Abstract
Purpose of review
HIV-infected children are at increased risk of developing cancer. Many of the cancers in HIV-infected children are linked to immunosuppression and oncogenic co-infections. Worldwide most HIV-infected children live in sub-Saharan Africa, but cancer data for this population are scarce. In this article we review the current literature on the epidemiology and prevention of cancer in HIV-infected children.
Recent findings
Combined antiretroviral therapy (cART) reduces the risk of developing cancer in HIV-infected children. Cancer risk remains increased in children who start cART at older ages or more advanced immunosuppression as compared to children who start cART at younger age and with mild immunosuppression. Starting cART before severe immunosuppression develops is key to prevent cancer in HIV-infected children but most children in low-income countries start cART at severe immunosuppression levels. Vaccination against high risk variants of human papilloma virus may protect again HPV-associated cancer later in life. However, tailoring of HPV-vaccination guidelines for HIV-infected children and young women awaits answers to determine the best vaccination strategies.
Summary
Better data on the short and long-term risk of developing cancer and the effects of preventive measures in HIV-infected children from regions with high burden of HIV/AIDS are urgently needed.
Keywords: cancer, HIV, children, prevention, epidemiology
Introduction
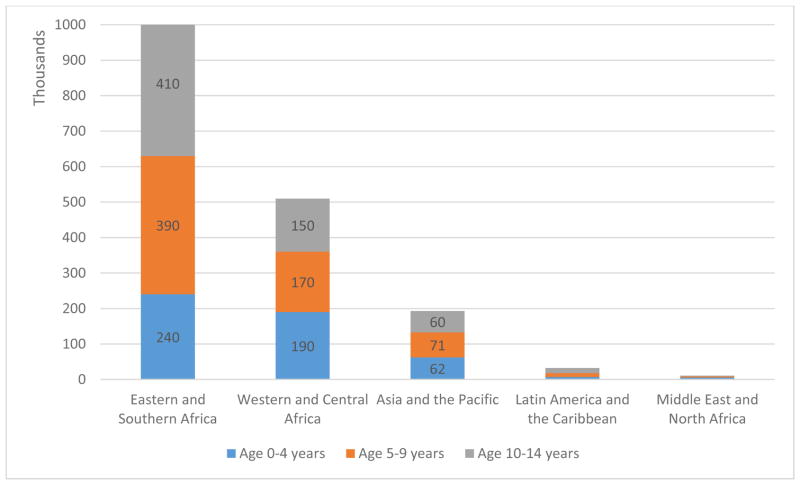
HIV-infected children have an increased cancer risk due to a compromised immune system and an increased susceptibility to oncogenic viruses [1,2]. Worldwide 1.8 million children aged 0–14 years are HIV-infected and at increased risk of developing cancer. Most of these children live in Africa: 1.04 million (58%) in Eastern and Southern Africa and 0.5 million (29%) in Western and Central Africa. In Asia-Pacific the total number of HIV-infected children is 193,000 (11% of HIV-infected children worldwide) and 32,200 (2%) in Latin America and the Caribbean’s (Figure 1). Cancer mortality in HIV-infected children remains high in resource-limited countries. Median survival in HIV-infected children and adolescents with Kaposi sarcoma (KS) was less than six months in a recent trial from Malawi [3]. In Uganda, median survival in HIV-infected children with Burkitt lymphoma was 11.8 months [4]. In a study from South Africa 10% of all HIV-infected children with cancer died of treatment related complications and severe infections [5]. Reliable estimates on the burden of cancer and measures to prevent cancer in HIV-infected children are warranted, especially in resource-limited countries with large burdens of HIV/AIDS. There is a paucity of cancer incidence studies in HIV-infected children from Sub-Saharan-Africa, where the majority of HIV-infected children live [6]. In this article we review the current literature on the epidemiology and prevention of cancer in HIV-infected children.
Figure 1. UNAIDS estimates for number of children [in thousands] living with HIV stratified by age group and region in 2016.
