Abstract
Different diseases of the optic disc may be caused by or lead to abnormal vasculature at the optic nerve head. Optical coherence tomography angiography (OCTA) is a novel technology that provides high resolution mapping of the retinal and optic disc vessels. Recent studies have shown the ability of OCTA to visualize vascular abnormalities in different optic neuropathies. In addition, quantified OCTA measurements were found promising for differentiating optic neuropathies from healthy eyes.
Keywords: Optical Coherence Tomography Angiography, Optic Disc, Optic Nerve Head, Glaucoma, Optic Neuropathy
INTRODUCTION
The blood flow of the optic nerve head (ONH) is supplied by two main sources; the central retinal artery supplies the superficial layers of the ONH (nerve fiber layer on the surface of the optic disc), and the posterior ciliary artery (PCA) circulation, with possible minor contribution from central retinal artery branches supplies the deeper layers (the prelaminar, lamina cribrosa, and retrolaminar regions).[1] Previous reports indicate that different types of optic neuropathies including glaucoma, ischemic optic neuropathy and hereditary optic neuropathy may be caused by the disorders involving the PCA.[1] Two main mechanisms can cause alterations in the blood flow to optic nerve head. A vascular event may be the trigger for the disease (such as ischemic optic neuropathy, and specific types of glaucomatous optic neuropathy). Alternatively, optic neuropathies due to non-vascular etiologies may lead to vascular changes in the ONH (e.g., hereditary optic neuropathies). Therefore, it is critical that any imaging method used to evaluate the role of the ONH microvascular disorders reveal reliable information about the microcirculation.
Different methods have been used to detect the ocular blood perfusion in clinical practice and experimental research. Fluorescein angiography and indocyanine green angiography are helpful in the qualitative evaluation of retinal and choroidal circulation, however, quantitative measurements of the ONH circulation cannot reliably be performed with these modalities.[2,3] Furthermore, injection of dye may be impractical for routine ONH vascular assessment because of the invasive nature and certain side effects such as nausea, anaphylaxis, etc.[4] Laser Doppler flowmetry, which samples capillary flow over a small retinal area, and laser speckle flowgraphy, which provides a spot sample of blood velocity have been developed to non-invasively measure the blood flow.[5,6] These modalities provide variable results and are not popular for clinical diagnostic applications. Magnetic resonance imaging (MRI) has been proposed to quantitatively image ONH perfusion; however, there are limitations including low spatial resolution, long acquisition time, movement artefacts and relatively high cost.[7] Ultrasound color Doppler imaging provides hemodynamic measurements of the major retrobulbar, retinal and choroidal vessels, but due to the limited resolution, it cannot provide precise measurements of retinal microcirculation.[8]
The development of optical coherence tomography (OCT) has revolutionized the practice and research in the field of the retinal and optic nerve diseases. OCT provides rapid, simple, precise and non-invasive imaging of the structures at the microscopic level. Since its first use in human, OCT has evolved significantly in speed, resolution, and imaging depth, especially with the spectral domain technology.[9,10] In addition to the ability of the OCT to show the structural changes in the retinal and ONH tissues, recent advances in the OCT technology allows functional assessment of ocular structures. Doppler OCT has been reported to be able to measure total human retinal blood flow around the ONH. However, this technology cannot resolve the microcirculation of the ONH, because the velocities in the small vessels are too low to be accurately measured by Doppler shift.[11,12]
Optical coherence tomography angiography (OCTA) is a novel technology that overcomes some of the limitations associated with previous imaging modalities. It uses variations in the intensity and/or phase properties of the OCT signals that result from movement of blood over multiple B-scans to generate high resolution map of the microcirculation.[12,13,14] These decorrelative measurement techniques are independent of flow orientation and can detect a larger dynamic range of flow velocities, allowing for better visualization of the capillary bed.[13,15] Swept-source OCT instruments use longer wavelength ranges (around 1050 nm) and faster scanning speeds. Therefore, the tissue penetration, signal-to-noise ratio, and spatial resolution is even more improved.[16]
In this article, we performed a comprehensive review of the current literature regarding the use of OCTA for visualization of the ONH vasculature and discussed relevant articles [Table 1].
Table 1.
Summary of the articles evaluated optical coherence tomography angiography of the optic nerve head
OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN THE HEALTHY OPTIC NERVE HEAD
Although fluorescein angiography can provide useful information about the larger vessels of the optic disc, a high resolution image of the papillary and peripapillary microvasculature cannot be practically obtained. Spaide et al[17] obtained spectral domain OCTA images from the ONH of 12 healthy subjects and compared the peripapillary capillary network with images obtained by fluorescein angiography. They reported that the radial peripapillary capillary network could not be visualized by fluorescein angiography, whereas the network was readily visualized in OCTA images.
