Abstract
During animal development and homeostasis, the structure of tissues, including muscles, blood vessels, and connective tissues, adapts to mechanical strains in the extracellular matrix (ECM). These strains originate from the differential growth of tissues or forces due to muscle contraction or gravity. Here we show using a computational model that by amplifying local strain cues, active cell contractility can facilitate and accelerate the reorientation of single cells to static strains. At the collective cell level, the model simulations show that active cell contractility can facilitate the formation of strings along the orientation of stretch. The computational model is based on a hybrid cellular Potts and finite-element simulation framework describing a mechanical cell-substrate feedback, where: 1) cells apply forces on the ECM, such that 2) local strains are generated in the ECM and 3) cells preferentially extend protrusions along the strain orientation. In accordance with experimental observations, simulated cells align and form stringlike structures parallel to static uniaxial stretch. Our model simulations predict that the magnitude of the uniaxial stretch and the strength of the contractile forces regulate a gradual transition between stringlike patterns and vascular networklike patterns. Our simulations also suggest that at high population densities, less cell cohesion promotes string formation.
Introduction
During embryonic development, a single fertilized egg cell grows into a complex functional organism (1). Even after years of studying morphogenesis, the organization of cells into tissues, organs, and organisms, it remains a puzzle how cells migrate and form the right pattern in the right part of the body at the right moment (2). Apart from chemical signals (3), mechanical signals play an equally important role in morphogenesis (4, 5). Static strains originating from differential growth of tissues are instrumental for the organization of cells in tissues in vivo. For example, in quail heart, the endocardium generates strains to which cardiomyocyte microtubules orient (6). Wing-hinge contractions in Drosophila cause anisotropic tension in the wing-blade epithelium, to which the cells align (7). Using a multiscale computational modeling approach, here we unravel how static strains, e.g., resulting from the differential growth of tissues, may drive the organization of cells and tissues.
In vitro and in silico experiments have helped to unravel the cellular mechanisms underlying the adaptation of tissues to strain. Myocytes (8), mesenchymal stem cells (9), muscle cells, and endothelial cells (10) orient in parallel to uniaxial static stretch. Furthermore, fibroblasts organize into stringlike structures in parallel to the stretch orientation (11), whereas endothelial cells form monolayers of cells oriented in parallel to the stretch (10).
Active cell traction forces play a crucial role in the alignment of cells to static uniaxial stretch. Using contact guidance, cells can adjust their orientation to the fibers that align with strain (12, 13). Then, by pulling on the matrix, cells can further align the fibers (14). Such mechanical cell-fiber feedback can coordinate cell alignment (15, 16, 17) and string formation (18) along strain. However, in vitro observations suggest that cell alignment to uniaxial stretch may not necessarily be driven by fiber alignment. Mesenchymal stem cells align along the orientation of strain on a nonfibrous matrix (9). In stretched collagen matrices, fibroblasts were found to align along strain in the absence of fiber alignment (11, 19). Other authors observed that collagen fibers aligned only after the cells had aligned (20, 21). Moreover, fibroblasts can orient along the uniaxial stretch even if fibronectin fibers were aligned perpendicular to the stretch (22). Altogether, these results suggest that cells can orient to stretch independently of the fiber orientation.
Mathematical modeling is a helpful tool to explore what biophysical mechanisms can explain the alignment of cells to strain. Previous mathematical models (23, 24) were based on optimization principles. Bischofs and Schwarz (23) proposed that cells minimize the amount of work needed for contracting the matrix. For dipolar cells, the work was minimized if they oriented in parallel with the uniaxial stretch. If the cells were assumed to generate strains in their local environment, cells formed strings that aligned with an external strain field (23, 25, 26). Based on the observation that cells reorganize focal adhesions and stress fibers to maintain constant local stresses, De et al. (24) proposed that cells adapt their contractility and orientation to find the minimal local stress in the matrix. They showed that the local stress becomes minimal if a dipolar cell orients in parallel to uniaxial stretch, as in this configuration the cell traction forces counteract the uniaxial stretch.
In this work, we explain cellular alignment to strain based on a mesoscopic, experimentally testable cellular mechanism. To simulate this mechanism, we propose a hybrid computational model in which the cellular Potts model (CPM) (27) is coupled to a finite-element model (FEM) of the matrix. The computational model (28) captures the mechanical cross talk between the extracellular matrix (ECM) and the cells as follows: 1) cells apply forces on the ECM (29); 2) the resulting strains in the ECM are calculated using a finite-element method; and 3) cells extend protrusions oriented along strain (21).
Based on experimental observations of fibroblasts on elastic substrates (30) and on modeling studies (24), it has been suggested that cellular traction forces may dominate over, or even counteract with, global strain cues. Paradoxically, our model suggests that contractile cells locally increase the global uniaxial strain that facilitates cell alignment to static uniaxial stretch. Our model also suggests that by contracting the matrix, cells can form strings in parallel to the orientation of uniaxial stretch. Finally, our simulations show that differences in cell cohesion and population density may determine under what conditions cells form strings, and when they only align on an individual level.
Materials and Methods
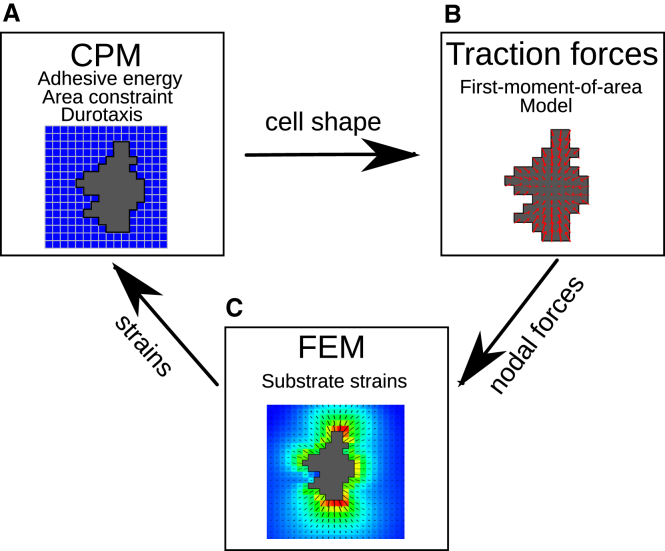
We extended our previous hybrid, cell-based, and continuum model (28) of mechanical cell-ECM feedback to include the effects of static strain. Fig. 1, A–C, gives an overview of the model structure. Active cell motility is simulated using the CPM (27). The CPM is coupled to a finite-element method that is used to calculate substrate deformations. A time step of the simulation proceeds as follows. Based on the local strains in the matrix and the interactions with adjacent cells, the CPM calculates the cell shapes (Fig. 1 A). Based on the cell shapes, the traction forces that cells apply on the ECM are determined using the empirically validated first-moment-of-area (FMA) model, as proposed by Lemmon and Romer (29) (Fig. 1 B). The FEM calculates the deformation of the substrate resulting from these forces (Fig. 1 C). Subsequently, the strains in the ECM influence cell movement in the CPM. More precisely, we assume that cells preferentially extend along the orientation of high strain.
Figure 1.
Structure of the coupled CPM-FEM model. (A) CPM calculates cell shapes in response to local ECM strains; (B) calculation of cellular traction forces based on cell shapes (29); and (C) substrate strains due to cellular traction forces. To see this figure in color, go online.