Latin America and the Caribbean: 7,200 children aged 0–4 years; 11,000 children aged 5–9 years; 14,000 children aged 10–14 years. Middle East and North Africa: 5,200 children aged 0–4 years; 3,400 children aged 5–9 years; 2,300 children aged 10–14 years. Estimates for Western, Central and Eastern Europe, Central Asia and North America are not available (NA). Source: unpublished UNAIDS 2016 estimates
Methods
We searched Medline for the period 08/2011-08/2016 using the following search terms: (((HIV[Title/Abstract] OR AIDS[Title/Abstract])) AND (cancer[Title/Abstract] OR neoplasm*[Title/Abstract] OR tumor*[Title/Abstract] OR tumour*[Title/Abstract])) AND (infant* OR children). Additional searches were done for specific topics of interest, such as African countries and prevention. We included articles that were published in the last five years and highlighted those of special interest from the last 18 months. Cancer treatment and outcome is not covered in this article.
Epidemiology of cancer in HIV-infected children
Producing reliable cancer incidence estimates in HIV-infected pediatric populations is a challenge: HIV-cohorts may not record cancer cases and cancer registries may not record HIV-infection status. Record linkages between data of HIV-treatment programs or HIV-registries and cancer registries have been identified as a potential solution to this problem [7,8]. Three recent studies from the USA, Taiwan and South Africa (SA) have used this method to estimate cancer incidence rates in HIV-infected children [9–11]. The SA study linked data at five pediatric cART programs using probabilistic record linkage methods with four referral pediatric oncology units [9]. Amongst a total of 11,707 children, 47 prevalent and 24 incident cancer cases were identified. The overall cancer incidence rate was 82/100,000 person-years. The most frequent cancers were KS and non-Hodgkin lymphoma (NHL) with incidence rates of 34 and 31/100,000 person-years, respectively. The incidence rate for all non AIDS-defining cancers (NADC) combined was 17/100,000 person-years. Risk factors identified were older age at starting cART (10 years versus <3 years, adjusted hazard ratio (HR) 7.3, 95%CI 2.2–24.6) and severe and advanced immunodeficiency as compared to mild or no immunodeficiency at enrolment into program (adjusted HR 3.5, 95%CI 1.1–12). The risk of developing cancer was reduced by 70% in children receiving combination antiretroviral therapy (cART) compared to children not receiving cART (adjusted HR 0.29, 95%CI 0.09–0.86). There were insufficient incident cancer cases to identify risk factors for specific cancers. This was one of the first studies to show a protective effect of cART on the risk of developing cancer at patient level in children. Although the study used data from referral pediatric oncology units to improve cancer ascertainment, under-ascertainment cannot be excluded given that children diagnosed with cancer may not reach specialized services. The study highlighted the need for early HIV diagnosis and cART initiation before advanced immunodeficiency develops to further reduce the burden of cancer in HIV-infected children. A recent multi-regional analysis has shown that many children in low-income countries start cART at severe immunosuppression levels (62% and 65% in girls and boys) compared to high income countries (21% and 28% in girls and boys) [12]. Reasons for the late start are manifold and several studies evaluated acceptance of HIV testing, linkage to care and cART initiation [13]. However, few reported long-term outcomes [14] and none provided cancer data.
Simard et al. investigated the long-term cancer risks in young adults diagnosed with AIDS during childhood [10]. The study was based on the US HIV/AIDS Cancer Match Study using record linkage between HIV and cancer registries. Participants were followed up for up to 10 years. Compared to the general population children diagnosed with AIDS had an increased risk of developing KS, NHL and NADCs, with leiomyosarcoma being the most frequent NADC. Comparing cancer incidence in the pre-cART with the cART era the risk of developing KS was reduced by almost 90% (relative risk (RR) 0.13, 95%CI 0.20–0.74) and the NHL risk by 60% (RR 0.40, 95%CI 0.21–0.75). The risk of developing NADCs did not decrease with the advent of cART (RR 0.98, 95%CI 0.33–2.86). The study highlighted that the risk of developing certain cancers remains increased in persons diagnosed with AIDS during childhood and the need for continued cancer monitoring in this population even with the initiation of cART. With improved access to care in low- and middle-income countries there are now growing numbers of HIV-infected children who start cART earlier and who live into adolescence and adulthood. Studies looking at long-term cancer risk in persons with long-term exposure to HIV-infection, immunosuppression and cART are urgently needed for African and other affected countries.
Lastly, the study from Taiwan used record linkage methods to estimate cancer incidence in HIV-infected children using the Nationwide Health Insurance database [11]. However, with 207 included children the study was rather small.