OCTA images allow the peripapillary vascular network to be easily evaluated in different layers. In normal eyes, a dense microvascular network with no focal capillary dropout can be observed around most healthy optic discs [Figure 1]. Falavarjani et al[18] showed that in retinal nerve fiber layer (RNFL) and full thickness retinal slabs, the peripapillary capillary network was more visible immediately adjacent to the border of the disc and around the major vascular arcades, and its clarity decreased centrifugally toward the periphery. The capillary network was less visible in the ganglion cell layer, inner nuclear layer and choriocapillaris slabs.
Figure 1.
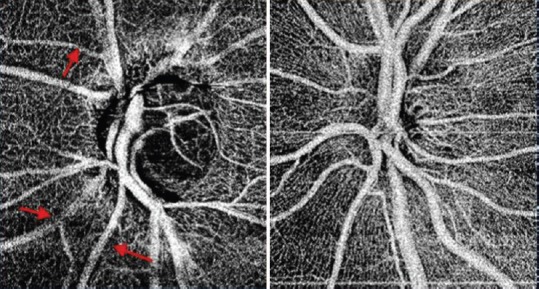
Optical coherence angiography of the optic nerve head of a glaucomatous disc (left) and a healthy disc (right). In addition to the general reduction in the visibility of the disc and peripapillary microvasculature in the glaucomatous disc, focal areas of vascular attenuation are visible (arrows).
Mase et al[19] analyzed the features of the radial peripapillary capillary network, located in the RNFL, using wide-field montage OCTA in 20 healthy human eyes. They found the radial peripapillary capillary densities decrease centrifugally at 0.5, 2.5, and 5 mm from the optic disc edge and correlated significantly with the RNFL thickness.
Chen et al[20] evaluated the repeatability and reproducibility of measurements of blood perfusion in the ONH using optical microangiography based OCTA (Carl Zeiss Meditec Inc., Dublin, California) in 10 healthy eyes. ONH perfusion was quantified as flux, vessel area density, and normalized flux within the ONH for the prelaminar, lamina cribrosa, and the full ONH. They found high repeatability and inter-observer reproducibility in all three layers using three metrics.
OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN GLAUCOMATOUS OPTIC NEUROPATHY
Figure 1 shows OCTA of the optic disc from a patient with glaucomatous optic neuropathy. Jia et al[12] developed a specific algorithm for OCTA called split-spectrum amplitude-decorrelation angiography (SSADA). The algorithm is based on the variation of reflectance amplitude which is sensitive to motion and flow in all directions. They used a swept light source with a central wavelength of 1050 nm and an axial resolution of 5.3 µm. They recruited 4 pre-perimetric glaucoma patients and four normal subjects in order to obtain ONH blood flow with OCTA. They targeted two regions, the whole disc and a temporal ellipse area within the disc, for comparison between two groups. By excluding the major superior and inferior branches of the retinal vessels on the temporal side, they focused on the ONH microvascular beds. They found that in early glaucoma the reduction of ONH microvascular flow was much more dramatic than that of whole ONH circulation. They suggested that quantification performed on microvascular perfusion might be sensitive for detecting ONH circulatory changes in early glaucoma patients.
In a separate study, Jia et al[21] compared the optic disc perfusion between 24 normal subjects and 11 patients with glaucoma using SSADA technology. They demonstrated that OCTA can detect reduced disc perfusion in a group of patients with early glaucoma with 100% sensitivity and specificity. They emphasized that this reduction in flow index is not a result of rim loss or cupping in glaucomatous eyes, and they showed a strong link between the disc flow index and the visual field pattern standard deviation.
Akagi et al[22] investigated the characteristics of the ONH microvascular structure using OCTA in 60 eyes with primary open angle glaucoma (POAG) with hemifield visual field defects and compared the results with 21 normal eyes. They found the peripapillary vessel densities to be significantly reduced at the corresponding location of the visual field defects in both non–highly myopic and highly myopic glaucomatous eyes compared with the normal eyes. The microvascular reduction was associated with visual field defects significantly in the peripapillary retina and partially in the optic disc.
Leveque et al[23] reported the use of a spectral domain OCTA instrument to measure ONH perfusion in 43 patients with open angle glaucoma, seven patients with angle-closure glaucoma and 30 normal subjects. They showed a significant difference in ONH vascular density between glaucoma and normal patients in the total disc and temporal vascular areas, with a decrease of ONH perfusion as compared with controls by 24.8% and 22.9%, respectively. They also observed a significant correlation between ONH perfusion and structural and functional glaucoma damage.