CPM
The CPM (27) describes cells on a regular lattice as a domain of connected lattice sites, , of identical spin, or cell identifier, . Sites of spin identify sections of the substrate that are covered by a biological cell, whereas sites of spin identify exposed substrate sites. The configuration of cells evolves according to the Hamiltonian,
| (1) |
The first term is a surface area constraint, with , the number of lattice sites covered by cell s, A(s) a target area, and λ a Lagrange multiplier. The second term represents the interfacial energies in the system, e.g., due to cell adhesion and cortical tensions. Here, is the interfacial energy between an adjacent lattice site pair and is the Kronecker delta. The contact energy Jcc regulates the degree of cell-cell adhesion, with lower values of Jcc corresponding to strong cell-cell adhesion.
To mimic cellular protrusions and retractions of the cells, the CPM iteratively picks a random lattice site and attempts to copy its spin into an adjacent site . The algorithm then calculates the energy change ΔH associated with the copy attempt based on the Hamiltonian (Eq. 1) and any additional energy changes associated with the copy direction (31), in this case, ΔHdir. With ΔHdir we express the cellular response to matrix strains, as outlined below. The copy is accepted if , or with Boltzmann probability to allow for stochasticity of cell movements. is a cellular temperature whose magnitude gives the amount of random cell motility. An additional connectivity constraint rejects copy attempts that would split cells into disconnected patches. During one Monte Carlo step (MCS), N copy attempts are made, with , i.e., the number of sites in the lattice.
To simulate the response of cells to strains in the substrate, we assumed that local strains promote cellular protrusion and inhibit cellular retractions. Such a mechanism is motivated by focal adhesions, large integrin complexes that bind the cell to the matrix and maturate on stiffer matrices (32). We assume a strain-stiffening material, so that focal adhesions mature on highly strained areas. We thus set
| (2) |
where λstrain is a parameter that describes the sensitivity of cells to strain; , is the direction of copying; and E1 and E2 and and are the eigenvalues and eigenvectors of ε that represent the principal strains and strain orientation in the target site . We use if a cell is extending and if a cell is retracting, to impose that strain stiffening of the matrix promotes extensions and inhibits retractions. At cell-cell interfaces we assume that the forces due to strain on the extending cell and the retracting cell are balanced, i.e., if and . We thus assume that neither of the two cells involved in the copy attempt benefits more from occupying a strained lattice site than another cell. The sigmoid function expresses that a minimum stiffness, , is required for focal adhesion maturation. We assume that cells perceive strain stiffening of the matrix, described by the function , where is a stiffening parameter. Compared to our previous implementation of this model (28), slight adaptations have been made in the Hamiltonian, which are discussed in the Supporting Material. They do not affect the qualitative behavior of the model. The parameter values used in this study are reported in Table S1 in the Supporting Material. We use a discretization of Δx = 2.5 μm. Based on single cell dispersion rates in our model, we previously estimated the time interval Δt corresponding to one MCS to be between Δt = 0.5 s and Δt = 3 s (28).
FEM of compliant substrate
A FEM (33) is used to calculate the strain on the substrate resulting from forces applied to the substrate. The substrate is assumed isotropic and linearly elastic. For simplicity, we applied infinitesimal strain theory, assuming that material properties, including local density and stiffness, are unchanged by deformations. So, the strain tensor is given by
| (3) |
where is the substrate deformation.
The elements of the FEM coincide with the lattice sites of the CPM, i.e., the deformation in a lattice site is approximated by an interpolation of the shape functions , for n = 1, 2, 3, 4 corresponding to the four nodes (corners) of lattice element e:
| (4) |
where is the substrate deformation at node n. We used conventional linear shape functions for four-noded quadrilateral elements (33). The FEM is iterated until equilibrium (, where K denotes the stiffness matrix (33)), to calculate the deformation at each node. All figures in this article show the strain in equilibrium. The terms Eij in the strain tensor of element e are thus given by
| (5) |
In our simulations, the unstretched substrate is used as a reference configuration for the displacements due to uniaxial stretch and cell contractility. This simplifies our calculations and speeds them up. For details, see van Oers et al. (28).
Cellular traction forces
To model the traction forces that cells apply on the substrate, we make use of an experimentally validated, predictive model, called the FMA model (29). The FMA model is based on the assumption that the network of actin fibers acts in the cells as a single, cohesive unit. In the context of our hybrid CPM-FEM, we implement the FMA model as follows. Defining lattice nodes as the corners of the CPM lattice sites, each lattice node i covered by a CPM cell pulls on every other node j within the same cell, with a force of magnitude proportional to the distance between the nodes, , and , if line piece (i, j) intersects with the cell boundary (see Lemmon and Romer (29); and see Fig. S1, A–D, and the Supporting Material for details). The total force on node i then becomes
| (6) |
In accordance with the assumption that the cytoskeleton has uniform contractility, the line pieces have a constant tension per unit distance μ (29). For convex cells, the resultant forces point toward the cell’s center of mass. For nonconvex cells, the resultant forces are directed toward the individual, convex compartments that the cell shape is composed of (see the Supporting Material).
Results
This work proposes a computational model for the collective response of cells to uniaxial stretch in compliant tissues. In the model, cells apply contractile forces onto a compliant substrate. The resulting strains in the matrix affect the motility of the cell itself and the motility of its neighbors. In all of the simulations described in this article, we stretched a substrate of Young’s modulus 12 kPa with a stress of = 1000 N/m2 applied to the boundary of the matrix in the FEM. This results in a static strain of ∼8% on the matrix. The cellular tension μ (see Eq. 6) was set to 0.0025 nN/μm, resulting in local strains around the tips of elongated cells of up to 2%, amplifying the static strain to values of ∼10%.
Individual cell response to uniaxial stretch is amplified by cell contractility
To elucidate how cell traction forces affect individual cell response to uniaxial stretch in our model, we simulated the response of a single cell to uniaxial stretch applied in the vertical orientation. This was carried out both in the presence (μ > 0) and in the absence (μ = 0) of active cell contraction. Fig. 2 A shows a noncontractile cell on a uniaxially stretched ECM after 500 MCS; the cell elongates slightly along the stretch orientation, in accordance with our previous results (28). Fig. 2 B shows the same simulation setup in the presence of active cell contraction. The contractile cell elongates more strongly than the noncontractile cell (Fig. 2 A). Interestingly, the cell orients itself along the strain orientation, despite the fact that the contractile forces (Eq. 6) counteract the uniaxial stretch. This choice for Δx is based on balance between precision and computation time. To confirm that the model is scalable, we repeated the simulation on grids that were refined by a factor of 2 (Δx = 1.25 μm; Fig. S3 A) and 4 (Δx = 0.625 μm; Fig. S3 B), and observed qualitatively similar behavior. Out of the batch of 100 simulations, in 38 of the simulations, the middle part of contractile cells became rather slender (Fig. S2 C), resulting in a cell shape that seems unrealistic, as elongated cells are typically reported to have a spindlelike shape. The area conservation (Eq. 1) imposes that extensions are, on average, balanced by retractions. Because contractile cells reduce the uniaxial stretch around the center of the cell, retractions are most likely to occur here, resulting in a slender middle part.