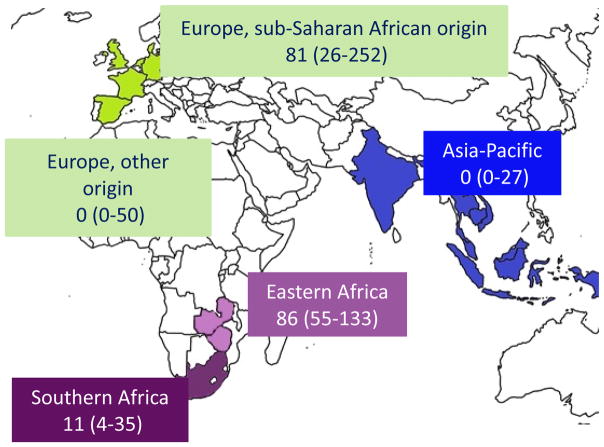
Incidence rates for KS, NHL and NADC differ substantially between these three studies from different geographic areas (table 1). It is also difficult to compare these studies given the differences in study design, inclusion criteria and analysis methods. This problem was addressed in a recent multiregional cohort study that directly compared the risk of developing KS in children starting cART in different geographic regions. Of the 24,991 HIV-infected children from Eastern Africa, Southern Africa and Europe starting cART, 26 children developed incident KS [15]. The study showed that KS (caused by an infection with Human Herpes Virus 8 (HHV-8)), is a frequent cancer in sub-Saharan Africa (SSA) and in SSA migrants in Europe, but cases are rare in children outside of this regions. Incidence rates per 100,000 person-years were 86 in Eastern Africa, 11 in Southern Africa, and 81 in children of SSA origin living in Europe, but zero in Asia-Pacific and European children of non- SSA origin (Figure 2). Higher age at starting cART and advanced HIV/AIDS stage were risk factors for developing KS. Regional differences persisted after adjusting for potential confounders and may point to higher HHV-8 co-infection rates in HIV-infected children from SSA. However, data for HHV-8 seroprevalence were not available in that study. In conclusion, the study underscores the need to start cART early in HIV-infected children at high risk for KS before advanced immunosuppression develops.
Table 1.
Literature review 2011–2016: cancer incidence in HIV-infected children in the era of on Combination Antiretroviral Therapy
| Study | Countries | Calendar years | Children included | Total follow-up time [years] | Cancer cases identified | Incidence rate per 100,000 person-years |
|---|---|---|---|---|---|---|
| Simard et al 2012, AIDS Cancer Match Study [10] | USA | 1996 – 2007 | 1,370 | 17,734 | 37* | Overall: 213 KS: 17 NHL: 132 NADC: 63 |
| Chen et al 2015, NHIRD [11] | Taiwan | 1998–2009 | 230 | NR | 7 | Overall: NR KS: 150 NHL: 75 NADC: NR |
| Bohlius et al 2016, IeDEA-SA [9] | South Africa | 2005 – 2011 | 11,707 | 29,348 | Total 71, prevalent 47, incident 24** | Overall: 82 KS: 34 NHL: 31 NADC: 17 |
Cancer incidence rate estimates in HIV-infected children published in the last five years.
included children and adolescents diagnosed with AIDS only;
prevalent defined as: before cohort enrolment, incident: after cohort enrolment. NHIRD National Health Insurance Research Database; IeDEA-SA International Epidemiological Databases to Evaluate AIDS in Southern Africa; NR not reported.
Figure 2. Kaposi Sarcoma Risk in HIV-Infected Children and Adolescents on Combination Antiretroviral Therapy from sub-Saharan Africa, Europe and Asia.
Kaposi Sarcoma incidence rates per 100,000 person-years with 95% confidence intervals, adapted from [15]
Early cART initiation may also help to reduce the risk of new HHV-8 infections in children living in endemic areas. Recent studies demonstrated that HIV-infected children have a higher risk of co-infection with HHV-8 compared to HIV-uninfected children [16,17]. An observational study from Zambia suggested that in HIV-infected children receiving cART the risk of HHV-8 infection was similar to HIV-uninfected children [17]. In contrast, in HIV-infected children not receiving cART the risk of acquiring HHV-8 was six-times higher compared to HIV-uninfected children (incidence rate ratio 5.97, 95%CI 3.13–11.41) [17].
