds.[2,3,4]
The most frequent cause of corneal graft failure is rejection, which is an immune reaction against the donor cornea. Thirty percent of transplanted corneas experience at least one episode of immune reaction, and in low-risk cases one third of these, eventually lead to graft failure.[5,6,7] The reported incidence of corneal graft rejection varies from 2.3% to 68%,[8] and this depends on the presence of high-risk factors, most notably corneal neovascularization.[5,6]
Corneal transplantation in a non-vascularized and non-inflamed host bed, which is termed as low-risk (LR) corneal graft, does not require any systemic immunosuppression or Human Leukocyte Antigens (HLA) tissue matching.[7,8,9] This high success rate is completely overshadowed by results of grafts placed in inflamed and vascularized host beds, so-called high-risk (HR) corneal transplantation, in which the graft has a 5-year survival rate below 35% with immunosuppression.[7,8,9] These outcomes are considerably worse than those following first grafts of the kidneys, heart, or liver.[10]
In this review, first we will provide an overview on risk factors for corneal transplant rejection and prophylactic measures to prevent transplant rejection in HR settings. Then, in more detail, we will review the systemic immunosuppressive therapies that are currently available to prevent rejection in HR corneal transplantation.
HIGH-RISK CORNEAL TRANSPLANTATION
In HR corneal transplantation, allograft rejection represents the main cause of corneal graft failure,[10,11,12] with rejection rates of 50-70%, even with maximal immunosuppression.[10,13]
Multiple risk factors have been identified to increase the rate of immune reaction [Table 1]. Re-grafting of a failed transplant or performing a transplant in the context of pre-existing conditions, such as ocular surface inflammatory or infective disease, makes the eye more susceptible to an alloimmune reaction.[14] Moreover, a clinical history of glaucoma and previous surgeries, particularly glaucoma surgery, raises the risk of graft rejection. This risk also increases by performing an eccentric transplantation or using a larger donor graft, which increases the exposure of alloantigens to the immune system. Finally, the risk of rejection is higher in very young recipients, during pregnancy, or after blood transfusion.[14]
Table 1.
Risk factors for corneal graft rejection
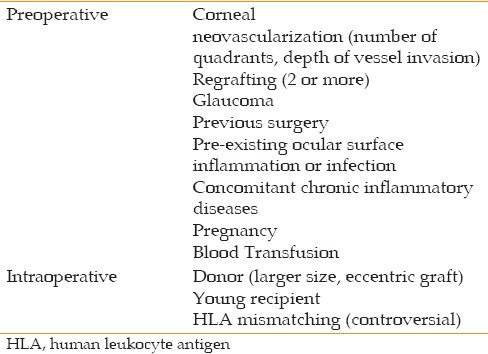
Host bed vascularity is, however, by far the most common risk factor for corneal allograft rejection. The normal cornea is devoid of lymph and blood vessels, and actively maintains its avascularity in a condition known as corneal angiogenic privilege.[15] In a LR environment, the vascular sprouting subsequent to surgery is quickly inhibited, and the angiogenic privilege is restored. In HR recipients, loss of angiogenic privilege leads to blood and lymphatic vessel invasion into the corneal graft. These corneal neovessels are critical in delivering immune effector cells to the graft site.[16] In addition, clinically invisible lymphatics play a critical role in trafficking alloantigens to host T cells in lymphoid tissue, which in turn initiate immune reaction and promote graft rejection.[15]
The extent of corneal neovascularization correlates with the risk of allograft rejection, and several studies have implicated that the involvement of deeper corneal layers by neovascularization is important in inciting an immune alloantigenic response.[12,14,17] It is well known that the number of corneal quadrants invaded by vessels as well as the number of vessels in each quadrant increase the risk of rejection.[11] Khodadoust et al defined the rejection risks based on the degree of host bed vascularization: avascular, mild (1-3 vessels), moderate (4-10 vessels) and heavy (>10 vessels). They noted that, in heavily vascularized eyes, 65% of grafts started to reject, and all grafts finally failed despite strong immunosuppressive treatment.[18]
Management of High-risk Corneal Transplantation
Management of corneal transplantation varies depending on the patient's degree of risk. Postoperative prophylactic immunosuppressive regimens can be devised according to the level of risk.[19]
In LR recipients, topical corticosteroids are the routine regimen during postoperative management and also for the treatment of endothelial graft rejections which occurs uncommonly in these cases.[20] In the past, this treatment has proven effective in improving survival rates of LR corneal transplantation patients.[21] However, the duration of prophylactic treatment with topical corticosteroids is still variable among surgeons.[20]
New surgical techniques to improve graft outcomes have been focused on lamellar keratoplasty to prevent the risk of rejection by reducing the amount of allogeneic tissue transplanted and also avoiding endothelial cells which are the target for alloimmune responses.[22] In addition, technical advances, such as globe fixation and Flieringa ring, graft suturing techniques, donor storage media and other technical factors (e.g., avoiding larger and eccentric grafts, and using nylon sutures instead of silk) have been reported to decrease graft failure rates due to non-immune factors.[23] However, survival of corneal grafts at high risk for rejection has improved little over the past decades,[24] even with new surgical devices and techniques, and new therapeutic strategies.
Interestingly, responders to the periodical survey of the Cornea Society felt that graft rejection occurs much less frequently after endothelial keratoplasty as compared to penetrating keratoplasty, considering the lack of difference in rejection prophylaxis or treatment strategies between the two procedures.[20] In addition, recent studies have significantly shown lower rates of corneal graft rejection following endothelial keratoplasty rather than penetrating keratoplasty.[25,26] For example, Anshu and colleagues reported rejection rates of 18%, 12%, and <1% after penetrating keratoplasty, Descemet stripping endothelial keratoplasty (DSEK), and Descemet membrane endothelial keratoplasty (DMEK), respectively.[27] However, it should be noted that in HR settings, the survival of endothelial keratoplasty is also very low.[28,29] Moreover, in HR settings, many corneas may have neovascularization and scarring which make them unsuitable for endothelial keratoplasty alone.
The human leukocyte antigen (HLA)-matching strategy in HR corneal transplantation is used only in some European centers; better survival rates have been shown mostly with minor ABO-antigen matching.[30,31]
The surgical timing of corneal transplantation, moreover, can have some effect on the survival of HR transplants. In mice, regression of lymphatic vessels occurs 6 months after any inflammatory process, and surgery at this time point results in a better outcome.[32] Thus, if possible, keratoplasty should be performed in an inflammation-free stage of the disease. However, further studies are needed to investigate these insights within the context of human corneal transplantation.
A novel treatment strategy for HR corneal transplantation is inhibiting corneal angiogenesis via blocking the vascular endothelial growth factor (VEGF).[6] Topical treatment with specific antibodies, trap proteins, or receptor antagonists has been shown to successfully prevent rejection in complicated grafts.[33,34] In addition, gene therapy has been used to maintain an immunosuppressive environment in the host bed,[35,36] and to inhibit the direct pathway of the antigen-presentation process by depleting antigen-presenting cells (APCs) in the donor cornea.[24]
Although the above-mentioned measures can be used to reduce the risk of rejection in HR corneal transplants, the key step in the management of these patients is the use of immunosuppressive therapy to dampen the immunologic pathways leading to rejection. The importance of postoperative immunosuppressive regimens for reducing the incidence of graft failure is supported by findings of the Collaborative Corneal Transplantation Study (CCTS),[37] which showed an improved graft survival with a strict topical corticosteroid regimen. However, doubts have been raised as to whether all patients in the CCTS were truly at high risk, because the authors attributed the improved graft survival found in their HR cases to the use of intensive topical steroid therapy postoperatively, close personal follow-up, and excellent patient compliance and understanding. On the other hand, the Castroviejo Cornea Society survey reported that 85% of patients at high risk of allograft rejection received therapeutic agents other than topical corticosteroids.[38]
In HR corneal transplantation, the use of corticosteroids is the first choice for prophylactic treatment. Prednisolone 1% or dexamethasone 0.1% drops represent the treatment of choice among Cornea Society members, and in the survey of United Kingdom cornea surgeons, the Bowman Club, these corticosteroids were administered between 6 to 8 times per day, including at night time. The duration of this regimen varies widely among the surgeons, with an average of 8 months among respondents in the Cornea Society survey.[20] The duration is also dependent on whether the host is phakic or pseudophakic. In the latter case, corticosteroids can be continued longer. However, because of the reduced efficacy in HR settings, several studies are currently investigating the effect of subconjunctival or systemic steroid treatment to achieve a better outcome.
SYSTEMIC THERAPY
The aims of managing HR corneal transplants are, first, to inhibit or cause regression of corneal lymph- and hemangiogenesis[16,39,40,41] and subsequently, to prevent or reverse immune-mediated graft rejection.