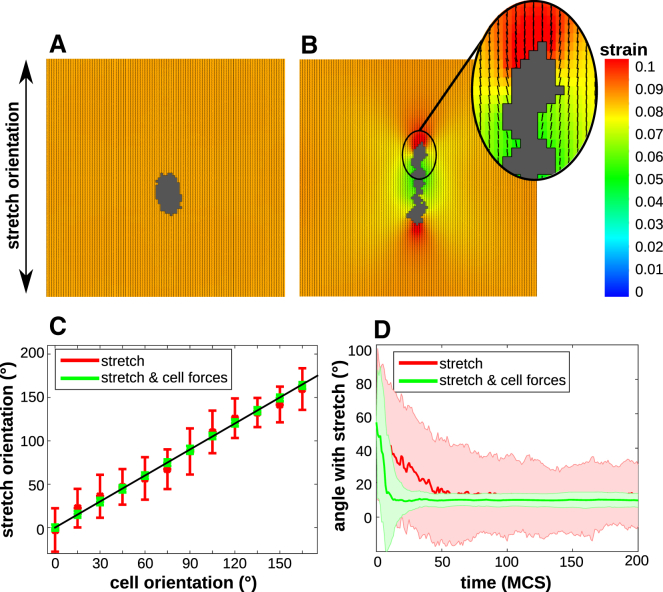
Figure 2.
(A) Noncontractile cell on substrate stretched along 0° at 500 MCS; (B) contractile cell on substrate stretched along 0° at 500 MCS. (A and B) Colors: principal strain magnitude; orientation and length of black line pieces: orientation and magnitude of principal strain. (C) Cell orientation as a function of stretch orientation at 500 MCS, averaged over n = 100 simulations (error bars: standard deviations; black line shows linear fit). (D) Time series of the orientation of a single cell on a substrate stretched over 0°, averaged over n = 100 simulation (shaded regions: standard deviations). Color coding (C and D): red (biggest standard deviations): noncontractile cells; green (smallest standard deviations): contractile cells. To see this figure in color, go online.
To study single cell orientation in more detail and check the isotropy of the model, we performed 100 simulations of single contractile and noncontractile cells for 500 MCS, using stretch angles in the range 0–180° with increments of 15° on a 100 × 100 lattice, representing a piece of tissue of 250 × 250 μm. Cells with a diameter of seven lattice sites were initiated in the middle of the matrix. The cell orientation was estimated from the inertia tensor of the cells (see Supporting Material). Fig. 2 C plots the cell orientation as a function of the orientation of stretch for cells without active contraction (red boxes) and with active contraction (green boxes). In both conditions, the cells follow the strain orientation on average. However, the cells that apply active contractile forces on the matrix followed the orientation with much higher accuracy, as evidenced by the much smaller standard deviations. Also, the eccentricities of the contractile cells were much more narrowly distributed than those of noncontractile cells (Fig. S2 A). Fig. 2 D shows that the contractile cells oriented more quickly to the stretch orientation than the noncontractile cells. This behavior was only observed on matrices of intermediate stiffness (Fig. S2 B). On soft substrates, cells remain small (28) and as a result do not apply sufficient force on the matrix. On a very stiff matrix, cells protrude in all directions (28) and thus they cannot orient along a specific angle.
Altogether, the simulated contractile cells aligned with the strain more accurately than the noncontractile cells. This can be explained by a positive feedback loop among cell shape, cell traction forces, and strain stiffening. Cells elongate slightly in response to uniaxial stretch. Due to the anisotropic cell shape, cells pull harder on the matrix around the tip of the cells, because the distance between the tip of the cell and the cell interior increases (see Eq. 6). So, the matrix stiffens around the tip of the cell, which further promotes cell elongation along uniaxial stretch.
Cell contractility enables cells to align with each other in parallel to uniaxial stretch
We next looked at the alignment of neighboring cells in uniaxially stretched matrices. We simulated the response of two circular cells placed horizontally next to each other on a substrate with a static strain along the vertical axis, both in the presence (μ > 0) and in the absence (μ = 0) of active cell contraction. Fig. 3 A shows a pair of cells on a statically stretched matrix at 500 MCS; the cells elongate slightly and do not align in a head-to-tail fashion. Fig. 3 B shows the same simulation setup in the presence of active cell contraction. In contrast to noncontractile cells, a pair of contractile cells assumes a head-to-tail configuration. Also, similar to the response of a single cell found in the previous section, both cells elongate more strongly than the noncontractile cell in Fig. 3 A. Notably, the pair of contractile cells assume a head-to-tail configuration along the orientation of uniaxial stretch.
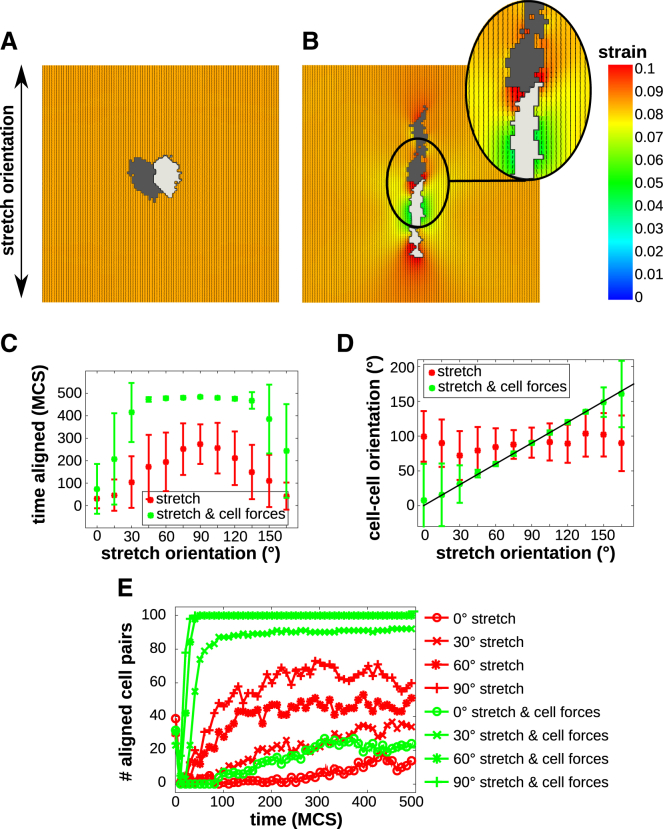
Figure 3.
(A) Noncontractile cell pair on substrate stretched along 0° at 500 MCS; (B) contractile cell pair on substrate stretched along 0° at 500 MCS. (A and B) Colors: principal strain magnitude; orientation and length of black line pieces: orientation and magnitude of principal strain. (C) Fraction of time a cell pair is aligned, averaged over n = 100 simulations; upper data points for contractile cells and lower data points for non-contractile cells; (D) angle of the line connecting the center of masses as a function of stretch orientation at 500 MCS, averaged over n = 100 simulations, contractile cells follow the fitted line. (C and D) Error bars: standard deviations. (E) Time series of the number of cell pairs that are aligned on stretched substrates with different stretch orientations (circle: 0°; cross: 30°; star: 60°; plus-sign: 90°). Upper lines are for contractile cells and lower lines are for non-contractile cells. (C–E) Red: noncontractile cells; green: contractile cells. To see this figure in color, go online.
To study this head-to-tail alignment in more detail, we performed 100 simulations of paired cells for 500 MCS for both scenarios on a 200 × 200 lattice, corresponding to 500 × 500 μm, for stretch angles in the range 0–180° with increments of 15°. Two cells with a diameter of seven lattice sites were initiated in the middle of the matrix, eight lattice sites apart. Cell-cell alignment was quantified by evaluating the triangle (A, B, C), where A and B are the center of masses of the two cells and C is the point where the lines describing the orientations of the two cells intersect. Table S2 describes how this triangle is used to decide whether a pair of cells is aligned or not. Fig. 3 C plots the fraction of time that cells are aligned on a stretched substrate as a function of stretch orientation for cells without active contraction (red boxes) and with active contraction (green boxes). Contractile cells align more often with each other than noncontractile cells. To confirm that cells align along the stretch orientation, we measured the orientation of the line connecting the center of masses of the two cells. Fig. 3 D plots this cell-cell angle as a function of stretch orientation; a pair of contractile cells aligns along stretch, compared to noncontractile cells that stick around their initial position (placed horizontally next to each other) and thus keep their initial alignment angle of 90°.