Prevention of Cancer in HIV infected and exposed children
In this section we review the latest World Health Organization (WHO) guidelines for the early testing and treatment of HIV-infected children and its implications, including cancer risks in HIV and antiretroviral drugs (ARV) exposed but uninfected children. Lastly we discuss vaccination against Human Papilloma Virus (HPV) in HIV-infected children to prevent cervical cancer later in life.
Early testing and treatment
In the WHO “Consolidated guidelines on HIV testing services” (2015), special mention is made of HIV-testing for infants/children and adolescents [18]. Scaling up of Early Infant Diagnosis (EID) is thought to be improved by task-shifting to trained and supervised lay providers. The use of point-of-care HIV testing is carefully considered with rapid diagnostic tests for HIV-serology recommended to assess HIV-exposure in infants less than four months, to rule out HIV in asymptomatic HIV-exposed infants at nine months and to diagnose HIV in children older than 18 months. The development of point-of-care virological assays could further improve EID access [18,19]. WHO suggests governments reconsider the age of consent to allow greater autonomy for HIV-testing among adolescents. adolescents [18]. However, to optimally prevent malignancy and achieve mortality and morbidity benefits, HIV diagnosis needs to be linked to prompt cART initiation and long-term retention on treatment, which could prove challenging in resource-limited settings.
Updated treatment recommendations support the initiation of cART for HIV-infected adolescents and children regardless of WHO clinical stage and CD4 cell count, particularly infants diagnosed within the first year of life. Priority is given to children two years and younger, children between two and five years with WHO clinical stage 3 or 4 or CD4 count ≤750cell/mm3 or CD4 percentage <25% and children older than five years (including adolescents) with WHO stage 3/4 or CD4 count ≤350cell/mm3 [19].
The new guidelines however, introduce the concern of cumulative effects of ARV therapy on a growing child as a result of early cART initiation with life-long duration. Possible strategies to reduce overall lifetime exposure and mitigate cumulative side-effects could include early time-limited cART. The CHER study (Children with HIV Early Antiretroviral randomized trial) investigated the effects of early time-limited cART initiation compared to deferred cART in infants [20]. HIV-infected infants 6-12 weeks of age with CD4 percentage >25% were randomized to deferred therapy (ART-Def), early cART restricted to 40 weeks (ART-40W) or early cART restricted to 96 weeks (ART-96W) with 3.5 years follow-up. Hazard ratios for death for cART-40W and cART-96W were 0.40 (p = 0.02) and 0.45 (p=0.36) respectively compared to cART-Def. Cumulative probability of clinical disease progression or death by 3.5 years was 41% in cART-Def, 28% in the cART-40W and 21% in the cART-96W groups. The CHER trial reported that primary clinical, immunological and virological endpoints for early-time limited cART were superior to deferred cART in asymptomatic HIV-infected infants. However, there was no comparison between time-limited and continuous early cART, follow-up was too short to fully assess the effects of the different strategies on incident malignancy and the strategy may not be feasible in lower-resource settings because of its complexity and the need for close clinical and CD4 monitoring. Overall, the idea of time-limited cART in infants is appealing but data regarding its efficacy compared to continuous cART is lacking.
Cancer risk in HIV and ARV exposed but uninfected children
While ARV prophylaxis for the prevention of mother-to-child HIV transmission has been tremendously successful, new challenges regarding the health outcomes of HIV and ARV exposed, uninfected infants are anticipated. Amongst these is the risk of cancer amongst infants exposed to HIV and ARV perinatally. Few studies have comprehensively investigated this risk with most studies hampered by short-term follow-up periods [21–25]. In the most recent study with a 16-year follow-up period in New Jersey, USA, cancer registry data was linked to HIV case surveillance data to determine the risk of cancer amongst HIV-exposed, uninfected infants perinatally exposed to ARV prophylaxis [26]. For 3,805 HIV-exposed uninfected children the incidence of all cancer types was 13.7/100,000 person-years (95%CI 3.7–35.2). Cancer incidence was not significantly different between HIV-exposed children unexposed to ARV prophylaxis (22.5/100,000 person-years) and children exposed to ARV prophylaxis (14.3/100,000 person-years). Another study showed an increased risk of developing cancer in children exposed to didanosine in-utero as compared to the other ARVs [25]. The authors concluded that didanosine should be strictly contraindicated in pregnancy. Continued vigilance of the health effects of ARV prophylaxis in infants is required as the prevention of mother-to-child transmission program gains momentum and success globally in heterogeneous settings.