Systemic immunosuppressive agents are administered on a prophylactic basis in patients with a very high risk for rejection, especially those with salvageable vision in only one eye. However, a defined treatment strategy for different degrees of high risk status is not available, thus indications for this systemic treatment are often based on the surgeon's judgment. This implies wide variability in treatment and also makes it more difficult to compare different studies.
While systemic corticosteroids and immunosuppressive agents may be partially effective in preventing graft rejection, their use is limited due to a wide range of ocular side effects, including infection, cataract formation and glaucoma, as well as potential systemic life-threatening systemic side effects, which may outweigh any improvement in corneal graft survival.[42,43,44,45]
In contrast to the outcomes reported for grafts of vascularized organs, the long-term survival of corneal grafts, in general, has not improved over recent decades, probably because the modern immunosuppression regimens that have exerted such a beneficial influence on other organ graft survival rates are generally neither effective nor appropriate for corneal transplantation.[46]
A number of shortcomings are evident in published reports on systemic immunosuppression in corneal transplant recipients:[47] (1) lack of risk factor stratification in some patients with failed grafts; (2) variable duration of prophylaxis;[48] (3) conflicting outcomes even in studies using similar regimens; and (4) comparison of patients with HLA-matched and non-matched donor corneas.[49,50] Comparison of results between studies is also difficult because of the use of oral steroids in the first weeks after transplantation in some studies.
Below, we summarize systemic immunosuppressive medications widely used to prevent graft rejection in HR corneal grafts.
Corticosteroids
Corticosteroids represent the key medication for management of corneal transplantation. They can be used pre-, intra-, or postoperatively. Systemic corticosteroids have been used, either as monotherapy or in combination with other immunosuppressive agents to prevent corneal graft rejection in HR settings as well as to treat acute rejection.[20,51,52] The report by the Cornea Society Survey[53] showed that oral prednisolone (40 to 80 mg/day for 2 to 7 days) is prescribed by 22% of the surgeons, almost always as an adjunct to topical treatment, and sometimes as prophylactic treatment for corneal graft rejection.[54] The usual dose is 60-80 mg daily (depending on body weight); however, the duration of treatment is widely variable, ranging from 1 day to 12 months.[40] Currently, there is no consensus on the best time to start and end immunosuppressive treatment in patients who have undergone a corneal transplantation.[55,56,57]
Kim et al, in an animal model of corneal transplantation, proposed that the preoperative use of corticosteroids, two weeks before grafting,[57] could be sufficient to prevent or decrease angiogenesis before the insult of surgery and, therefore, improve outcomes. In fact, some studies have revealed that pretreatment with corticosteroids decreases host bed neovascularization in both LR and HR corneal transplantations.[45]
Systemic administration of other steroids, such as a single-dose of intravenous have been considered by 14% of surgeons in HR situations such as in vascularized corneas and in cases with previous rejection. A single intravenous dose of 125 mg methylprednisolone sodium has been advocated for management of severe graft rejection in association with hourly topical corticosteroid drops.[58] Alternatively, it has been shown that a single 500 mg intravenous pulse of methylprednisolone in severe endothelial rejection appears to be at least as effective as oral administration, while avoiding potential side effects of prolonged oral medication.[59,60] In a study by Hill et al, the pulse therapy group achieved a 79% graft survival rate as compared with 63% for the oral group. Among recovered grafts, only 25% of those in the pulse group developed an additional episode of rejection, while 67% of eyes in the oral group did. This suggests that pulse therapy may confer some degree of long-term protection.[60] However, another study focusing on administering a second pulse given either 24 or 48 hours after the first pulse did not report any significant improvement.[61]
Compared to the surveys conducted in 1989 and 2004, the 2011 Cornea Society survey showed an increased use of postoperative subconjunctival methylprednisolone injections, oral prednisolone, or intravenous methylprednisolone and hydrocortisone for routine management of corneal transplantation. Surprisingly, the use of subconjunctival steroid preparations was found to be higher in recipients at LR rather than those at HR for graft rejection (76% versus 54%, respectively). This may be due to the use of other topical and oral immunosuppressive agents in HR graft recipients.[20]
The major limitation of corticosteroid therapy is the substantial toxic profile of systemic and long-term usage; among others these include increased intraocular pressure, cataract formation, impaired wound healing, secondary Cushing syndrome, and predisposition to opportunistic infections, all necessitating careful monitoring.[62,63,64]
Cyclosporine A
Cyclosporine A (CsA) is a macrolide with powerful immunosuppressive activity that modulates T cell function.[65] It is an 11-amino acid peptide isolated from the fungus Tolypocladium inflatum. Cyclosporine A acts by binding to the intracellular protein cyclophilin, which inhibits the activity of calcineurin enzyme, blocking nuclear factor activation.[66] Cyclosporine A inhibits the interleukin (IL)-2 pathway, leading to a decrease in the synthesis and secretion of several pro-inflammatory cytokines such as IL-2, IL-4, interferon-γ (IFN- γ), tumor necrosis factor-α (TNF- α), and thus results in inhibition of helper and cytotoxic T cell differentiation. Cyclosporine A also improves allergic reactions by inhibiting mast cells, and plays a role in cell apoptosis in autoimmune diseases.[67]
Because of its mechanism of action, CsA has been extensively used to prevent immune rejection after solid-organ transplantation.[68,69] However, it can cause strong adverse events, such as hypertension, nephrotoxicity, hepatotoxicity and gastrointestinal toxicity, when high doses are taken orally or intravenously for a long time.[70,71]
In ophthalmology, CsA has been used both topically and orally for a multitude of indications. To avoid the systemic side effects and deliver greater amounts of the medication into the eye, topical CsA has been prescribed for years and has gained popularity among ophthalmologists to treat different immune disorders, including dry eye disease, vernal keratoconjunctivitis, and ocular graft-versus-host disease.[72,73,74] Oral CsA has also been used to treat a variety of diseases including uveitis, Vogt-Koyanagi-Harada disease, and Graves' orbitopathy.[75,76]
For corneal transplantation, both systemic and topical CsA have been used to prevent graft rejection. Previous studies on oral CsA are summarized below. The use of oral CsA for prevention of immune rejection in HR corneal transplantation has been studied and described in the literature for decades, but to date a consensus has not been reached about the real effectiveness of this medication. Several authors have published results showing that systemic CsA administration can improve the outcome of HR corneal transplantation. Table 2 summarizes the related articles. In a prospective study, Hill and colleagues reported that CsA-treated corneal graft recipients had a significantly higher rejection free rate as compared to the untreated control group (73% and 49% respectively, P < 0.001).[42] These authors also described that graft rejection is decreased when CsA is taken for 12 months instead of a shorter period of 4 to 6 months.[77] Other retrospective studies have further suggested the benefit of oral CsA regarding survival and rejection rates [Table 1].[78,79,80]
Table 2.
Previous studies on systemic cyclosporine administration for high-risk corneal transplantation
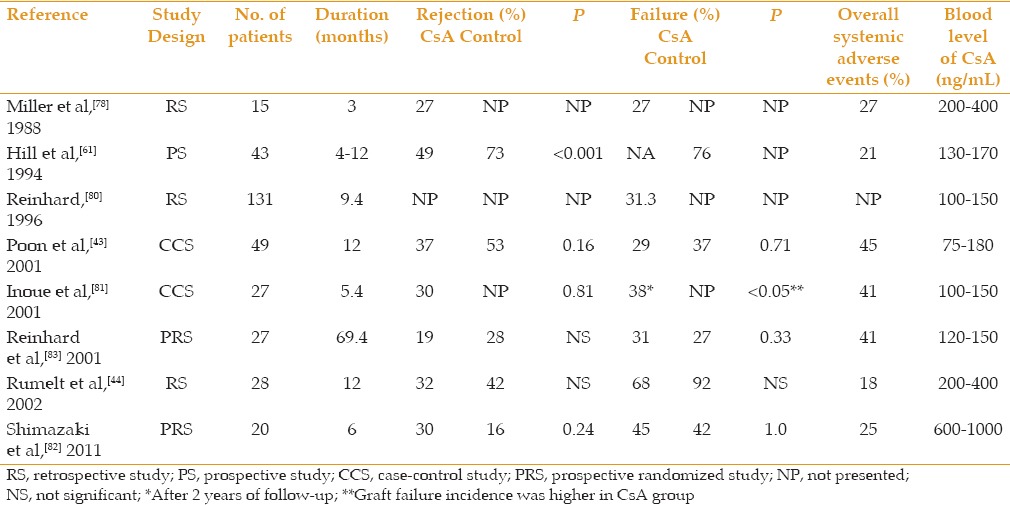
In contrast, several studies have reported either no or only limited effectiveness of oral CsA administration in HR corneal transplantation.[43,44,81,82] A retrospective study observed only a limited effect of CsA in preventing immune reactions and reported no significant difference between the control and treatment groups in terms of rejection or failure rates.[44] Poon et al as well as Inoue et al, in retrospective case-control reports, also failed to find a significant difference in rejection and failure rates between the CsA-treated group and the control group.[43,81] In a recent prospective, randomized trial, Shimazaki et al observed no statistically significant effect from the systemic use of CsA regarding the rates of rejection (30% and 16%, respectively) and failure (45% and 42%, respectively), although all rejection episodes in the treatment group occurred after the drug was discontinued.[82] In agreement with Reinhard et al,[80] the authors hypothesized that oral CsA may be effective while in use and could shift the immune response from acute to chronic. This would “postpone” a rejection episode to a chronic clinical course, which could be diagnosed and treated more efficiently.[80,82] In a different prospective randomized trial, Reinhard et al compared oral CsA to oral mycophenolate mofetil (MMF), and found no statistical difference between the groups in graft rejection and failure.[83]
The questionable effects of oral CsA in preventing corneal graft rejection may be due to the fact that, although it achieves a high concentration in the serum, it does not reach the aqueous humor and thus may not be able to prevent the loss of ocular immune privilege following HR corneal transplantation.[82,84] In addition, the severity of primary host disease, duration of treatment, and occurrence of side effects can all cloud the real effect of the drug.