Fig. 3 E plots the number of cell pairs that aligned as a function of time in n = 100 simulations. This shows that with stretch ∼0°, cells cannot always align. This is because, after initial elongation, the tips of the cells are not in each other’s vicinity, such that the cells cannot sense each other’s strain. Interestingly, this phenomenon may provide an explanation for an experimental observation reported by Winer et al. (34). Studying the behavior of endothelial cells on compliant matrices, they observed that cell pairs aligned more on 2 mg/mL polyacrylamide gels than on a softer, 1 mg/mL gel on which cell assumed an extremely elongated shape. They hypothesized that this “extremely elongated shape of the cells, and thus the shape of the resulting strain field, reduced the probability that a second cell would come in contact with the affected gel”. To test this hypothesis in our model, we increased the probability of a cell to come into contact with the strain field of the other cell, by increasing the cellular temperature T. Increasing T increases the probability that a cell makes a protrusion. Fig. S4 A shows the fraction of time a pair of contractile cells is aligned as a function of T and Fig. S4 B shows how the number of cell pairs that aligns depends on T. This illustrates that pairs of cells more readily align at higher values of T. These simulation results thus match the hypothesis of Winer et al. (34). At motilities >∼ T = 20, cell motility became randomized to the extent that the cells could no longer align.
In summary, in our model, pairs of contractile cells aligned in head-to-tail configurations along the orientation of stretch, whereas noncontractile cells oriented with the stretch, but not in a head-to-tail fashion. The bipolar strain fields around the contractile cells were instrumental for this cell-cell alignment.
Cell contractility facilitates the self-organization of cells into strings oriented parallel to uniaxial stretch
After identifying the orientation response of a pair of cells, we asked how cell contractility affects the alignment of a large group of cells. We simulated a group of cells on a stretched matrix, both in the presence (μ > 0) and in the absence (μ = 0) of active cell contraction. The behavior of the model does not depend on the stretch orientation, so we only show the results for stretching in the vertical orientation in the next sections. Fig. 4 A shows a group of cells on a statically stretched matrix in the vertical orientation at 3000 MCS: the cells have elongated slightly and have not migrated away from their initial position. Fig. 4 B shows the same simulation setup in the presence of active cell contraction. The contractile cell aligned locally with one another in a head-to-tail configuration, as observed in our simulation of paired cells. This cell-cell alignment enables cells to form strings along the orientation of uniaxial stretch, as observed experimentally by Eastwood et al. (11)
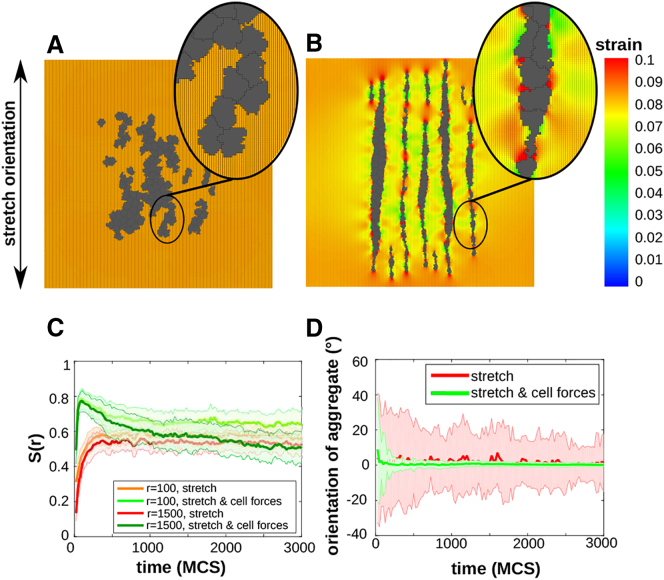
Figure 4.
(A) Noncontractile cells on substrate stretched along 0° at 3000 MCS; (B) contractile cells on substrate stretched along 0° at 3000 MCS. (A and B) Colors: principal strain magnitude; orientation and length of black line pieces: orientation and magnitude of principal strain. (C) Time series of orientational order parameter S(r), averaged over n = 25 simulations (red (bottom curve): r = 100 μm for noncontractile cells; orange (second bottom curve): r = 1500 μm for noncontractile cells; green (top curve): r = 100 μm for contractile cells; dark-green (second curve from top): r = 1500 μm for contractile cells). (D) Time series of the orientation of cell aggregates on a substrate stretched over 0° at 3000 MCS, averaged over n = 25 simulations (shaded regions: standard deviations). (C and D) Red (biggest standard deviations): noncontractile cells; green (smallest standard deviations): contractile cells. To see this figure in color, go online.
To study this behavior in more detail, we performed 25 simulations of a group of cells on a 400 × 400 lattice, representing a piece of tissue of 1 × 1 mm, for 3000 MCS for both scenarios, for a stretch angle of 0°. Cells are initially placed uniformly inside a region of 200 × 200 lattice sites in the middle of the matrix, as to minimize boundary effects. Cells are initially one lattice site in size. The density of cells was d = 0.15, yielding around 120 cells. To characterize the collective orientation of cells, we measured a 2D orientational order parameter S(r), with range r (μm), defined for the CPM as in Palm and Merks (35). Briefly, , where is the center of mass of cell s and is the angle between the orientation of the cell of spin s and a local director, i.e., the average orientation of the cells within a radius r around the centroid of cell s (see the Supporting Material for detail). S(r) ranges from S(r) = 0 for configurations of randomly oriented cells, to S(r) = 1 for fully aligned cells. Fig. 4 C plots the orientational order parameter as a function of time, showing a local orientational order (r = 100 μm) for noncontractile cells (orange curve) and for contractile cells (green curve), and the global orientational order (r = 1500 μm) for noncontractile cells (red curve) and for contractile cells (dark-green curve). Contractile cells achieve a higher local and similar global ordering than noncontractile cells. Note that contractile cells initially obtain a high orientational order, close to 0.8. Because cells initially have enough space, they elongate well. When cells start to adhere to another and form strings, cells in the interior of a string cannot orient well, such that the global orientational order parameter decreases. This is a model artifact that we investigated further in the Supporting Material and will address in the Discussion.
To confirm that, by contracting the matrix, cells coalign into strings oriented along uniaxial stretch, we measured the orientation of cell aggregates, with a cell aggregate defined as a connected patch of cells (see the Supporting Material for details on the calculation). Fig. 4 D plots the orientation of cell aggregates as a function of time, of noncontractile cells (green curve) and contractile cells (red curve). In both conditions, cells form aggregates with an orientation ∼0°, which is the orientation of stretch. The aggregates formed by contractile cells follow the stretch orientation more accurately, as shown by the smaller standard deviations, indicating that strings have formed.
In our model, contractility facilitates the formation of strings of cells along the stretch orientation, in agreement with experimental observations (11). We have shown previously that in unstrained matrices, contractile cells organize into networklike structures (28). We next studied what level of uniaxial stretching is needed for cells to prefer a stringlike organization instead of a networklike organization.