HPV-vaccination for the primary prevention of invasive cervical cancer
HIV-infected individuals are at higher risk for all types of HPV-related cancer compared to the HIV-uninfected individuals [27]. Bi-, quadri- and the newly introduced nanovalent HPV-vaccine offer effective protection against infection with high risk HPV variants and have acceptable safety profiles in the general population [28]. Vaccinations are recommended for girls and women aged 9 years up to 25 years old prior to HPV exposure [29]. There are few studies investigating the immunogenicity and safety of the HPV-vaccination in HIV-infected populations [30]. However, from the available evidence, both the bi- and quadrivalent HPV-vaccines have demonstrated high seroconversion rates and acceptable safety profiles in various HIV-infected populations (children, female adolescents and adults). The nanovalent vaccine, is still untested in the HIV-infected population [31]. Seroconversion rates for the quadrivalent vaccine of more than 92% were reported in HIV-infected boys and girls aged 7–12 years [32] and HIV-infected young women 16–23 years (ART and non-ART groups) [33]. However Levin et al reported lower antibody titres to HPV- 6 and 18 in HIV-infected children (7–12 years) compared to historical controls [32]. Kahn et al also reported lower antibody titres in young HIV-infected women (16–23 years) not on cART [33], but similar antibody titres for women receiving cART compared to the HIV-uninfected comparison group [33].
In a study of the bivalent vaccine in HIV-infected women in South Africa (18–25 years), all study participants remained seropositive for HPV-16 and -18 at 12 month follow-up [34]. Antibody titres were 50% and 70% lower at 7 and 12 months in the HIV-infected group compared to the HIV-uninfected group [30,34]. However, HPV-16 and HPV-18 geometric mean titres in the HIV-infected group remained 26- and 16-fold higher at one year than those reported in healthy women (15–25 years) who cleared a natural infection. The bivalent vaccine was reported to have an acceptable safety and reactogenicity profile in HIV-infected young women in this trial.
While the immunogenicity and safety of HPV-vaccination in HIV-infected children and young women has been demonstrated [30,32–34], no long-term follow-up of HIV cohorts receiving HPV-vaccination have been reported. Therefore the duration of vaccine-induced immunogenicity and the clinical significance of the lower antibody titre in HIV-infected children and young women receiving the bi- or quadrivalent HPV-vaccination remain unknown. Tailoring of HPV-vaccination guidelines for HIV-infected children and young women awaits answers to pertinent questions such as the adequacy of a two-dose quadrivalent vaccination schedule, the evaluation of the newly available nano-valent HPV-vaccine, and the requirement/ timing of a booster dose in this group.
Conclusion
Many of the cancers occurring in HIV-infected children are linked to immunosuppression and oncogenic co-infections. Starting cART before severe immunosuppression develops and vaccination against oncogenic viruses appear key to prevent cancer in HIV-infected children. Better data on the short and long-term risk of developing cancer and the effects of preventive measures in HIV-infected children from regions with high burden of HIV/AIDS are urgently needed.
Key points.
HIV-infected children are at increased risk of developing cancer due to immunosuppression and most cancers are associated with oncogenic co-infections.
The majority of HIV-infected children live in Sub-Saharan-Africa, but cancer studies in HIV-infected children from this region are scarce.
Starting combined antiretroviral therapy (cART) before severe immunosuppression develops is key to prevent cancer in HIV-infected children but most children in low-income countries start cART at severe immunosuppression levels.
Vaccination against high risk variants of human papilloma virus may protect again cancer later in life, however, tailoring of HPV-vaccination guidelines for HIV-infected children and young women awaits answers to determine the best vaccination strategies.
Acknowledgments
We would like to thank Eliane Rohner for providing a map with global Kaposi sarcoma incidence rate estimates and Mary Mahy and Juliana Daher from UNAIDS for providing numbers of children living with HIV stratified by age group.