Variable results on the effectiveness of CsA for preventing corneal graft rejection may also be partly explained by different dosage and blood levels of CsA in different studies. In most studies, blood levels of 75 to 400 ng/mL had been maintained during the treatment period.[43,44,78,81] However, a recent study used blood concentrations after 2 hours as the method to monitor the concentration of the drug, and kept it between 600 and 1000 ng/mL.[82] The latter approach has been used after renal transplantation, and may be more accurate in controlling drug concentration for transplantation follow-up.[85] Due to its variable absorption, monitoring of CsA blood levels as well as liver and renal function tests are mandatory.[86] Poon et al reported a 45% incidence of adverse events during oral administration of cyclosporine, with kidney dysfunction as the most common side effect.[43] While other studies did not report such a high percentage of side effects, they did describe systemic adverse events including nausea, hypertension and elevated blood levels of creatinine and urea.[44,77,78,81,82]
Tacrolimus
Tacrolimus, or FK506, is a macrolide antibiotic with potent immunosuppressive activity, which is isolated from the soil fungus Streptomyces Tsukubaensis. This calcineurin inhibitor has a mode of action similar to that of CsA. Its mechanism of action initiates with binding to a class of peptidyl-prolyl cis-trans isomerases, known as FK506-binding proteins (FKBPs), especially FKBP-12.[87,88] Subsequently, a complex of tacrolimus-FKBP-12, calcineurin, calcium and calmodulin is created, leading to inhibition of calcineurin's ability to dephosphorylate the nuclear factor of activated T cells. Thus, the initial phase of T cell activation is blocked, resulting in inhibition of T-lymphocyte signal transduction and IL-2 transcription. Additionally, release of TNF-α, IFN-γ and other cytokines is also affected by tacrolimus.[89,90] Tacrolimus has been reported to entail fewer systemic side effects than CsA, even given that its immunosuppressive effect is 25-100 times more powerful than CsA.[91,92] Clinical trials have shown that its efficacy is greater than CsA for liver, kidney and pulmonary transplantations, and the medication is also less prone to induce systemic hypertension and lipid abnormalities.[93,94,95]
In ophthalmology, tacrolimus has been effectively used to treat immune-mediated ocular disorders such as atopic keratoconjunctivitis,[96] posterior uveitis,[97] and chronic graft-versus-host disease.[98,99] Furthermore, because of its potent immunosuppressive effects, tacrolimus has been used in various studies to prevent graft rejection in HR hosts as detailed below.
Systemic tacrolimus (2-12 mg daily) has been shown to reduce graft rejection in HR corneal transplantation, with a graft survival of 65%.[100] The therapeutic target range of 1 to 12 mg/L was achieved with a mean daily dose of 4.4 mg. However, the optimum length of treatment is still not known.[101] Hypertension (23%) is the most common side effect of tacrolimus treatment, followed by headaches, malaise and gastrointestinal upset.[100]
In a prospective study, Yamazoe et al showed that treatment with tacrolimus (10-20 ng/mL) resulted in significantly fewer graft rejection episodes and longer graft survival than CsA, probably due to its more effective suppression of alloimmunity.[102] Moreover, patients treated with tacrolimus tolerated the drug better than those treated with CsA; however, 20% discontinued tacrolimus treatment because of renal dysfunction and muscle pain, which might also be related to previous CsA treatment they had received. Furthermore, 30% of the grafts failed without rejection, possibly because of previous history of glaucoma.
In another study, Joseph et al found that only 8 of the 43 corneal transplant patients who used tacrolimus prophylactically (1 mg twice daily) had rejection episodes, and 5 of these experienced rejection related graft failure.[100] These patients might have benefited from higher doses of tacrolimus or a combination treatment with another immunosuppressive agent.
In an animal model of corneal transplantation, a combination of FK506 and MMF delayed graft rejection for 3 weeks. The Gray's survival model, stratified according to the type of recipient (low risk and high risk), showed that tacrolimus monotherapy more effectively reduced the risk of rejection than did MMF monotherapy.[103]
Mycophenolate Mofetil
Mycophenolate mofetil (MMF) is another type of immunosuppressant used systemically after organ transplantation.[104] It is the pro-drug of the active substance mycophenolic acid (MPA), a potent inhibitor of inosine monophosphate dehydrogenase, which inhibits the de novo synthesis of guanosine nucleotides, resulting in selective inhibition of T- and B-lymphocyte proliferation.[105,106] As T and B cells are predominantly dependent on de novo synthesis of guanosine nucleosides, the purine biosynthesis of these cells is relatively selectively inhibited. Other cells are either not, or less, affected by MMF as the result of their ability to use alternative salvage pathways of purine synthesis.[105,107]
Mycophenolate mofetil has been demonstrated to be effective and safe in kidney and heart transplantations at a dose of 3 g per day and for liver transplantation using a dose of 2 g per day.[108,109,110,111] The most common reported side effects are infections, anemia, leukopenia, and gastrointestinal disturbances; however, blood monitoring is generally unnecessary and reserved for special situations, such as severe adverse events or treatment failure.[111]
In ophthalmology, MMF has been used to treat uveitis and HR corneal transplantation.[62,76,83] In a preliminary report of a prospective, randomized, multicenter trial with 86 patients, Reinhard et al described an immune reaction free rate of 89% in the MMF-treated group in contrast to 67% in the control group (P = 0.03) during the first year after surgery.[112] In this study, both groups received early postoperative systemic corticosteroids and topical corticosteroids for 5 months. Patients classified as high-risk, in the MMF-treated group, received 2 g of MMF per day for 6 months in addition to the corticosteroid treatment. After a mean follow-up period of 3 years in the same cohort, Birnbaum et al reported a reaction-free graft survival rate of 83% in the MMF-treated group and 64.5% in the control group (P = 0.04).[113] Regarding graft failure, no statistically significant difference was found between the two groups, although the percentage of graft failures as a result of rejection was noticeably higher in the control group (20% vs. 78%). Mycophenolate mofetil was relatively well tolerated, as many of the reported side effects were not likely due to MMF administration, and only 2 patients out of 57 in the MMF group had to stop taking the medication because of the side effects. The main adverse events were gastrointestinal disturbances, arterial hypertension and hyperlipidemia. Similarly, to test MMF as an agent for prophylaxis in HR keratoplasty, Mayer et al conducted a trial in which 10 patients with prior herpetic disease underwent penetrating keratoplasty and received MMF 2 g per day for 1 year. According to the authors, there were two instances of mild rejection with no influence on endothelial cell density and no recurrence of herpetic disease.[114]