The results of varying uniaxial stretch are shown in Fig. 5. How the amount of uniaxial stretch affects string formation, depends on the magnitude of the cell traction forces. When we varied the uniaxial stretch and fixed the cell traction force to the default parameter value (middle row in Fig. 5 A), we observed that cells can more easily align in a head-to-tail configuration and form strings (Fig. 5 C) if stretching is lower than the default value (Fig. 5 D). Indeed, the global ordering decreases as a function of uniaxial stretch (middle set of bar plots in Fig. 5 B). In our model, this is explained as follows. Due to the assumed strain stiffening behavior, the cells spread more (28) on highly stretched matrices. Then, within strings, cells have less space and orient less well. If cells apply little traction (first row in Fig. 5 A), they do not form strings with small uniaxial stretch, but do when stretching is increased. Then, with even more uniaxial stretch, cells orient along stretch, but do not forms strings, similar to noncontractile cells (Fig. 4 B). Indeed, the global orientational order parameter shows a biphasic dependence on uniaxial stretching (first set of bar plots in Fig. 5 B). This is explained in our model as follows. Cell forces cannot sufficiently amplify a small uniaxial stretch and thus more uniaxial stretch is needed to instigate string formation. However, at higher uniaxial stretch, the cell traction forces are insufficiently strong to amplify the uniaxial strain, and as a result cells do not form strings. Note that these cells do not form networks with little uniaxial stretch, as they do not sufficiently contract the matrix to align with other cells. If cells are highly contractile (last row in Fig. 5 A) they form networks, similar to cells on nonstretched matrices (Fig. 1 F). Higher uniaxial stretching transforms a network into an oriented network (Fig. 5 E) and subsequently into strings. Indeed, the global order has a biphasic dependence on stretching (last set of bar plots in Fig. 5 B). This is because with too little uniaxial stretch, cell-generated strains dominate the global strain cue and thus cells do not collectively orient. Of course, if we would increase uniaxial stretch even more, cells would align but no longer form strings.
Figure 5.
(A) Contractile cells on substrate stretched along 0° at 3000 MCS simulated with various values of cell traction force and matrix stretching force; (B) global orientational order parameter S(r = 1500 μm) at 3000 MCS, averaged over n = 25 simulations; error bars: standard deviations, bars in bar chart are sorted according to value of σstretch as indicated in figure inset. (C) Zoom-in of cell configuration of μ = 0.0025, Fstretch = 100; (D) zoom-in of cell configuration of μ = 0.0025, Fstretch = 1000; (E) zoom-in of cell configuration of μ = 0.01, Fstretch = 500. (A, C, and E) Colors: principal strain magnitude; orientation and length of black line pieces: orientation and magnitude of principal strain. To see this figure in color, go online.
To better understand the results in Fig. 5, recall that cells extend toward areas that are stiffened by strain, as described by the sigmoid function (Fig. S5 B), where . Fig. S5 A shows that cells can only form strings when the matrix is stiffened to values above (Fig. S5 D). If the matrix is not stiffened, or becomes too rigid, the cells will not align (Fig. S5, C and E). To relate this to Fig. 5, instead of strain, we plotted normalized stiffness values in Fig. S6. This shows that when the uniaxial stretch stiffens the matrix to values around and cell traction forces then stiffen the matrix more, strings can be formed. However, strings cannot be formed when the matrix is stiffened too much by either the cells or the uniaxial stretch.
Altogether, the results suggest that an optimal balance between uniaxial stretch and cell contractility is needed for cells to form strings.
Decreasing cell-cell adhesion promotes string formation in populations with high cell density
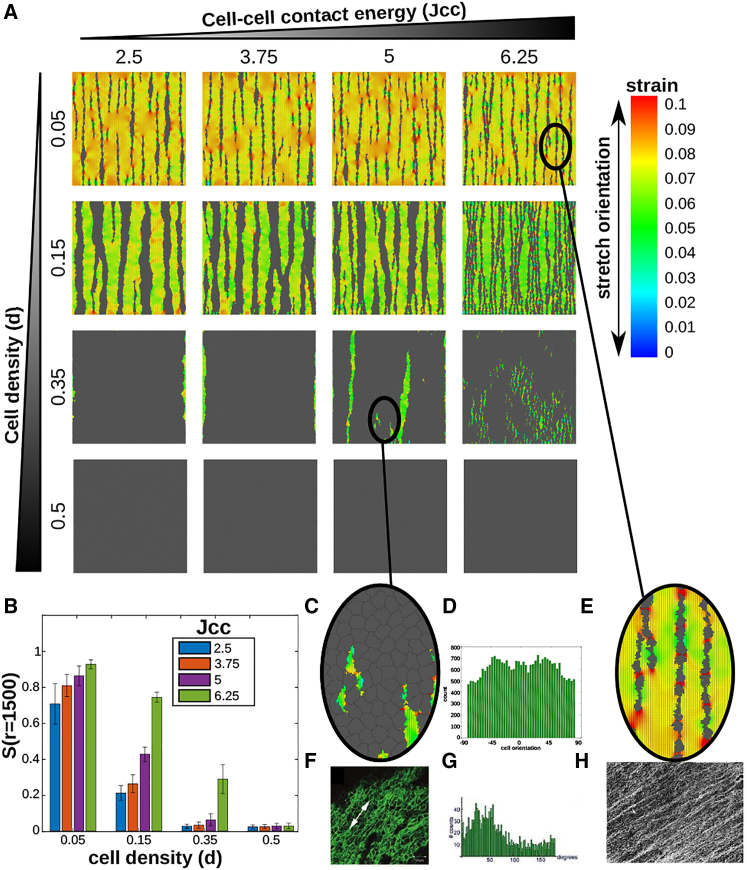
Experimental work has reported two alternative cellular responses to uniaxial strain. Fibroblasts seeded at a density of 106 cells/mL form strings along the orientation of uniaxial stretch (11). Three-dimensional (3D) cultures of endothelial cells at much higher density of 107–109 cells/mL align along the stretch orientation, but do not form strings (10). Thus, the differences between these two experiments could be due to cellular densities, or to specific differences between fibroblasts and endothelial cells. In particular, endothelial cells have stronger cell-cell adhesion than fibroblasts, as the endothelial-specific VE-cadherins have stronger bond strengths than the N-cadherins found in fibroblast cell-cell junctions (36). Fig. 6 A shows an overview of the final configurations of simulations in which we systematically varied cell density and cell-cell contact energies; Fig. 6 B shows the corresponding global orientational order parameters. To better mimic variable densities of cells, we initialized cells on the whole grid of 400 × 400 lattice sites. The configurations shown in Fig. 6 A suggest that fewer, thicker strings are formed if the cells adhere more strongly to one another (i.e., low Jcc). Also, the global order parameter increases as the cell-cell contact energies increase (Fig. 6 B), suggesting that nonadhering cells respond more easily to the strain cue. At a seeding density d = 0.35 and mildly repellent cell-cell adhesion settings of Jcc = 5, the final configurations (Fig. 6 C) and the distribution of cell orientations (Fig. 6 D) qualitatively resemble the experiments by van der Schaft et al. (10) (Fig. 6, F and G). Decreasing cell-cell adhesion and cell densities to d = 0.05 Mid Jcc = 6.25 produces configurations similar to Eastwood et al. (11) (Fig. 6, E and H). Currently, in completely confluent cell layers with high cell-cell adhesion (d = 0.35, d = 0.5, Jcc = 3.75, 2.5 in Fig. 6), the cells do not align at all, because in our model the cells cannot respond to strain at cell-cell interfaces. We investigated this issue further in the Supporting Material and address this in the Discussion.
Figure 6.