Financial support and sponsorship
This study was supported by the National Institute of Allergy and Infectious Diseases (Grant number 5U01-AI069924-05) of the National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Stefan DC. Malignancies in children with HIV infection [Internet] In: Yarchoan R, editor. Cancers in People with HIV and AIDS: Progress and Challenges. Springer Science + Business Media; 2014. pp. 349–357. [Google Scholar]
- 2.Goncalves PH, Montezuma-Rusca JM, Yarchoan R, Uldrick TS. Cancer prevention in HIV-infected populations. Semin Oncol. 2016;43:173–188. doi: 10.1053/j.seminoncol.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chagaluka G, Stanley C, Banda K, Depani S, Nijram’Madzi J, Katangwe T, Israels T, Bailey S, Mukaka M, Molyneux E. Kaposi’s sarcoma in children: An open randomised trial of vincristine, oral etoposide and a combination of vincristine and bleomycin [Internet] Eur J Cancer. 2014;50:1472–1481. doi: 10.1016/j.ejca.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Orem J, Maganda A, Mbidde EK, Weiderpass E. Clinical characteristics and outcome of children with Burkitt lymphoma in Uganda according to HIV infection. [Internet] Pediatr Blood Cancer. 2009;52:455–8. doi: 10.1002/pbc.21769. [DOI] [PubMed] [Google Scholar]
- 5.Davidson A, Wainwright RD, Stones DK, Kruger M, Hendricks M, Geel J, Poole J, Reynders D, Omar F, Mathew R, et al. Malignancies in South African children with HIV. [Internet] J Pediatr Hematol Oncol. 2014;36:111–7. doi: 10.1097/MPH.0b013e31829cdd49. [DOI] [PubMed] [Google Scholar]
- 6*.Rees CA, Keating EM, Lukolyo H, Danysh HE, Scheurer ME, Mehta PS, Lubega J, Slone JS Baylor Pediatric HIV-Related Malignancy Consortium. Mapping the Epidemiology of Kaposi Sarcoma and Non-Hodgkin Lymphoma Among Children in Sub-Saharan Africa: A Review. [Internet] Pediatr Blood Cancer. 2016;63:1325–31. doi: 10.1002/pbc.26021. Systematic and comprehensive literature review on Kaposi Sarcoma and non-Hodgkin lymphoma in HIV-infected and -uninfected children in Africa. The study clearly documents the need for research on cancer in HIV-infected children in African settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggar RJ. Risk of Cancer in Children With AIDS [Internet] Jama. 2000;284:205. [PubMed] [Google Scholar]
- 8.Mbulaiteye SM, Katabira ET, Wabinga H, Parkin DM, Virgo P, Ochai R, Workneh M, Coutinho A, Engels EA. Spectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match Study. [Internet] Int J Cancer. 2006;118:985–990. doi: 10.1002/ijc.21443. [DOI] [PubMed] [Google Scholar]
- 9**.Bohlius J, Maxwell N, Spoerri A, Wainwright R, Sawry S, Poole J, Eley B, Prozesky H, Rabie H, Garone D, et al. Incidence of AIDS-Defining and Other Cancers in HIV-Positive Children in South Africa: Record Linkage Study. [Internet] Pediatr Infect Dis J. 2016 doi: 10.1097/INF.0000000000001117. Recent cohort study using probabilistic record linkage methods between HIV data and cancer registry data to improve ascertainmrnt of cancer in HIV-infected children in an African setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simard EP, Shiels MS, Bhatia K, Engels EA. Long-term cancer risk among people diagnosed with AIDS during childhood. [Internet] Cancer Epidemiol Biomarkers Prev. 2012;21:148–54. doi: 10.1158/1055-9965.EPI-11-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Jen I, Chen Y-H, Lin M-W, Bhatia K, Sharp GB, Law MG, Arthur Chen Y-M. Cancer incidence in a Nationwide HIV/AIDS patient cohort in Taiwan in 1998-2009. [Internet] J Acquir Immune Defic Syndr. 2014;65:463–72. doi: 10.1097/QAI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Koller M, Patel K, Chi BH, Wools-Kaloustian K, Dicko F, Chokephaibulkit K, Chimbetete C, Avila D, Hazra R, Ayaya S, et al. Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. [Internet] J Acquir Immune Defic Syndr. 2015;68:62–72. doi: 10.1097/QAI.0000000000000380. Large scale multiregional study to document CD4 cell counts and CD4 percentages at start of antiretroviral therapy in HIV-infected children at a global scale, unfortunately no cancer data reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohn J, Whitehouse K, Tuttle J, Lueck K, Tran T. Articles Paediatric HIV testing beyond the context of prevention of mother-to-child transmission: a systematic review and meta-analysis [Internet] Lancet HIV. 2016;3018:1–9. doi: 10.1016/S2352-3018(16)30050-9. [DOI] [PubMed] [Google Scholar]