There are only a few published articles comparing the safety and efficacy of CsA and MMF. In a prospective randomized trial including 41 patients, Reis et al showed no significant difference between CsA and MMF in terms of rejection episodes (10% vs. 9.5%, respectively), graft survival (100% in both groups), and occurrence of adverse events in a HR setting during a follow-up of about 10 months.[115] The authors suggested that MMF or CsA in combination with a short postoperative course of oral corticosteroids are similarly effective in preventing acute rejection following HR corneal transplantation. Moreover, they concluded that the broad and safe therapeutic range (2 or 3 g/day) of MMF avoids major side effects and also frequent checkups; therefore, MMF is more likely to be administered in patients with suboptimal compliance who fail to visit the ophthalmologist or general practitioner on a regular basis. Reinhard et al published the results of the same trial with a follow-up of after 3 years, and still reported no significant difference between CsA and MMF treatment groups in graft survival (74% vs. 69%, respectively) and rejection-free rate (73% vs. 53%, respectively, P = 0.46), even though the CsA group had a lower rejection percentage.[83]
In a retrospective study with 417 HR keratoplasties, Birnbaum et al reported a significantly greater effect from MMF in preventing graft rejections as compared to CsA after 3 years of follow-up (72% vs. 60%, respectively, P = 0.03), but with no significant difference in terms of graft survival (87% vs. 77%, respectively).[62] However, this result may be in part due to patient selection, as according to the authors, the CsA group had more severely HR patients than did the MMF group. On the other hand, hosts that received combined CsA and MMF did not show a significant difference in immune reaction rates as compared with the other two. In fact, they found a lower rate of survival with combined treatment (67%, P < 0.01) when compared to the other two groups; this finding could be explained by patient selection bias with initially worse prognosis. In terms of safety, MMF-treated patients presented a lower percentage of side effects than did CsA-treated patients.[62]
Another retrospective study had a large MMF group of 79 patients and a small CsA group of 5 patients, and demonstrated that the combination of the immunosuppressive agents with systemic acyclovir in patients with previous herpetic keratitis achieved a graft survival rate of 86.9% and rejection-free rate of 72.9%. Comparison between the groups could not be made due to the small sample size in the CsA group.[116] It is known that MMF has a synergistic effect with acyclovir, thus this combination might be beneficial for patients undergoing keratoplasty after herpetic corneal disease.[114,117]
In 2004, among the members of the Cornea Society that responded to a survey, only 14% and 0% of them were using systemic CsA or MMF, respectively, for prevention of HR corneal transplantation.[54] These percentages may be higher currently due to the number of published studies about this matter. Several clinicians agree that MMF seems to have the same capacity as CsA in reducing the severity of shifting the immune reactions from severe to mild.[83,116] In addition, MMF's broad therapeutic range, a smaller percentage of severe side effects, and lower cost due to less blood monitoring, makes the drug more convenient for ophthalmic patients.[83,115]
Rapamycin (Sirolimus, Rapamune)
Rapamycin is a bacterial macrolide isolated from Streptomyces hygroscopicus, which has antifungal and immunosuppressive properties.[118] Rapamycin forms a complex with the immunophilin FK binding-protein (FKBP-12), and the rapamycin-FKBP-complex inhibits the mammalian target of rapamycin (mTOR).[119] Despite having a structure similar to that of tacrolimus, rapamycin is not a calcineurin inhibitor and thus not nephrotoxic. It acts by decreasing IL-2-mediated activation of T-lymphocytes with a blood concentration range of 12-20 ng/mL.[118,120] Rapamycin has been shown to inhibit the growth factor induced proliferation of fibroblasts, endothelial cells and smooth muscle cells, which are also beneficial in solid organ transplantation.[121,122]
In a prospective pilot study, Birnabaum et al showed a comparable efficacy regarding immune reaction-free corneal graft survival with the use of rapamycin and MMF. Specifically, no immune reaction was observed in either group at 6 months, while only two reversible immune reactions occurred in the rapamycin group within 2 years of follow-up.[123]
Chatel et al also performed a prospective study on 6 patients with HR corneal transplantation. After 1 year of combination therapy with MMF and rapamycin, and then two years of rapamycin monotherapy, they showed 3 rejections episodes with 1 year of follow-up in 6 patients, of whom 1 had an irreversible rejection.[124] In addition, all patients developed hypercholesterolemia within 6 months of therapy. Therefore, the authors concluded that the combination of rapamycin and MMF was effective in extending corneal transplant survival, but generally the treatment was not well tolerated. Finally, the broad spectrum of side effects following treatment with rapamycin observed in these studies as well as in other solid organ transplantation studies, especially arterial thrombosis, appears to limit its safe usage.[125,126]
Novel Strategies for Immunomodulation
Several immunoregulatory approaches have been studied in animal models in recent years and have shown interesting results in increasing the survival of corneal grafts.
Suppression of antigen-presenting cell (APC) maturation by malononitrilamide (FK778) has been demonstrated to prolong corneal allograft survival in a rat keratoplasty model.[127] In addition, antibody based therapeutic agents have been developed to modulate the rejection of vascularized organ allografts. Specific systemic treatment with intact antibody can certainly prevent or delay corneal graft rejection in experimental animals.[128,129] A wide variety of polyclonal, monoclonal and recombinant antibodies have been tested targeting immune cell determinant or co-stimulatory molecules, such as IL-1 blockade,[130] leukocyte function antigen-1(LFA-1), very late antigen-1 and 4 (VLA-1, VLA-4),[131,132] CD40–CD154 pathway,[133,134] CD28 and CD3 (CD80 and CD86).[135] It has been reported that Cytotoxic T Lymphocyte Antigen 4 protein (CTLA4-Ig), a fusion protein acting on B7-CD28 binding, can prolong corneal allograft survival after systemic injections in animal models by directly inhibiting T cell activation.[136] However, the efficacy of antibody therapy in humans is often limited by systemic side effects and the development of anti-idiotypic and anti-isotypic antibodies in the recipient.[137] In addition, the blood eye and ocular surface barriers limit access of whole antibodies into the eye. In fact, few of these studies in experimental animals[39,134,138,139,140,141,142,143] have been subsequently translated into clinical trials.[144,145]
It is worthy of note that recently there have been novel approaches to immunomodulate the alloimmunity focusing on morpholine oligonucleoitides,[146] cell-specific gene therapy,[142] RNA interference,[147] anti-VEGF therapy,[148] tolerogenic APC,[149] and IL-2 therapy[150] with promising experimental results.
CONCLUSION
The cornea is the most commonly transplanted tissue. A significant number of grafts are performed in the inflamed and vascularized environment of high-risk hosts, which puts the patients at high risk of allograft rejection. The management of HR corneal transplantation is very challenging because of the low efficacy of long-term immunosuppressive therapy and their side effects. Many studies have recently been performed to find an immunosuppressive monotherapy or a combined therapeutic regimen that shows long-term efficacy with no major side effects. However, customized immunomodulation treatment appears to be the best management option for these difficult cases, for example, improved efficacy of CsA in patients with atopic keratoconjuctivitis, or of MMF in subjects with history of ocular HSV infections. Moreover, the use of MMF is suggested in patients with compliance issues or with systemic comorbidities.