In a prospective observational study, Liu et al[24] evaluated OCTA images from 12 glaucomatous and 12 age-matched normal eyes. Peripapillary flow index and peripapillary vessel density in glaucomatous eyes were significantly lower than those in normal eyes and were highly correlated with visual field pattern standard deviation in glaucomatous eyes.
Wang et al[25] performed a prospective, cross-sectional observational study on 62 eyes with open angle glaucoma, and 20 normal control eyes. They found a decrease in the disc flow index and vessel density in the glaucomatous eyes, which was correlated with the severity of glaucoma damage. Furthermore, their analysis showed that altered flow index and vessel density were correlated with visual field mean deviation, RNFL thickness and ganglion cell layer thickness. Altered flow index and vessel density values were also found to be good indicators of eyes with open angle glaucoma, especially in severe stages of the disease.
Yarmohammadi et al[26] evaluated peripapillary retinal vasculature in 23 healthy subjects, 37 glaucoma suspects, and 104 glaucoma patients. They demonstrated that the vessel density measured in the RNFL was statistically different among the three groups. They found that age-adjusted mean vessel density was significantly lower in open angle glaucoma eyes compared with glaucoma suspects and healthy eyes and vessel density had diagnostic accuracy similar to RNFL thickness measurements for differentiating between healthy and glaucoma eyes. The same group evaluated the association between vessel density measurements using OCTA and severity of visual field loss in POAG eyes.[27] They found that decreased vessel density was significantly associated with the severity of visual field damage independent of the structural loss. They concluded that OCTA is a promising technology in glaucoma management, potentially enhancing the understanding of the role of vasculature in the pathophysiology of the disease.
Suh et al[28] investigated 82 POAG patients with and without focal lamina cribrosa (LC) defects (41 eyes of 41 patients in each group) matched by severity of visual field damage to assess whether vessel density measured by OCTA is reduced in glaucomatous eyes with focal LC defects. They showed that OCTA measured vessel density was significantly lower in POAG eyes with focal LC defects than in eyes without LC defect. The reduction of vessel density was also found to be spatially correlated with the location of the LC defect.
Rao et al[29] evaluated the diagnostic ability of peripapillary vessel density measurements on OCTA in POAG and primary angle-closure glaucoma (PACG), and to compare these with peripapillary RNFL thickness measurements. In this cross-sectional study, 48 eyes of 33 healthy control subjects, 63 eyes of 39 patients with POAG and 49 eyes of 32 patients with PACG underwent OCTA and RNFL imaging with spectral domain OCT. They found that the diagnostic ability of peripapillary vessel density parameters of OCTA, especially the inferotemporal sector measurement, was good in POAG and PACG. Diagnostic abilities of vessel density measurements were comparable to RNFL measurements in both POAG and PACG.
Lee et al[30] investigated the topographic relationship between the decreased parapapillary microvasculature and RNFL in POAG. The vascular impairment was found as an area of decreased density of the microvascular network of the retina in all POAG eyes and exactly coincided with the RNFL defect.
Scripsema et al[31] compare perfused peripapillary capillary density in POAG, normal tension glaucoma (NTG), and normal patients using OCTA. The comparison between groups indicated that annular capillary vessel density for both 3.5- and 4.5-mm scans was significantly reduced in both POAG and NTG groups compared to normal eyes. Annular capillary density was also significantly reduced in the POAG group when compared to the NTG group.
Our group[32] conducted a prospective observational study which included 20 eyes with POAG, 20 pre-perimetric glaucoma eyes and 16 normal eyes. The optic disc region was imaged by a 1050 nm wavelength swept source OCT system (DRI OCT Triton, TOPCON inc. Tokyo, Japan). Vessel density was assessed as ratio of the area occupied by the vessels in the optic nerve head, in the 3 mm papillary and in the peripapillary region, defined as a 700 μm wide elliptical annulus around the disc. All vessel density measurements showed a stepwise decrease from normal eyes to pre-perimetric glaucoma eyes to glaucoma eyes. Furthermore, this difference in vessel density was seen in all three of the anatomic sites measured: optic nerve head, papillary, and peripapillary regions. The study suggested that retinal vascular changes may develop early in the glaucomatous process.
OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN OTHER OPTIC NERVE DISEASES
Wang et al[33] performed OCTA to examine the ONH and retinal microcirculation changes in 59 eyes of 35 patients with multiple sclerosis (MS) with or without a history of optic neuropathy (ON) and compared the results with 21 healthy eyes. Their results showed that the ONH flow index of MS + ON eyes was significantly lower than the values of the control group and the MS − ON group. They also found that the MS + ON eyes had a significantly higher percentage of abnormally low ONH flow index than the control eyes.