- 14.Chibwesha CJ, Chi BH. Expanding coverage of paediatric HIV testing [Internet] Lancet HIV. 2016;3018:16–17. doi: 10.1016/S2352-3018(16)30064-9. [DOI] [PubMed] [Google Scholar]
- 15**.Pediatric AIDS-Defining Cancer Project Working Group for IeDEA Southern Africa, TApHOD, and COHERE in EuroCoord. Kaposi Sarcoma Risk in HIV-Infected Children and Adolescents on Combination Antiretroviral Therapy From Sub-Saharan Africa, Europe, and Asia. [Internet] Clin Infect Dis. 2016 doi: 10.1093/cid/ciw519. International multiregional study comparing directly the risk of developing Kaposi sarcoma in HIV-infected children starting cART in Asia, Africa and Europe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohner E, Wyss N, Heg Z, Faralli Z, Mbulaiteye SM, Novak U, Zwahlen M, Egger M, Bohlius J. HIV and human herpesvirus 8 co-infection across the globe: Systematic review and meta-analysis. Int J Cancer. 2016;138:45–54. doi: 10.1002/ijc.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Olp LN, Minhas V, Gondwe C, Kankasa C, Wojcicki J, Mitchell C, West JT, Wood C. Effects of Antiretroviral Therapy on Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Transmission among HIV-Infected Zambian Children. J Natl Cancer Inst. 2015;107:1–8. doi: 10.1093/jnci/djv189. Observational study demonstrating a benefical effect of antireptrovial therapy on the risk of acquiring human-herpes-virus 8, the underlying cause of Kaposi sarcoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.WHO. World Heal Organ. 2015. Consolidated guidelines on HIV testing services 2015. World Health Organisation comprehensive guidelines on HIV testing services (HST). The document consolidates previous and new HST recommendations that address the exisitng gaps and limitations to current approaches. It highlights tailored approaches to HST for key populations. Essential reading for programme managers, healthcare workers and other stakeholdes in HIV care. [PubMed] [Google Scholar]
- 19*.WHO. CONSOLIDATED GUIDELINES ON THE USE OF ANTIRETROVIRAL DRUGS FOR TREATING AND PREVENTING HIV INFECTION. 2015. Following an extensive review of evidence in 2015, the WHO has updated its 2013 guidelines on the use of ARV's including ten new recommendations to improve the quality and efficiency of HIV services provided to the HIV-infected population with an emphasis on earlier initiation of ART for improved clinical outcomes. [Google Scholar]
- 20.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, Josipovic D, Liberty A, Lazarus E. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral ( CHER ) randomised trial. Lancet. 2013;382:1555–1563. doi: 10.1016/S0140-6736(13)61409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culane M, Fowler MG, Lee S, McSherry G, Brady M, O’Donnell K, Mofenson L, Gortmaker S, Shapiro D, Scott G, et al. Lack of Long-term Effects of In Utero Exposure to Zidovudine Among Uninfected Children Born to HIV-Infected Women. JAMA. 1999;281:151–157. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 22.Brogly S, Williams P, Seage GRI, Van Dyke RB. In utero nucleoside reverse transcriptase inhibitor exposure and cancer in HIV-uninfected children: an update from the Pediatric AIDS Clinical Trials Group 219 an 219C cohorts. J Acquir Immune Defic Syndr. 2006;41:530–536. doi: 10.1097/01.qai.0000194735.66322.d9. [DOI] [PubMed] [Google Scholar]