In addition to application of novel immunosuppressive drugs and targeted biologic treatments that block specific pathways implicated in transplant immunity, there are a number of tolerance-inducing protocols including expansion of regulatory T cells (T reg) through interleukin-2 therapy or use of tolerogenic APCs that may hold promise in promoting corneal graft survival without the toxic side effects of systemic immunosuppressive medications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Crawford AZ, Patel DV, McGhee C. A brief history of corneal transplantation: From ancient to modern. Oman J Ophthalmol. 2013;6(Suppl 1):S12–S17. doi: 10.4103/0974-620X.122289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KA, Coster DJ. The immunobiology of corneal transplantation. Transplantation. 2007;84:806–813. doi: 10.1097/01.tp.0000285489.91595.13. [DOI] [PubMed] [Google Scholar]
- 3.Sit M, Weisbrod DJ, Naor J, Slomovic AR. Corneal graft outcome study. Cornea. 2001;20:129–133. doi: 10.1097/00003226-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sangwan VS, Ramamurthy B, Shah U, Garg P, Sridhar MS, Rao GN. Outcome of corneal transplant rejection: A 10-year study. Clin Experiment Ophthalmol. 2005;33:623–627. doi: 10.1111/j.1442-9071.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 5.Dohlman TH, Omoto M, Hua J, Stevenson W, Lee SM, Chauhan SK, et al. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 2015;99:678–686. doi: 10.1097/TP.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 6.Dastjerdi MH, Saban DR, Okanobo A, Nallasamy N, Sadrai Z, Chauhan SK, et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010;51:2411–2417. doi: 10.1167/iovs.09-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: Emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Lam H, Dana MR. Corneal graft rejection. Int Ophthalmol Clin. 2009;49:31–41. doi: 10.1097/IIO.0b013e3181924e23. [DOI] [PubMed] [Google Scholar]
- 9.Zhu SN, Yamada J, Streilein JW, Dana MR. ICAM-1 deficiency suppresses host allosensitization and rejection of MHC-disparate corneal transplants. Transplantation. 2000;69:1008–1013. doi: 10.1097/00007890-200003150-00061. [DOI] [PubMed] [Google Scholar]
- 10.Qazi Y, Hamrah P. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. J Clin Cell Immunol. 2013;2013(Suppl 9) doi: 10.4172/2155-9899.S9-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Australian Corneal Graft Registry. 1990 to 1992 report. Aust N Z J Ophthalmol. 1993;21(2 Suppl):1–48. [PubMed] [Google Scholar]
- 12.Williams KA, Roder D, Esterman A, Muehlberg SM, Coster DJ. Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology. 1992;99:403–414. doi: 10.1016/s0161-6420(92)31960-8. [DOI] [PubMed] [Google Scholar]
- 13.Williams KA, Lowe M, Bartlett C, Kelly TL, Coster DJ. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation. 2008;86:1720–1724. doi: 10.1097/TP.0b013e3181903b0a. [DOI] [PubMed] [Google Scholar]
- 14.Maguire MG, Stark WJ, Gottsch JD, Stulting RD, Sugar A, Fink NE, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101:1536–1547. doi: 10.1016/s0161-6420(94)31138-9. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan SK, Dohlman TH, Dana R. Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity. J Clin Cell Immunol. 2014;5 doi: 10.4172/2155-9899.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 17.Kamp MT, Fink NE, Enger C, Maguire MG, Stark WJ, Stulting RD. Patient-reported symptoms associated with graft reactions in high-risk patients in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Cornea. 1995;14:43–48. [PubMed] [Google Scholar]
- 18.Khodadoust AA, Silverstein AM. Studies on the nature of the privilege enjoyed by corneal allografts. Invest Ophthalmol. 1972;11:137–148. [PubMed] [Google Scholar]
- 19.Hill JC. Immunosuppression in corneal transplantation. Eye. 1995;9(Pt 2):247–253. doi: 10.1038/eye.1995.48. [DOI] [PubMed] [Google Scholar]
- 20.Kharod-Dholakia B, Randleman JB, Bromley JG, Stulting RD. Prevention and treatment of corneal graft rejection: Current practice patterns of the Cornea Society (2011) Cornea. 2015;34:609–614. doi: 10.1097/ICO.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 21.Dua HS, Azuara-Blanco A. Corneal allograft rejection: Risk factors, diagnosis, prevention, and treatment. Indian J Ophthalmol. 1999;47:3–9. [PubMed] [Google Scholar]
- 22.Han DC, Mehta JS, Por YM, Htoon HM, Tan DT. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009;148:744–751. doi: 10.1016/j.ajo.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Lee RM, Lam FC, Georgiou T, Paul B, Then KY, Mavrikakis I, et al. Suturing techniques and postoperative management in penetrating keratoplasty in the United Kingdom. Clin Ophthalmol. 2012;6:1335–1340. doi: 10.2147/OPTH.S35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Shen L, Jin Y, Saban DR, Chauhan SK, Dana R. Depletion of passenger leukocytes from corneal grafts: An effective means of promoting transplant survival? Invest Ophthalmol Vis Sci. 2009;50:3137–3144. doi: 10.1167/iovs.08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ang M, Wilkins MR, Mehta JS, Tan D. Descemet membrane endothelial keratoplasty. Br J Ophthalmol. 2016;100:15–21. doi: 10.1136/bjophthalmol-2015-306837. [DOI] [PubMed] [Google Scholar]
- 26.Price FW, Jr, Feng MT, Price MO. Evolution of Endothelial Keratoplasty: Where Are We Headed? Cornea. 2015;34(Suppl 10):S41–S47. doi: 10.1097/ICO.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 27.Anshu A, Price MO, Price FW., Jr Risk of corneal transplant rejection significantly reduced with Descemet's membrane endothelial keratoplasty. Ophthalmology. 2012;119:536–540. doi: 10.1016/j.ophtha.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Coster DJ, Lowe MT, Keane MC, Williams KA. A comparison of lamellar and penetrating keratoplasty outcomes: A registry study. Ophthalmology. 2014;121:979–987. doi: 10.1016/j.ophtha.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Keane MC, Galettis RA, Mills RA, Coster DJ, Williams KA. A comparison of endothelial and penetrating keratoplasty outcomes following failed penetrating keratoplasty: A registry study. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-307792. (in press) [DOI] [PubMed] [Google Scholar]
- 30.Khaireddin R, Wachtlin J, Hopfenmuller W, Hoffmann F. HLA-A, HLA-B and HLA-DR matching reduces the rate of corneal allograft rejection. Graefes Arch Clin Exp Ophthalmol. 2003;241:1020–1028. doi: 10.1007/s00417-003-0759-9. [DOI] [PubMed] [Google Scholar]
- 31.Volker-Dieben HJ, Claas FH, Schreuder GM, Schipper RF, Pels E, Persijn GG, et al. Beneficial effect of HLA-DR matching on the survival of corneal allografts. Transplantation. 2000;70:640–648. doi: 10.1097/00007890-200008270-00018. [DOI] [PubMed] [Google Scholar]
- 32.Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 33.Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, et al. Novel anti (lymph) angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Dohlman TH, Di Zazzo A, Omoto M, Hua J, Ding J, Hamrah P, et al. E-Selectin Mediates Immune Cell Trafficking in Corneal Transplantation. Transplantation. 2016;100:772–780. doi: 10.1097/TP.0000000000001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams KA, Coster DJ. Gene therapy for diseases of the cornea – A review. Clin Experiment Ophthalmol. 2010;38:93–103. doi: 10.1111/j.1442-9071.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 36.Parker DG, Brereton HM, Klebe S, Coster DJ, Williams KA. A steroid-inducible promoter for the cornea. Br J Ophthalmol. 2009;93:1255–9. doi: 10.1136/bjo.2009.159137. [DOI] [PubMed] [Google Scholar]
- 37.The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 38.Hill JC. Immunosuppression in corneal transplantation. Eye. 1995;9(Pt 2):247–253. doi: 10.1038/eye.1995.48. [DOI] [PubMed] [Google Scholar]
- 39.Ling S, Lin H, Xiang D, Feng G, Zhang X. Clinical and experimental research of corneal lymphangiogenesis after keratoplasty. Ophthalmologica. 2008;222:308–316. doi: 10.1159/000144030. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann BO, Luetjen-Drecoll E, Bock F, Wiegand SJ, Hos D, Dana R, et al. Transient postoperative vascular endothelial growth factor (VEGF)-neutralisation improves graft survival in corneas with partly regressed inflammatory neovascularisation. Br J Ophthalmol. 2009;93:1075–1080. doi: 10.1136/bjo.2008.145128. [DOI] [PubMed] [Google Scholar]