Falavarjani et al[18] described the optic disc and peripapillary microvascular changes in OCTA images of 21 eyes of 12 patients with optic disc edema, pseudo-edema and optic atrophy. They reported a dilated and tortuous prelaminar capillary network with an increase or decrease in the visibility of the peripapillary capillary network in disc edema, and the decreased visibility of peripapillary capillary network corresponding to the region or sector of RNFL thinning in eyes with optic atrophy. Also, they found statistically significantly lower vessel density in eyes with optic atrophy. Also, a significant correlation was found between vessel density and RNFL thickness.
Garcia et al[34] reported application of the OCTA to show peripapillary microvasculature in a patient with extensive peripapillary nerve fiber myelination.
DISCUSSION
OCTA is a new technology with a great potential for use in the clinical setting. Compared with FA and ICGA, the current retinal angiographic gold standards, OCTA has advantages including that it is fast and non-invasive, acquires volumetric scans with simultaneous structural and functional information that can be segmented to specific depths, and that it provides accurate size and localization information. However, there are several limitations associated with OCTA imaging. Unlike Doppler OCT, which provides absolute volumetric flow in microliters/minute, OCTA only yields a flow index in arbitrary units. Different types of artifacts may affect interpretation and measurements in OCTA images.[35] Specifically, flow projection artifact from superficial blood vessels prevents clinicians from separately measuring superficial and deep ONH flow. In addition, since the disc flow measurements calculate both disc and retinal circulations they cannot separate PCA and retinal circulations.
Despite these limitations, OCTA images can help our understanding of the pathogenesis of ONH diseases. The results of available studies suggest that OCTA images are able to clearly show different changes in the microvascular network at the optic disc and peripapillary area. In addition, quantification of the peripapillary vascular network is now feasible. However, further well-designed studies are needed to show the benefit of incorporating the OCTA into clinical practice.
Financial support and sponsorship
Dr. Sadda is a consultant for Carl Zeiss Meditec, Optos, Allergan, Genentech, Alcon, Novartis and Roche. He receives research funding from Carl Zeiss Meditec, Optos, Allergan and Genentech. He also receives honoraria from Carl Zeiss Meditec, Optos and Allergan. Dr Sadun is a consultant for Stealth biotechnology, and receives an Edison unrestricted grant support. Other authors have no financial interest to declare.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–748. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regillo CD. The present role of indocyanine green angiography in ophthalmology. Curr Opin Ophthalmol. 1999;10:189–196. doi: 10.1097/00055735-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Keane PA, Sadda SR. Retinal imaging in the twenty- first century: State of the art and future directions. Ophthalmology. 2014;121:2489–2500. doi: 10.1016/j.ophtha.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 4.Stein MR, Parker CW. Reactions following intravenous fluorescein. Am J Ophthalmol. 1971;72:861–868. doi: 10.1016/0002-9394(71)91681-3. [DOI] [PubMed] [Google Scholar]
- 5.Avila CP, Jr, Bartsch DU, Bitner DG, Cheng L, Mueller AJ, Karavellas MP, et al. Retinal blood flow measurements in branch retinal vein occlusion using scanning laser Doppler flowmetry. Am J Ophthalmol. 1998;126:683–690. doi: 10.1016/s0002-9394(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 6.Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010;88:723–729. doi: 10.1111/j.1755-3768.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- 7.Peng Q, Zhang Y, Nateras OS, van Osch MJ, Duong TQ. MRI of blood flow of the human retina. Magn Reson Med. 2011;65:1768–1775. doi: 10.1002/mrm.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kay MD. Color Doppler imaging in disorders of the orbit, retina, and optic nerve. Semin Ophthalmol. 1995;10:242–250. doi: 10.3109/08820539509060978. [DOI] [PubMed] [Google Scholar]
- 9.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27:45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Fawzi AA, Varma R, Sadun AA, Zhang X, Tan O, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011;52:840–845. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia Y, Morrison JC, Tokayer J, Tan O, Lombardi L, Baumann B, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012;3:3127–3137. doi: 10.1364/BOE.3.003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmud MS, Cadotte DW, Vuong B. Review of speckle and phase variance optical coherence tomography to visualize microvascular networks. J Biomed Opt. 2013;18:50901. doi: 10.1117/1.JBO.18.5.050901. [DOI] [PubMed] [Google Scholar]