- 23.Hankin C, Lyall H, Peckham C, Tookey P. Monitoring death and cancer in children born to HIV-infected women in England and Wales: use of HIV surveillance and national routine data. AIDS. 2007;21:867–869. doi: 10.1097/QAD.0b013e3280b01822. [DOI] [PubMed] [Google Scholar]
- 24.Benhammou V, Warszawski J, Bellec S, Doz F, Andre N, Lacour B, Levine M, Bavoux F, Tubiana R, Mandelbrot L, et al. Incidence of cancer in children perinatally exposed to nucleoside reverse transcriptase inhibitors. Aids. 2008;22:2165–2177. doi: 10.1097/QAD.0b013e328311d18b. [DOI] [PubMed] [Google Scholar]
- 25*.Hleyhel M, Goujon S, Delteil C, Vasiljevic A, Luzzi S, Stephan JL, Reliquet V, Jannier S, Tubiana R, Dollfus C, et al. Risk of cancer in children exposed to didanosine in utero. Aids. 2016 doi: 10.1097/qad.0000000000001051. Evaluation of cancer incidence amongst HIV-uninfected children exposed to nucleos(t)ide reverse transcriptase inhibitors in utero.Didanosine, still in use in some African countries, accounted for one-third of cancers in exposed children and was associated with a higher cancer risk in multivariate analysis. [DOI] [PubMed] [Google Scholar]
- 26*.Ivy W, 3rd, Nesheim SR, Paul SM, Ibrahim AR, Chan M, Niu X, Lampe MA. Cancer Among Children With Perinatal Exposure to HIV and Antiretroviral Medications--New Jersey, 1995–2010. J Acquir Immune Defic Syndr. 2015;70:62–66. doi: 10.1097/QAI.0000000000000695. Children perinatally exposed to ARV's in New Jersey from 1995–2008 were cross-referenced with the New Jersey State Cancer Registry. Cancer incidence was similar in ARV exposed and unexposed children and numbers of cases amongst ARV exposed children did not differ significantly from cases expected from state and national general population estimates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 28**.Van Damme P, Olsson SE, Block S, Castellsague X, Gray GE, Herrera T, Huang L-M, Kim DS, Pitisuttithum P, Chen J, et al. Immunogenicity and Safety of a 9-Valent HPV Vaccine [Internet] Pediatrics. 2015;136:e28–39. doi: 10.1542/peds.2014-3745. Multi-centre study (72 sites) investigating the immunogencity of 9-Valent HPV-vaccine in girls and boys aged 9-15 years compared to young women aged 16–26 years. 9vHPV was well tolerated in girls and boys with immune responses non-inferior to those of young women, supporting that efficacy findings in young women could be bridged to younger girls and boys (9–15 years) [DOI] [PubMed] [Google Scholar]
- 29.WHO. Human papillomavirus vaccines: WHO position paper, October 2014. World Heal Organ Wkly Epidemiol Rec. 2014;89:465–492. [PubMed] [Google Scholar]
- 30.Toft L, Tolstrup M, Storgaard M, Ostergaard L, Søgaard OS. Vaccination against oncogenic human papillomavirus infection in HIV-infected populations: review of current status and future perspectives. [Internet] Sex Health. 2014;11:511–23. doi: 10.1071/SH14015. [DOI] [PubMed] [Google Scholar]
- 31*.Kojic EM, Rana AI, Cu-uvin S. Human Papillomavirus Vaccination in HIV-infected Women: Need for Increased Coverage. Expert Rev Vaccines. 2016;15:105–117. doi: 10.1586/14760584.2016.1110025. Summary of available evidence on the use of HPV-vaccines amongst HIV-infected women with expert commentary on the gaps in available evidence and the barriers to HPV-vaccine access for HIV-infected women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin MJ, Moscicki A-B, Song L-Y, Fenton T, Meyer WA, Read JS, Handelsman EL, Nowak B, Sattler CA, Saah A, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. [Internet] J Acquir Immune Defic Syndr. 2010;55:197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn JA, Xu J, Kapogiannis BG, Rudy B, Gonin R, Liu N, Wilson CM, Worrell C, Squires KE. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV- infected young women. [Internet] Clin Infect Dis. 2013;57:735–44. doi: 10.1093/cid/cit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, Durand C, Hervé C, Descamps D. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study [Internet] Vaccine. 2013;31:5745–53. doi: 10.1016/j.vaccine.2013.09.032. [DOI] [PubMed] [Google Scholar]