- 41.Hos D, Saban DR, Bock F, Regenfuss B, Onderka J, Masli S, et al. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol. 2011;129:445–452. doi: 10.1001/archophthalmol.2011.42. [DOI] [PubMed] [Google Scholar]
- 42.Hill JC. Systemic cyclosporine in high-risk keratoplasty: Long-term results. Eye. 1995;9(Pt 4):422–428. doi: 10.1038/eye.1995.99. [DOI] [PubMed] [Google Scholar]
- 43.Poon AC, Forbes JE, Dart JK, Subramaniam S, Bunce C, Madison P, et al. Systemic cyclosporin A in high risk penetrating keratoplasties: A case-control study. Br J Ophthalmol. 2001;85:1464–469. doi: 10.1136/bjo.85.12.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rumelt S, Bersudsky V, Blum-Hareuveni T, Rehany U. Systemic cyclosporin A in high failure risk, repeated corneal transplantation. Br J Ophthalmol. 2002;86:988–992. doi: 10.1136/bjo.86.9.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchiyama E, Papaliodis GN, Lobo AM, Sobrin L. Side-effects of anti-inflammatory therapy in uveitis. Semin Ophthalmol. 2014;29:456–467. doi: 10.3109/08820538.2014.959203. [DOI] [PubMed] [Google Scholar]
- 46.Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209–222. doi: 10.1007/s10792-007-9099-9. [DOI] [PubMed] [Google Scholar]
- 47.Arentsen JJ. Corneal transplant allograft reaction: Possible predisposing factors. Trans Am Ophthalmol Soc. 1983;81:361–402. [PMC free article] [PubMed] [Google Scholar]
- 48.Coster DJ. The Montgomery Lecture. Some factors which affect the visual outcome of corneal transplantation. Eye. 1991;5(Pt 3):265–278. doi: 10.1038/eye.1991.43. [DOI] [PubMed] [Google Scholar]
- 49.Sanfilippo F, MacQueen JM, Vaughn WK, Foulks GN. Reduced graft rejection with good HLA-A and B matching in high-risk corneal transplantation. N Engl J Med. 1986;315:29–35. doi: 10.1056/NEJM198607033150105. [DOI] [PubMed] [Google Scholar]
- 50.Belin MW, Bouchard CS, Frantz S, Chmielinska J. Topical cyclosporine in high-risk corneal transplants. Ophthalmology. 1989;96:1144–1150. doi: 10.1016/s0161-6420(89)32756-4. [DOI] [PubMed] [Google Scholar]
- 51.Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: Current practice patterns (2004) Cornea. 2006;25:286–290. doi: 10.1097/01.ico.0000178731.42187.46. [DOI] [PubMed] [Google Scholar]
- 52.Rapuano CJ, Cohen EJ, Brady SE, Arentsen JJ, Laibson PR. Indications for and outcomes of repeat penetrating keratoplasty. Am J Ophthalmol. 1990;109:689–695. doi: 10.1016/s0002-9394(14)72437-7. [DOI] [PubMed] [Google Scholar]
- 53.Koay PY, Lee WH, Figueiredo FC. Opinions on risk factors and management of corneal graft rejection in the United kingdom. Cornea. 2005;24:292–296. doi: 10.1097/01.ico.0000138841.44926.f8. [DOI] [PubMed] [Google Scholar]
- 54.Abudou M, Wu T, Evans JR, Chen X. Immunosuppressants for the prophylaxis of corneal graft rejection after penetrating keratoplasty. Cochrane Database Syst Rev. 2015:CD007603. doi: 10.1002/14651858.CD007603.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimazaki J, Iseda A, Satake Y, Shimazaki-Den S. Efficacy and safety of long-term corticosteroid eye drops after penetrating keratoplasty: A prospective, randomized, clinical trial. Ophthalmology. 2012;119:668–673. doi: 10.1016/j.ophtha.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Claerhout I, Beele H, Verstraete A, Van den Broecke C, Kestelyn P. The effect of duration and timing of systemic cyclosporine therapy on corneal allograft survival in a rat model. Graefes Arch Clin Exp Ophthalmol. 2001;239:152–157. doi: 10.1007/s004170000242. [DOI] [PubMed] [Google Scholar]
- 57.Kim HK, Choi JA, Uehara H, Zhang X, Ambati BK, Cho YK. Presurgical corticosteroid treatment improves corneal transplant survival in mice. Cornea. 2013;32:1591–1598. doi: 10.1097/ICO.0b013e31829ebb0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braude LS, Chandler JW. Corneal allograft rejection. The role of the major histocompatibility complex. Surv Ophthalmol. 1983;27:290–305. doi: 10.1016/0039-6257(83)90229-1. [DOI] [PubMed] [Google Scholar]
- 59.Hill JC, Maske R, Watson P. Corticosteroids in corneal graft rejection. Oral versus single pulse therapy. Ophthalmology. 1991;98:329–333. doi: 10.1016/s0161-6420(91)32291-7. [DOI] [PubMed] [Google Scholar]
- 60.Hill JC, Maske R, Watson PG. The use of a single pulse of intravenous methylprednisolone in the treatment of corneal graft rejection. A preliminary report. Eye. 1991;5(Pt 4):420–424. doi: 10.1038/eye.1991.67. [DOI] [PubMed] [Google Scholar]
- 61.Hill JC, Ivey A. Corticosteroids in corneal graft rejection: Double versus single pulse therapy. Cornea. 1994;13:383–388. doi: 10.1097/00003226-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Birnbaum F, Bohringer D, Sokolovska Y, Sundmacher R, Reinhard T. Immunosuppression with cyclosporine A and mycophenolate mofetil after penetrating high-risk keratoplasty: A retrospective study. Transplantation. 2005;79:964–968. doi: 10.1097/01.tp.0000158022.62059.f2. [DOI] [PubMed] [Google Scholar]
- 63.Costa DC, de Castro RS, Kara-Jose N. Case-control study of subconjunctival triamcinolone acetonide injection vs intravenous methylprednisolone pulse in the treatment of endothelial corneal allograft rejection. Eye. 2009;23:708–714. doi: 10.1038/eye.2008.289. [DOI] [PubMed] [Google Scholar]
- 64.Mochizuki M. Immunotherapy in ocular diseases. Nihon Ganka Gakkai Zasshi. 1992;96:1608–1634. [PubMed] [Google Scholar]
- 65.Borel JF, Feurer C, Magnee C, Stahelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977;32:1017–1025. [PMC free article] [PubMed] [Google Scholar]
- 66.Braida M, Knop J. Effect of cyclosporin A on the T-effector and T-suppressor cell response in contact sensitivity. Immunology. 1986;59:503–507. [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: Focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22:1–15. doi: 10.1111/j.1399-0012.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 69.Beauchesne PR, Chung NS, Wasan KM. Cyclosporine A: A review of current oral and intravenous delivery systems. Drug Dev Ind Pharm. 2007;33:211–220. doi: 10.1080/03639040601155665. [DOI] [PubMed] [Google Scholar]
- 70.Calne RY. Cyclosporin in cadaveric renal transplantation: 5-year follow-up of a multicentre trial. Lancet. 1987;2:506–507. doi: 10.1016/s0140-6736(87)91809-5. [DOI] [PubMed] [Google Scholar]
- 71.Greenberg A, Thompson ME, Griffith BJ, Hardesty RL, Kormos RL, el-Shahawy MA, et al. Cyclosporine nephrotoxicity in cardiac allograft patients-A seven-year follow-up. Transplantation. 1990;50:589–593. doi: 10.1097/00007890-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28:1091–1096. doi: 10.1097/ICO.0b013e3181a16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wan KH, Chen LJ, Rong SS, Pang CP, Young AL. Topical cyclosporine in the treatment of allergic conjunctivitis: A meta-analysis. Ophthalmology. 2013;120:2197–2203. doi: 10.1016/j.ophtha.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 74.Utine CA, Stern M, Akpek EK. Clinical review: Topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18:352–361. doi: 10.3109/09273948.2010.498657. [DOI] [PubMed] [Google Scholar]
- 75.Wiersinga WM. Graves' orbitopathy: Management of difficult cases. Indian J Endocrinol Metab. 2012;16(Suppl 2):S150–s152. doi: 10.4103/2230-8210.104026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pasadhika S, Rosenbaum JT. Update on the use of systemic biologic agents in the treatment of noninfectious uveitis. Biologics. 2014;8:67–81. doi: 10.2147/BTT.S41477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill JC. Systemic cyclosporine in high-risk keratoplasty. Short- versus long-term therapy. Ophthalmology. 1994;101:128–133. doi: 10.1016/s0161-6420(13)31253-6. [DOI] [PubMed] [Google Scholar]
- 78.Miller K, Huber C, Niederwieser D, Gottinger W. Successful engraftment of high-risk corneal allografts with short-term immunosuppression with cyclosporine. Transplantation. 1988;45:651–653. doi: 10.1097/00007890-198803000-00030. [DOI] [PubMed] [Google Scholar]
- 79.Sundmacher R, Reinhard T, Heering P. Six years' experience with systemic cyclosporin A prophylaxis in high-risk perforating keratoplasty patients. A retrospective study. Ger. J Ophthalmol. 1992;1:432–436. [PubMed] [Google Scholar]