- 14.Chalam K V, Sambhav K. Optical coherence tomography angiography in retinal diseases. J Ophthalmic Vis Res. 2016;11:84–92. doi: 10.4103/2008-322X.180709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fingler J, Zawadzki RJ, Werner JS, Schwartz D, Fraser SE. Volumetric microvascular imaging of human retina using optical coherence tomography with a novel motion contrast technique. Opt Express. 2009;17:22190–22200. doi: 10.1364/OE.17.022190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potsaid B, Baumann B, Huang D. Ultrahigh speed 1050nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second. Opt Express. 2010;18:20029–20048. doi: 10.1364/OE.18.020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaide RF, Klancnik JM, Cooney MJ. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 18.Ghasemi Falavarjani K, Tian JJ, Akil H, Garcia GA, Sadda SR, Sadun AA. Swept source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina. 2016;36(Suppl 1):S168–77. doi: 10.1097/IAE.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 19.Mase T, Ishibazawa A, Nagaoka T, Yokota H, Yoshida A. Radial Peripapillary Capillary Network Visualized Using Wide-Field Montage Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57:504–510. doi: 10.1167/iovs.15-18877. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Bojikian KD, Xin C, Wen JC, Gupta D, Zhang Q, et al. Repeatability and reproducibility of optic nerve head perfusion measurements using optical coherence tomography angiography. J Biomed Opt. 2016;21:65002. doi: 10.1117/1.JBO.21.6.065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, et al. Optical Coherence Tomography Angiography of Optic Disc Perfusion in Glaucoma. Ophthalmology. 2014;121:1322–1332. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akagi T, Nakanishi H, Tereda N, Morooka S, Yamada H, Hasegawa T, et al. Microvascular Density in Glaucomatous Eyes with Hemifield Visual Field Defects: An Optical Coherence Tomography Angiography Study. Am J Ophthalmol. 2016;168:237–249. doi: 10.1016/j.ajo.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Lévêque PM, Zéboulon P, Brasnu E, Baudouin C, Labbé A. Optic Disc Vascularization in Glaucoma: Value of Spectral-Domain Optical Coherence Tomography Angiography. J Ophthalmol. 2016;2016:6956717. doi: 10.1155/2016/6956717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Jia Y, Takusagawa HL, Pechauer AD, Edmunds B, Lombardi L, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. 2015;133:1045–1052. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Jiang C, Ko T, Kong X, Yu X, Min W, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: An optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2015;253:1557–1564. doi: 10.1007/s00417-015-3095-y. [DOI] [PubMed] [Google Scholar]
- 26.Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalastas PI, Fatehee N, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci. 2016;57:451–459. doi: 10.1167/iovs.15-18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Yousefi S, Saunders LJ, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology. 2016;123:2498–2508. doi: 10.1016/j.ophtha.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh MH, Zangwill LM, Manalastas PIC, Belghith A, Yarmohammadi A, Medeiros FA, et al. Optical Coherence Tomography Angiography Vessel Density in Glaucomatous Eyes with Focal Lamina Cribrosa Defects. Ophthalmology. 2016;123:2309–2317. doi: 10.1016/j.ophtha.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao HL, Kadambi SV, Weinreb RN, Puttaiah NK, Pradhan ZS, Rao DA, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2016-309377. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Lee EJ, Lee KM, Lee SH, Kim TW. OCT Angiography of the peripapillary retina in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2016;57:6265–6270. doi: 10.1167/iovs.16-20287. [DOI] [PubMed] [Google Scholar]
- 31.Scripsema NK, Garcia PM, Bavier RD, Chui TY, Krawitz BD, Mo S, et al. Optical Coherence Tomography Angiography analysis of perfused peripapillary capillaries in primary open-angle glaucoma and normal tension glaucoma. Invest Ophthalmol Vis Sci. 2016;57:OCT611–OCT620. doi: 10.1167/iovs.15-18945. [DOI] [PubMed] [Google Scholar]
- 32.Ghasemi Falavarjani K, Tian JJ, Akil H, Garcia GA, Sadda SR, Sadun AA. Swept source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina. 2016;36(Suppl 1):S168–S177. doi: 10.1097/IAE.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Jia Y, Spain R, Potsaid B, Liu JJ, Baumann B, et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol. 2014;98:1368–1373. doi: 10.1136/bjophthalmol-2013-304547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia GA, Tian JJ, Apinyawasisuk S, Kim S, Akil H, Sadun AA. Clues from Crouzon: Insight into the potential role of growth factors in the pathogenesis of myelinated retinal nerve fibers. J Curr Ophthalmol. 2016;28:232–236. doi: 10.1016/j.joco.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghasemi Falavarjani K, Al-Sheikh M, Akil H, Sadda SR. Image artefacts in swept-source optical coherence tomography angiography. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2016-309104. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