(A) Contractile cells on substrate stretched along 0° at 3000 MCS simulated with various values of cell density and cell-cell contact energy. (B) Global orientational order parameter S(r = 1500 μm) at 3000 MCS, averaged over n = 25 simulations (error bars: standard deviations; bars in bar chart are sorted according to value of Jcc as indicated in figure inset. (C) Zoom-in of cell configuration of Jcc = 5, d = 0.35. (D) Cell orientations of Jcc = 6.25, d = 0.35. (E) Zoom-in of cell configuration of Jcc = 6.25, d = 0.05. (F) 3D culture of endothelial cells on uniaxially stretched matrix, taken from van der Schaft et al. (10). (G) Orientation of 3D culture of endothelial cells on uniaxially stretched matrix, taken from van der Schaft et al. (10). (H) Fibroblasts on uniaxially stretched matrix, taken from Eastwood et al. (11). (A, C, and E) Colors, principal strain magnitude: orientation and length of black line pieces: orientation and magnitude of principal strain. To see this figure in color, go online.
Discussion
In this article we have presented a computational model to show that active cell contraction can facilitate cellular alignment to the orientation of static uniaxial stretch. The computational model describes motile cells living on top of an elastic substrate, and is based on only a few, experimentally validated assumptions: 1) cells exert contractile forces on the substrate, which locally generate strains in the substrate (29, 37); 2) cells move by repeatedly attempting to extend or retract pseudopods at random; and 3) along the substrate strain orientation, pseudopod extensions are promoted and pseudopod retractions are inhibited (21), a procedure mimicking the maturation of focal adhesions under strain (32).
We have shown previously (28) that these assumptions suffice to reproduce 1) the elongation of single cells on compliant substrates, 2) the alignment of two adjacent cells, and at the collective level 3) the formation of vascularlike network structures and angiogenesis-like sprouting structures. Here we show that a refined version of this model also reproduces experimentally observed behavior of fibroblasts, endothelial cells, and myocytes on statically, uniaxially stretched substrates: 1) cells tend to align in parallel to the uniaxial stretch orientation (8, 9, 10) (compare to Fig. 2); 2) cells align with one another in parallel to the uniaxial stretch orientation (Fig. 3); and 3) collectively, the cells form strings oriented along the stretch (Eastwood et al. (11) and Fig. 4) and they elongate along the stretch in close to confluent layers of cells (van der Schaft et al. (10) and Fig. 6). Although the assumed response to strains (assumption 3) makes the simulated cells orient to the stretch without contractility (see van Oers et al. (28) and Fig. 2 A), active contractility makes cells elongate more strongly (Fig. 2 B), and allows them to respond to strain cues more accurately (Fig. 2 C) and more rapidly (Fig. 2 D) than noncontractile cells. Thus, a crucial factor for these phenomena is the balance between active cell contractility and the magnitude of the uniaxial stretch cue. Provided the cellular traction forces are sufficiently strong, the cells will collectively organize into oriented strings even in response to very subtle strain cues (Fig. 5). For stronger cell contractility, however, the local strains will override the global strain cue and the cells will organize into networklike patterns as reported previously (see Fig. 5 A, lower-left panels; also compare to van Oers et al. (28)). The reported model behavior holds for substrates with stiffness of ∼10–∼16 kPa, a wider range than the autonomous cell elongation reported previously (28). Note that the exact magnitude of this range depends on the parameter settings and in particular threshold E0 in sigmoid function , whose values were kept unchanged relative to van Oers et al. (28) (and see Table S1).
Experimental validation of our model predictions would need to focus both on the response to uniaxial static stretch of single cells and on the collective behavior of multiple cells. Single cells in our models elongate more easily and reorient more easily to uniaxial static stretch if they contract the matrix. At the multicellular level, contractility induces string formation on uniaxially statically stretched matrices. A number of published in vitro experiments already support the single cell behavior that our model predicts. For example, oxidatively modified low density lipoprotein stimulates the contractility of human aortic endothelial cells, which correlates with increased cell elongation (38). Fibroblasts moving on stretched collagen gels align their trajectories more strongly to the strain orientation than less contractile neutrophils (39). To validate single cell response to uniaxial static stretch, we propose experiments in which cells with different contractilities are seeded on a uniaxially stretched matrix as, e.g., in Haston et al. (39). Treatment with lysophosphatidic acid can stimulate Rho-mediated contractility (26, 40), while treating cells with blebbistatin or cytochalasin D inhibits contractility (34). At the multicellular level, with increasing uniaxial stretch, our model system switches gradually between networks and strings (Fig. 5). Previous cell culture studies (10, 11) have not varied the strain magnitude, but in uniaxially, statically stretched ex ovo chick chorioallantoic membranes, blood vessels realign along stretch (41). Further in vitro experiments could vary the magnitude of the uniaxial stretch and the degree of contractility using chemical treatments (see, e.g., Klumpers et al. (42) for a suitable experimental system). The cell density and the cell-cell adhesion strength also influenced the ability of cells to form strings. At high cell densities, simulated cells are less able to form strings, while decreasing cell-cell adhesion restores string formation. Uniaxial stretching experiments where cell-seeding densities are varied and cell-cell adhesion is controlled, by inhibiting or knocking out Cadherins, could validate these predictions.
Although our model is currently not resolved to molecular detail, its simulation results do suggest a mechanistic explanation for the response of cells to static uniaxial stretch. Previous theoretical models (23, 24) proposed that cells actively regulate their orientation to optimize a local mechanical property. Bischofs and Schwarz (23) represented cells as active dipoles, and showed that the dipole can minimize the amount of work required to contract the matrix by orienting along the external strain (23). This optimization principle was motivated by force-induced focal adhesion maturation: maximum forces will develop at the focal adhesions that are displaced the least. Based on observations suggesting that cells maintain a constant local stress in their microenvironment, De et al. (24) proposed that dipolar cells actively regulate their orientation and contractility to maintain a constant optimal amount of local stresses in the matrix. In this model, the dipolar cells reorient to the uniaxial stretch and gradually reduce the magnitude of their contractility to reduce the stress in the matrix. Mechanistic rationales certainly motivated these optimization principles, but the mechanisms were not modeled explicitly and a dipole shape was presumed. Our approach instead aims to derive single-cell phenomena and collective cellular responses to strain from a small set of experimentally plausible assumptions at the subcellular level. This work is only a first step toward this aim. Currently, the local substrate strains regulate the protrusion and retraction probabilities based on a phenomenological function (Eq. 2), which simulates focal adhesion maturation. In our ongoing work we are refining this part of the model by introducing explicit kinetic models of the focal adhesions.
This coarse-grained description has suggested new mechanisms for the experimental observations listed above, but due to a number of technical limitations it still fails to reproduce others. We cannot yet reproduce cell alignment to uniaxial stretch in a completely confluent layer, because the strain-bias of the cell protrusions and retractions is cancelled out at cell-cell interfaces (see Eq. 2 and Fig. S7, A and B). As a first exploration of the behavior of our model in absence of this effect, we ran a series of simulations in which we differentiated the probability of the retractions relative to extensions. With an decreased retraction probability , fully confluent cell layers collectively oriented in parallel to stretch (Fig. S7, C and D). In contrast, in simulations with an increased retraction probability , the cells oriented themselves perpendicular to the stretch orientations in a confluent layer (Fig. S7, E and F). Another result of the absence of strain-effects at cell-cell boundaries is that contractile cells do not achieve a high global ordering within strings (Fig. 4 A); this is because cells in the interior of the strings do not elongate. When the retraction probability is decreased , the contractile cells reach a higher global ordering (S(r = 1500 μm) = 0.71) compared to the noncontractile cells (S(r = 1500 μm) = 0.51) (Fig. S8 A). In simulations in which the retraction probability is increased , the contractile cells reached a lower global ordering (S(r = 1500 μm) = 0.37) compared to the noncontractile cells (S(r = 1500 μm) = 0.64), as some cells in the interior of strings started to align perpendicular to strain (Fig. S8 B). Despite these quantitative differences, note that cells form strings irrespective of the specific modeling choices (Fig. S8, C and D). Also related to this modeling choice is the apparent unrealistic cell shape as presented in Fig. S2 C. Such cells appear less frequently in simulations where retraction probabilities are decreased (Fig. S8, C and D). This work primarily focused on the collective behavior of cells; in our ongoing work we are developing more detailed, single-cell models.