- 80.Reinhard T, Sundmacher R, Heering P. Systemic ciclosporin A in high-risk keratoplasties. Graefes Arch Clin Exp Ophthalmol. 1996;234(Suppl 1):S115–S121. doi: 10.1007/BF02343059. [DOI] [PubMed] [Google Scholar]
- 81.Inoue K, Kimura C, Amano S, Sato T, Fujita N, Kagaya F, et al. Long-term outcome of systemic cyclosporine treatment following penetrating keratoplasty. Jap J Ophthalmol. 2001;45:378–382. doi: 10.1016/s0021-5155(01)00339-2. [DOI] [PubMed] [Google Scholar]
- 82.Shimazaki J, Den S, Omoto M, Satake Y, Shimmura S, Tsubota K. Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am J Ophthalmol. 2011;152:33–39. doi: 10.1016/j.ajo.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 83.Reinhard T, Reis A, Bohringer D, Malinowski M, Voiculescu A, Heering P, et al. Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high-risk keratoplasty patients: 3 years' results of a randomized prospective clinical trial. Graefes Arch Clin Exp Ophthalmol. 2001;239:367–372. doi: 10.1007/s004170100285. [DOI] [PubMed] [Google Scholar]
- 84.Theng J, Zhou L, Tan D, La KW. Distribution of cyclosporin A in the cornea after topical or oral administration. J Ocul Pharmacol Ther. 2002;18:83–88. doi: 10.1089/108076802317233243. [DOI] [PubMed] [Google Scholar]
- 85.Pescovitz MD, Barbeito R. Two-hour post-dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clin Transplant. 2002;16:378–382. doi: 10.1034/j.1399-0012.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- 86.Panda A, Vanathi M, Kumar A, Dash Y, Priya S. Corneal graft rejection. Surv Ophthalmol. 2007;52:375–396. doi: 10.1016/j.survophthal.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 88.Siekierka JJ, Hung SH, Poe M, Lin CS, Sigal NH. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989;341:755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- 89.Shaw KT, Ho AM, Raghavan A, Kim J, Jain J, Park J, et al. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc Natl Acad Sci U S A. 1995;92:11205–11209. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tamura K, Fujimura T, Iwasaki K, Sakuma S, Fujitsu T, Nakamura K, et al. Interaction of tacrolimus (FK506) and its metabolites with FKBP and calcineurin. Biochem Biophys Res Commun. 1994;202:437–443. doi: 10.1006/bbrc.1994.1947. [DOI] [PubMed] [Google Scholar]
- 91.Geba GP, Ptak W, Askenase PW. Topical tacrolimus and cyclosporin A differentially inhibit early and late effector phases of cutaneous delayed-type and immunoglobulin E hypersensitivity. Immunology. 2001;104:235–242. doi: 10.1046/j.0019-2805.2001.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sawada S, Suzuki G, Kawase Y, Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987;139:1797–1803. [PubMed] [Google Scholar]
- 93.Friemann S, Feuring E, Padberg W, Ernst W. Improvement of nephrotoxicity, hypertension, and lipid metabolism after conversion of kidney transplant recipients from cyclosporine to tacrolimus. Transplant Proc. 1998;30:1240–1242. doi: 10.1016/s0041-1345(98)00226-7. [DOI] [PubMed] [Google Scholar]
- 94.Griffith BP, Bando K, Hardesty RL, Armitage JM, Keenan RJ, Pham SM, et al. A prospective randomized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994;57:848–851. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O'Grady JG, Burroughs A, Hardy P, Elbourne D, Truesdale A. Tacrolimus versus microemulsified ciclosporin in liver transplantation: The TMC randomised controlled trial. Lancet. 2002;360:1119–1125. doi: 10.1016/s0140-6736(02)11196-2. [DOI] [PubMed] [Google Scholar]
- 96.Stumpf T, Luqmani N, Sumich P, Cook S, Tole D. Systemic tacrolimus in the treatment of severe atopic keratoconjunctivitis. Cornea. 2006;25:1147–1149. doi: 10.1097/01.ico.0000240091.11854.14. [DOI] [PubMed] [Google Scholar]
- 97.Mochizuki M, Ikeda E, Shirao M, Fujito S, Yoshimura K, Shimada N. Preclinical and clinical study of FK506 in uveitis. Curr Eye Res. 1992;11(Suppl):87–95. doi: 10.3109/02713689208999516. [DOI] [PubMed] [Google Scholar]
- 98.Ogawa Y, Yamazaki K, Kuwana M, Mashima Y, Nakamura Y, Ishida S, et al. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci. 2001;42:111–119. [PubMed] [Google Scholar]
- 99.Abud TB, Amparo F, Saboo US, Di Zazzo A, Dohlman TH, Ciolino JB, et al. A Clinical Trial Comparing the Safety and Efficacy of Topical Tacrolimus versus Methylprednisolone in Ocular Graft-versus-Host Disease. Ophthalmology. 2016;123:1449–1457. doi: 10.1016/j.ophtha.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joseph A, Raj D, Shanmuganathan V, Powell RJ, Dua HS. Tacrolimus immunosuppression in high-risk corneal grafts. Br J Ophthalmol. 2007;91:51–55. doi: 10.1136/bjo.2006.097428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the management of high-risk corneal and limbal grafts. Ophthalmology. 2001;108:1838–1844. doi: 10.1016/s0161-6420(01)00759-x. [DOI] [PubMed] [Google Scholar]
- 102.Yamazoe K, Yamazoe K, Yamaguchi T, Omoto M, Shimazaki J. Efficacy and safety of systemic tacrolimus in high-risk penetrating keratoplasty after graft failure with systemic cyclosporine. Cornea. 2014;33:1157–1163. doi: 10.1097/ICO.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 103.Svozilkova P, Bysterska P, Masek K, Valenta Z, Zvarova J, Farghali H. Comparison of FK 506, mycophenolate mofetil, and aminoguanidine effects on delay of corneal allograft rejection in an experimental model of low-risk and high-risk keratoplasty. Immunopharmacol Immunotoxicol. 2006;28:335–40. doi: 10.1080/08923970600809447. [DOI] [PubMed] [Google Scholar]
- 104.Rose ML, Smith J, Dureau G, Keogh A, Kobashigowa J. Mycophenolate mofetil decreases antibody production after cardiac transplantation. J Heart Lung Transplant. 2002;21:282–285. doi: 10.1016/s1053-2498(01)00335-7. [DOI] [PubMed] [Google Scholar]
- 105.Morris RE, Hoyt EG, Murphy MP, Eugui EM, Allison AC. Mycophenolic acid morpholinoethylester (RS-61443) is a new immunosuppressant that prevents and halts heart allograft rejection by selective inhibition of T- and B-cell purine synthesis. Transplant Proc. 1990;22:1659–1662. [PubMed] [Google Scholar]
- 106.Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80(2 Suppl):S181–S190. doi: 10.1097/01.tp.0000186390.10150.66. [DOI] [PubMed] [Google Scholar]
- 107.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 108.Clayton PA, McDonald SP, Chapman JR, Chadban SJ. Mycophenolate versus azathioprine for kidney transplantation: A 15-year follow-up of a randomized trial. Transplantation. 2012;94:152–158. doi: 10.1097/TP.0b013e31825475a3. [DOI] [PubMed] [Google Scholar]
- 109.Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J, et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001;7:442–450. doi: 10.1053/jlts.2001.23356. [DOI] [PubMed] [Google Scholar]
- 110.Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. European Mycophenolate Mofetil Cooperative Study Group. Lancet. 1995;345:1321–1325. [PubMed] [Google Scholar]
- 111.Mycophenolate mofetil in renal transplantation: 3-year results from the placebo-controlled trial. European Mycophenolate Mofetil Cooperative Study Group. Transplantation. 1999;68:391–396. [PubMed] [Google Scholar]
- 112.Reinhard T, Mayweg S, Sokolovska Y, Seitz B, Mittelviefhaus H, Engelmann K, et al. Systemic mycophenolate mofetil avoids immune reactions in penetrating high-risk keratoplasty: Preliminary results of an ongoing prospectively randomized multicentre study. Transpl Int. 2005;18:703–708. doi: 10.1111/j.1432-2277.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- 113.Birnbaum F, Mayweg S, Reis A, Bohringer D, Seitz B, Engelmann K, et al. Mycophenolate mofetil (MMF) following penetrating high-risk keratoplasty: Long-term results of a prospective, randomised, multicentre study. Eye. 2009;23:2063–2070. doi: 10.1038/eye.2008.402. [DOI] [PubMed] [Google Scholar]
- 114.Mayer K, Reinhard T, Reis A, Voiculescu A, Sundmacher R. Synergistic antiherpetic effect of acyclovir and mycophenolate mofetil following keratoplasty in patients with herpetic eye disease:First results of a randomised pilot study. Graefes Arch Clin Exp Ophthalmol. 2003;241:1051–1054. doi: 10.1007/s00417-003-0724-7. [DOI] [PubMed] [Google Scholar]
- 115.Reis A, Reinhard T, Voiculescu A, Kutkuhn B, Godehardt E, Spelsberg H, et al. Mycophenolate mofetil versus cyclosporin A in high risk keratoplasty patients: A prospectively randomised clinical trial. Br J Ophthalmol. 1999;83:1268–1271. doi: 10.1136/bjo.83.11.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maier AK, Ozlugedik S, Rottler J, Heussen FM, Klamann MK, Huber KK, et al. Efficacy of postoperative immunosuppression after keratoplasty in herpetic keratitis. Cornea. 2011;30:1398–1405. doi: 10.1097/ICO.0b013e31821e65b3. [DOI] [PubMed] [Google Scholar]
- 117.Neyts J, Andrei G, De Clercq E. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob Agents Chemother. 1998;42:216–222. doi: 10.1128/aac.42.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sehgal SN, Molnar-Kimber K, Ocain TD, Weichman BM. Rapamycin: A novel immunosuppressive macrolide. Med Res Rev. 1994;14:1–22. doi: 10.1002/med.2610140102. [DOI] [PubMed] [Google Scholar]
- 119.Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, et al. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells. 2004;9:359–66. doi: 10.1111/j.1356-9597.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 120.Wiederrecht GJ, Sabers CJ, Brunn GJ, Martin MM, Dumont FJ, Abraham RT. Mechanism of action of rapamycin: New insights into the regulation of G1-phase progression in eukaryotic cells. Prog Cell Cycle Res. 1995;1:53–71. doi: 10.1007/978-1-4615-1809-9_5. [DOI] [PubMed] [Google Scholar]
- 121.Morales JM, Wramner L, Kreis H, Durand D, Campistol JM, Andres A, et al. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. Am J Transplant. 2002;2:436–442. doi: 10.1034/j.1600-6143.2002.20507.x. [DOI] [PubMed] [Google Scholar]
- 122.Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 123.Birnbaum F, Reis A, Bohringer D, Sokolowska Y, Mayer K, Voiculescu A, et al. An open prospective pilot study on the use of rapamycin after penetrating high-risk keratoplasty. Transplantation. 2006;81:767–772. doi: 10.1097/01.tp.0000191291.71003.1b. [DOI] [PubMed] [Google Scholar]
- 124.Chatel MA, Larkin DF. Sirolimus and mycophenolate as combination prophylaxis in corneal transplant recipients at high rejection risk. Am J Ophthalmol. 2010;150:179–184. doi: 10.1016/j.ajo.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 125.Trotter JF. Sirolimus in liver transplantation. Transplant Proc. 2003;35(3 Suppl):193S–200S. doi: 10.1016/s0041-1345(03)00234-3. [DOI] [PubMed] [Google Scholar]
- 126.Vanderheyden M, Goethals M, Wellens F. Right atrial thrombosis in a heart transplant recipient after initiation of sirolimus. Acta Cardiol. 2005;60:229–232. doi: 10.2143/AC.60.2.2005038. [DOI] [PubMed] [Google Scholar]
- 127.Birnbaum F, Schwartzkopff J, Scholz C, Reis A, Reinhard T. The new malononitrilamide immunosuppressant FK778 prolongs corneal allograft survival in the rat keratoplasty model. Eye. 2007;21:1516–23. doi: 10.1038/sj.eye.6702727. [DOI] [PubMed] [Google Scholar]
- 128.Asai T, Choi BK, Kwon PM, Kim WY, Kim JD, Vinay DS, et al. Blockade of the 4-1BB (CD137)/4-1BBL and/or CD28/CD80/CD86 costimulatory pathways promotes corneal allograft survival in mice. Immunology. 2007;121:349–358. doi: 10.1111/j.1365-2567.2007.02581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gebhardt BM, Shi W. Experimental corneal allograft rejection. Immunol Res. 2002;25:1–26. doi: 10.1385/IR:25:1:01. [DOI] [PubMed] [Google Scholar]
- 130.Dana MR, Yamada J, Streilein JW. Topical interleukin 1 receptor antagonist promotes corneal transplant survival. Transplantation. 1997;63:1501–1507. doi: 10.1097/00007890-199705270-00022. [DOI] [PubMed] [Google Scholar]
- 131.Hori J, Yamagami S, Obata H, Tsuru T, Isobe M. Effect of monoclonal antibody to VLA-4 on corneal allograft survival in mice. Transplant Proc. 1996;28:1990–1991. [PubMed] [Google Scholar]
- 132.Hori J, Isobe M, Yamagami S, Mizuochi T, Tsuru T. Specific immunosuppression of corneal allograft rejection by combination of anti-VLA-4 and anti-LFA-1 monoclonal antibodies in mice. Exp Eye Res. 1997;65:89–98. doi: 10.1006/exer.1997.0316. [DOI] [PubMed] [Google Scholar]
- 133.Qian Y, Boisgerault F, Benichou G, Dana MR. Blockade of CD40-CD154 costimulatory pathway promotes survival of allogeneic corneal transplants. Invest Ophthalmol Vis Sci. 2001;42:987–994. [PubMed] [Google Scholar]
- 134.Qian Y, Dana MR. Effect of locally administered anti-CD154 (CD40 ligand) monoclonal antibody on survival of allogeneic corneal transplants. Cornea. 2002;21:592–597. doi: 10.1097/00003226-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 135.Jun AS, Larkin DF. Prospects for gene therapy in corneal disease. Eye. 2003;17:906–911. doi: 10.1038/sj.eye.6700565. [DOI] [PubMed] [Google Scholar]
- 136.Comer RM, King WJ, Ardjomand N, Theoharis S, George AJ, Larkin DF. Effect of administration of CTLA4-Ig as protein or cDNA on corneal allograft survival. Invest Ophthalmol Vis Sci. 2002;43:1095–1103. [PubMed] [Google Scholar]
- 137.Bach JF, Fracchia GN, Chatenoud L. Safety and efficacy of therapeutic monoclonal antibodies in clinical therapy. Immunol Today. 1993;14:421–425. doi: 10.1016/0167-5699(93)90243-E. [DOI] [PubMed] [Google Scholar]
- 138.Ayliffe W, Alam Y, Bell EB, McLeod D, Hutchinson IV. Prolongation of rat corneal graft survival by treatment with anti-CD4 monoclonal antibody. Br J Ophthalmol. 1992;76:602–606. doi: 10.1136/bjo.76.10.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He YG, Ross J, Niederkorn JY. Promotion of murine orthotopic corneal allograft survival by systemic administration of anti-CD4 monoclonal antibody. Invest Ophthalmol Vis Sci. 1991;32:2723–2728. [PubMed] [Google Scholar]
- 140.Smolin G, Hyndiuk RA. Suppression of corneal graft reaction by antilymphocyte serum. 3. Effect of pretreatment of donor animals. Arch Ophthalmol. 1971;85:451–454. doi: 10.1001/archopht.1971.00990050453011. [DOI] [PubMed] [Google Scholar]
- 141.Kagaya F, Hori J, Kamiya K, Kaji Y, Oshika T, Amano S, et al. Inhibition of murine corneal allograft rejection by treatment with antibodies to CD80 and CD86. Exp Eye Res. 2002;74:131–139. doi: 10.1006/exer.2001.1109. [DOI] [PubMed] [Google Scholar]
- 142.Comer RM, King WJ, Ardjomand N, Theoharis S, George AJ, Larkin DF. Effect of administration of CTLA4-Ig as protein or cDNA on corneal allograft survival. Invest Ophthalmol Vis Sci. 2002;43:1095–1103. [PubMed] [Google Scholar]
- 143.Thiel MA, Takano T, Hawksworth N, Coster DJ, Williams KA. Low-dose, short-term treatment with anti-CD4 monoclonal antibody prolongs corneal allograft survival. Transplant Proc. 2001;33:635–636. doi: 10.1016/s0041-1345(00)02178-3. [DOI] [PubMed] [Google Scholar]
- 144.Schmitz K, Hitzer S, Behrens-Baumann W. Immune suppression by combination therapy with basiliximab and cyclosporin in high risk keratoplasty. A pilot study. Ophthalmologe. 2002;99:38–45. doi: 10.1007/pl00007114. [DOI] [PubMed] [Google Scholar]
- 145.Dick AD, Meyer P, James T, Forrester JV, Hale G, Waldmann H, et al. Campath-1H therapy in refractory ocular inflammatory disease. Br J Ophthalmol. 2000;84:107–109. doi: 10.1136/bjo.84.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cho YK, Zhang X, Uehara H, Young JR, Archer B, Ambati B. Vascular Endothelial Growth Factor Receptor 1 morpholino increases graft survival in a murine penetrating keratoplasty model. Invest Ophthalmol Vis Sci. 2012;53:8458–8471. doi: 10.1167/iovs.12-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tang XL, Sun JF, Wang XY, Du LL, Liu P. Blocking neuropilin-2 enhances corneal allograft survival by selectively inhibiting lymphangiogenesis on vascularized beds. Mol Vis. 2010;16:2354–2361. [PMC free article] [PubMed] [Google Scholar]
- 148.Yatoh S, Kawakami Y, Imai M, Kozawa T, Segawa T, Suzuki H, et al. Effect of a topically applied neutralizing antibody against vascular endothelial growth factor on corneal allograft rejection of rat. Transplantation. 1998;66:1519–1524. doi: 10.1097/00007890-199812150-00016. [DOI] [PubMed] [Google Scholar]
- 149.Hattori T, Saban DR, Emami-Naeini P, Chauhan SK, Funaki T, Ueno H, et al. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J Leukoc Biol. 2012;91:621–627. doi: 10.1189/jlb.1011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tahvildari M, Omoto M, Chen Y, Emami-Naeini P, Inomata T, Dohlman TH, et al. In Vivo Expansion of Regulatory T Cells by Low-Dose Interleukin-2 Treatment Increases Allograft Survival in Corneal Transplantation. Transplantation. 2016;100:525–532. doi: 10.1097/TP.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]