Apart from this coarse-graining of the focal adhesion dynamics and cell motility, our model also relies on other methodological simplifications. The finite-element description of the substrate assumes that the ECM is isotropic, nonfibrous, and linearly elastic. Because of these assumptions, our model best applies to nonfibrous matrices (e.g., synthetic polyacrylamide matrices), or to matrices with fibers much smaller than the size of the cells. Of course, more complex matrix mechanics can be modeled using FEM approaches. Interestingly, Aghvami et al. (43), who modeled an anisotropic fiber reinforced material, showed similar increased local strains around (nonmigratory) cells pulling on uniaxially stretched matrices as in our model. Alternative, agent-based approaches have been proposed for fibrous matrices (16, 17, 44); in comparison to these models, a particular advantage of our hybrid approach is in its scalability to multicellular systems. As a disadvantage relative to these agent-based approaches, our hybrid setup relies on an operator splitting approach, which alternates updates of the cell traction forces with the MCSs of the CPM. Although this process speeds up our computations and operator splitting approaches are routinely applied in hybrid modeling (see, e.g., Checa et al. (45) and Albert and Schwarz (46, 47)), it of course also introduces numerical errors: ideally we would recalculate the cellular traction forces and substrate strains after every copy attempt of the CPM. From a biophysical point of view the operator splitting assumption is valid if we can separate the timescales of the growth and degradation of focal adhesions, such that cell traction forces remain approximately constant during the time represented by one MCS. Indeed, focal adhesion dynamics occur at a timescale of minutes, which is longer than one MCS, which in our model is equivalent to 0.5–3 s (28). An ongoing improvement of our approach concerns the coupling between the cellular traction forces, as represented by the FMA model (Eq. 6), and the representation of these forces in the Hamiltonian (Eq. 1). In the basic CPM, the area conservation and adhesive energy terms in the Hamiltonian describe a pressure and approximate a membrane tension that together represent cell contractility. This allowed us to study the effects of cell-cell contact energies. These terms are not equal to the forces described by the FMA model. The strength of this model is that it produces experimentally validated strain fields. The decoupling of the CPM and the FMA model will become an issue at locations where the two sets of forces are unequal, e.g., at cell-cell interfaces and can affect the mesoscopic cell behavior. Because we are interested in how mesoscopic cell behavior affects the macroscopic level, i.e., collective behavior, these approximations and decoupling suffice here. In our ongoing work, we are adopting an approach proposed by Albert and Schwarz (46) to alleviate this issue.
In summary, we proposed a local cell-matrix feedback mechanism explaining the reorientation of cells to external stretch. In agreement with experimental observations, in this model cell contractility facilitates the reorientation of cells. The proposed mechanism also suffices for the formation of strings along the orientation of stretch. In our future work, we are refining the model by introducing explicit focal adhesion dynamics. This approach will pave the way for issues that our model cannot currently explain, including the response of cells to cyclic stretch (48, 49), and the role of cell-substrate adhesivity in the formation of networklike patterns (50) and collective cell behavior (47).
Author Contributions
E.G.R. and R.M.H.M. designed research; E.G.R. performed research and analyzed data; and E.G.R. and R.M.H.M. wrote the article.
Acknowledgments
This work is part of the research program “Innovational Research Incentives Scheme Vidi Cross-divisional 2010 ALW” with project number 864.10.009, which is (partly) financed by the Netherlands Organization for Scientific Research (NWO). We thank SURFsara (www.surfsara.nl) for the support in using the Lisa Compute Cluster.
Editor: Sean Sun.
Footnotes
Supporting Materials and Methods, eleven figures, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)34287-4.
Supporting Citations
Supporting Material
References
- 1.Friedl P., Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 2.Reig G., Pulgar E., Concha M.L. Cell migration: from tissue culture to embryos. Development. 2014;141:1999–2013. doi: 10.1242/dev.101451. [DOI] [PubMed] [Google Scholar]
- 3.Rogers K.W., Schier A.F. Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- 4.Siedlik M.J., Nelson C.M. Regulation of tissue morphodynamics: an important role for actomyosin contractility. Curr. Opin. Genet. Dev. 2015;32:80–85. doi: 10.1016/j.gde.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shawky J.H., Davidson L.A. Tissue mechanics and adhesion during embryo development. Dev. Biol. 2015;401:152–164. doi: 10.1016/j.ydbio.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garita B., Jenkins M.W., Linask K.K. Blood flow dynamics of one cardiac cycle and relationship to mechanotransduction and trabeculation during heart looping. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H879–H891. doi: 10.1152/ajpheart.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aigouy B., Farhadifar R., Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 8.Collinsworth A.M., Torgan C.E., Truskey G.A. Orientation and length of mammalian skeletal myocytes in response to a unidirectional stretch. Cell Tissue Res. 2000;302:243–251. doi: 10.1007/s004410000224. [DOI] [PubMed] [Google Scholar]
- 9.Liu C., Baek S., Chan C. Effect of static pre-stretch induced surface anisotropy on orientation of mesenchymal stem cells. Cell. Mol. Bioeng. 2014;7:106–121. doi: 10.1007/s12195-013-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Schaft D.W.J., van Spreeuwel A.C.C., Baaijens F.P.T. Mechanoregulation of vascularization in aligned tissue-engineered muscle: a role for vascular endothelial growth factor. Tissue Eng. Part A. 2011;17:2857–2865. doi: 10.1089/ten.TEA.2011.0214. [DOI] [PubMed] [Google Scholar]
- 11.Eastwood M., Mudera V.C., Brown R.A. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil. Cytoskeleton. 1998;40:13–21. doi: 10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Vader D., Kabla A., Mahadevan L. Strain-induced alignment in collagen gels. PLoS ONE. 2009;4:6e5902. doi: 10.1371/journal.pone.0005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaubaroux C., Perrin-Schmitt F., Hemmerlé J. Cell alignment driven by mechanically induced collagen fiber alignment in collagen/alginate coatings. Tissue Eng. Part C Methods. 2015;21:881–888. doi: 10.1089/ten.tec.2014.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebe R.J., Caldwell H., Milam S. Cells transmit spatial information by orienting collagen fibers. Matrix. 1989;9:451–458. doi: 10.1016/s0934-8832(11)80014-4. [DOI] [PubMed] [Google Scholar]
- 15.Takakuda K., Miyairi H. Tensile behaviour of fibroblasts cultured in collagen gel. Biomaterials. 1996;17:1393–1397. doi: 10.1016/0142-9612(96)87280-2. [DOI] [PubMed] [Google Scholar]
- 16.Reinhardt J.W., Krakauer D.A., Gooch K.J. Complex matrix remodeling and durotaxis can emerge from simple rules for cell-matrix interaction in agent-based models. J. Biomech. Eng. 2013;135:71003. doi: 10.1115/1.4024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhardt J.W., Gooch K.J. Agent-based modeling traction force mediated compaction of cell-populated collagen gels using physically realistic fibril mechanics. J. Biomech. Eng. 2014;136:021024. doi: 10.1115/1.4026179. [DOI] [PubMed] [Google Scholar]
- 18.Dyson R.J., Green J.E.F., Byrne H.M. An investigation of the influence of extracellular matrix anisotropy and cell-matrix interactions on tissue architecture. J. Math. Biol. 2016;72:1775–1809. doi: 10.1007/s00285-015-0927-7. [DOI] [PubMed] [Google Scholar]
- 19.Tondon A., Kaunas R. The direction of stretch-induced cell and stress fiber orientation depends on collagen matrix stress. PLoS One. 2014;9:e89592. doi: 10.1371/journal.pone.0089592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E.J., Holmes J.W., Costa K.D. Remodeling of engineered tissue anisotropy in response to altered loading conditions. Ann. Biomed. Eng. 2008;36:1322–1334. doi: 10.1007/s10439-008-9509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang Y., Wang X., Greisler H.P. Dynamic quantitative visualization of single cell alignment and migration and matrix remodeling in 3-D collagen hydrogels under mechanical force. Biomaterials. 2011;32:3776–3783. doi: 10.1016/j.biomaterials.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudera V.C., Pleass R., Brown R.A. Molecular responses of human dermal fibroblasts to dual cues: contact guidance and mechanical load. Cell Motil. Cytoskeleton. 2000;45:1–9. doi: 10.1002/(SICI)1097-0169(200001)45:1<1::AID-CM1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Bischofs I.B., Schwarz U.S. Cell organization in soft media due to active mechanosensing. Proc. Natl. Acad. Sci. USA. 2003;100:9274–9279. doi: 10.1073/pnas.1233544100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De R., Zemel A., Safran S.A. Dynamics of cell orientation. Nat. Phys. 2007;3:655–659. [Google Scholar]
- 25.Bischofs I.B., Schwarz U.S. Effect of Poisson ratio on cellular structure formation. Phys. Rev. Lett. 2005;95:068102. doi: 10.1103/PhysRevLett.95.068102. [DOI] [PubMed] [Google Scholar]
- 26.Bischofs I.B., Schwarz U.S. Collective effects in cellular structure formation mediated by compliant environments: a Monte Carlo study. Acta Biomater. 2006;2:253–265. doi: 10.1016/j.actbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Graner F., Glazier J.A. Simulation of biological cell sorting using a two-dimensional extended Potts model. Phys. Rev. Lett. 1992;69:2013–2016. doi: 10.1103/PhysRevLett.69.2013. [DOI] [PubMed] [Google Scholar]
- 28.van Oers R.F.M., Rens E.G., Merks R.M.H. Mechanical cell-matrix feedback explains pairwise and collective endothelial cell behavior in vitro. PLOS Comput. Biol. 2014;10:e1003774. doi: 10.1371/journal.pcbi.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemmon C.A., Romer L.H. A predictive model of cell traction forces based on cell geometry. Biophys. J. 2010;99:L78–L80. doi: 10.1016/j.bpj.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo C.M., Wang H.B., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rejniak K., Poplawski N.J., Anderson A. Magnetization to morphogenesis: a brief history of the Glazier-Graner-Hogeweg model. In: Glazier J.A., Balter A., editors. Single-Cell-Based Models in Biology and Medicine. Birkhäuser; Basel, Switzerland: 2007. pp. 79–106. [Google Scholar]
- 32.Pelham R.K., Wang Y.L. Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Bio Bull. 1998;194:348. doi: 10.2307/1543109. [DOI] [PubMed] [Google Scholar]
- 33.Davies A.J. Oxford University Press; Oxford, UK: 2011. The Finite Element Method: An Introduction with Partial Differential Equations. [Google Scholar]
- 34.Winer J.P., Oake S., Janmey P.A. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS One. 2009;4:e6382. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palm M.M., Merks R.M.H. Vascular networks due to dynamically arrested crystalline ordering of elongated cells. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2013;87:012725. doi: 10.1103/PhysRevE.87.012725. [DOI] [PubMed] [Google Scholar]
- 36.Panorchan P., George J.P., Wirtz D. Probing intercellular interactions between vascular endothelial cadherin pairs at single-molecule resolution and in living cells. J. Mol. Biol. 2006;358:665–674. doi: 10.1016/j.jmb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Califano J.P., Reinhart-King C.A. Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell. Mol. Bioeng. 2010;3:68–75. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byfield F.J., Tikku S., Levitan I. OxLDL increases endothelial stiffness, force generation, and network formation. J. Lipid Res. 2006;47:715–723. doi: 10.1194/jlr.M500439-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Haston W.S., Shields J.M., Wilkinson P.C. The orientation of fibroblasts and neutrophils on elastic substrata. Exp. Cell Res. 1983;146:117–126. doi: 10.1016/0014-4827(83)90330-0. [DOI] [PubMed] [Google Scholar]
- 40.Roy P., Petroll W.M., Jester J.V. Exertion of tractional force requires the coordinated up-regulation of cell contractility and adhesion. Cell Motil. Cytoskeleton. 1999;43:23–34. doi: 10.1002/(SICI)1097-0169(1999)43:1<23::AID-CM3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Belle J., Ysasi A., Mentzer S.J. Stretch-induced intussusceptive and sprouting angiogenesis in the chick chorioallantoic membrane. Microvasc. Res. 2014;95:60–67. doi: 10.1016/j.mvr.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klumpers D.D., Smit T.H., Mooney D.J. The effect of growth-mimicking continuous strain on the early stages of skeletal development in micromass culture. PLoS One. 2015;10:e0124948. doi: 10.1371/journal.pone.0124948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aghvami M., Barocas V.H., Sander E.A. Multiscale mechanical simulations of cell compacted collagen gels. J. Biomech. Eng. 2013;135:71004. doi: 10.1115/1.4024460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlüter D.K., Ramis-Conde I., Chaplain M.A.J. Computational modeling of single-cell migration: the leading role of extracellular matrix fibers. Biophys. J. 2012;103:1141–1151. doi: 10.1016/j.bpj.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Checa S., Rausch M.K., Duda G.N. The emergence of extracellular matrix mechanics and cell traction forces as important regulators of cellular self-organization. Biomech. Model. Mechanobiol. 2015;14:1–13. doi: 10.1007/s10237-014-0581-9. [DOI] [PubMed] [Google Scholar]
- 46.Albert P.J., Schwarz U.S. Dynamics of cell shape and forces on micropatterned substrates predicted by a cellular Potts model. Biophys. J. 2014;106:2340–2352. doi: 10.1016/j.bpj.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albert P.J., Schwarz U.S. Dynamics of cell ensembles on adhesive micropatterns: bridging the gap between single cell spreading and collective cell migration. PLOS Comput. Biol. 2016;12:e1004863. doi: 10.1371/journal.pcbi.1004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong D., Ji B., Dai L. Stability of adhesion clusters and cell reorientation under lateral cyclic tension. Biophys. J. 2008;95:4034–4044. doi: 10.1529/biophysj.108.131342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong Y., Kong D., Ji B. Frequency-dependent focal adhesion instability and cell reorientation under cyclic substrate stretching. Cell. Mol. Bioeng. 2011;4:442–456. [Google Scholar]
- 50.Califano J.P., Reinhart-King C.A. A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell. Mol. Bioeng. 2008;1:122–132. [Google Scholar]
- 51.Bresenham J.E. Algorithm for computer control of a digital plotter. IBM Syst. J. 1965;4:25–30. [Google Scholar]
- 52.Serra J. Academic Press; Orlando, FL: 1983. Image Analysis and Mathematical Morphology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.