Abstract
Mechanical loading is the primary functional determinant of bone mass and architecture, and osteocytes play a key role in translating mechanical signals into (re)modelling responses. Although the precise mechanisms remain unclear, Wnt signalling pathway components, and the anti-osteogenic canonical Wnt inhibitor Sost/sclerostin in particular, play an important role in regulating bone's adaptive response to loading. Increases in loading-engendered strains down-regulate osteocyte sclerostin expression, whereas reduced strains, as in disuse, are associated with increased sclerostin production and bone loss. However, while sclerostin up-regulation appears to be necessary for the loss of bone with disuse, the role of sclerostin in the osteogenic response to loading is more complex. While mice unable to down-regulate sclerostin do not gain bone with loading, Sost knockout mice have an enhanced osteogenic response to loading. The molecular mechanisms by which osteocytes sense and transduce loading-related stimuli into changes in sclerostin expression remain unclear but include several, potentially interlinked, signalling cascades involving periostin/integrin, prostaglandin, estrogen receptor, calcium/NO and Igf signalling. Deciphering the mechanisms by which changes in the mechanical environment regulate sclerostin production may lead to the development of therapeutic strategies that can reverse the skeletal structural deterioration characteristic of disuse and age-related osteoporosis and enhance bones' functional adaptation to loading. By enhancing the osteogenic potential of the context in which individual therapies such as sclerostin antibodies act it may become possible to both prevent and reverse the age-related skeletal structural deterioration characteristic of osteoporosis.
Graphical abstract
Highlights
-
•
Loading-related changes in osteocyte sclerostin expression spatially predict subsequent osteogenic responses.
-
•
Acute sclerostin down-regulation is not sufficient for maximal osteogenic responses to loading.
-
•
Inability to suppress sclerostin precludes bone gain following loading. Lack of sclerostin prevents bone loss in disuse.
-
•
Sclerostin influences the osteogenic context in which loading acts as its deletion enhances functional adaptation.
1. Introduction
Mechanical loading is the primary functional determinant of bone mass and architecture [1], [2]. Loading generates strain (percentage change in dimension) and other mechanically relevant stimuli (e.g. fluid flow shear stress) throughout the bone tissue and within the osteocyte canalicular network. Loading levels or distributions which engender strains beyond a habitual minimum effective strain (MES) trigger bone formation resulting in increased bone mass, improved bone architecture and thus re-establishment of habitual levels and distribution of strain [3], [4], [5]. Decreased loading, such as occurs during disuse, results in osteoclastic bone resorption and bone loss in an apparent attempt to also re-establish habitual levels and distribution of strain. This homeostatic feedback loop, described by Harold Frost as ‘the mechanostat’ [4], involves the site-specific co-ordinated (re)modelling activity of osteocytes, osteoblasts and osteoclasts [5].
Osteocytes are embedded in the mineralised matrix and were long thought to have little or no function, but are now known to play a particularly important role in coordinating local bone remodelling responses and have recently described as ‘master-regulators’ [6], [7], [8]. The Wnt antagonist Sost/sclerostin is almost exclusively expressed by osteocytes in the adult skeleton [9], and osteocytes are also an important source of receptor activator of nuclear factor κB ligand (Rankl) [10], which probably plays a key role in initiating repair in damaged bone; e.g. apoptosing osteocytes around microcracks secrete Rankl [11]. Canonical Wnt signalling in osteocytes also regulates bone resorption via the expression of osteoprotogerin (Opg); mice lacking β-catenin in osteocytes have dramatically reduced bone mass due to reduced Opg levels [12].
Given their location and morphology, with long interlinked dendritic processes forming a functional syncytium extending to the bone surfaces, osteocytes are ideally suited to sense load-associated strains, including shear strains across their membranes as fluid is displaced through their canalicular system. Osteocytes are now considered to be the primary mechanosensors which locally coordinate adaptive (re)modelling responses [13]. The experiment by Skerry et al. [2] that led us to acceptance of this hypothesis was the demonstration of rapid strain magnitude-related increases in the activity of the metabolism enzyme glucose-6-phosphate dehydrogenase (G6PD) in osteocytes in the turkey ulna following a short period of loading.
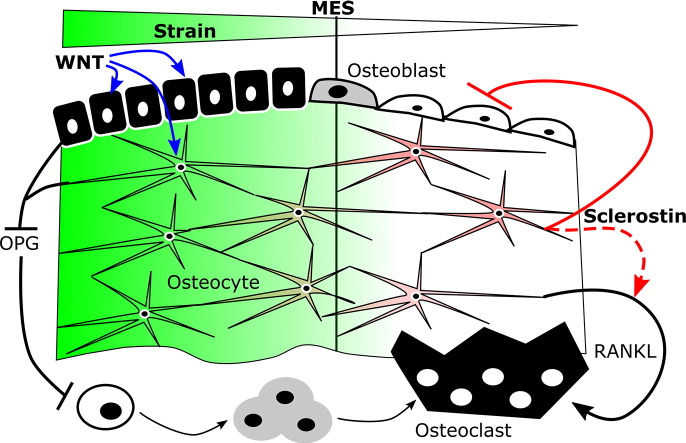
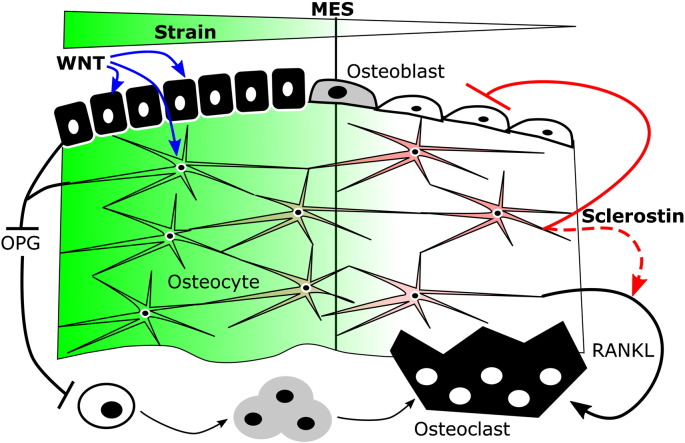
For many years after this, the mechanisms underlying the coordination of adaptive remodelling responses by osteocytes were largely unknown. Hypothesised mechanisms included direct cell-cell communication [14], [15] and/or the secretion of paracrine mediators such as prostaglandins (PG) or insulin-like growth factors (Igf). However, once sclerostin had been shown to be expressed in osteocytes [9], Robling et al. [16] convincingly demonstrated that one potentially important mechanism by which mechanical loading controls osteocyte activity is by regulating sclerostin expression. His demonstration that loading the mouse ulna down regulates sclerostin expression has been reproduced in a variety of experimental loading models [17], [18], [19], [20], [21], [22], [23] (Fig. 1). It then led to proposal of the simple model that local, loading-related down-regulation of osteocyte sclerostin increases bone formation by relieving inhibition of canonical Wnt signalling in osteoblasts while also, directly or indirectly through regulation of OPG, suppressing the resorptive activity of osteoclasts (Fig. 2). The responses of transgenic mice with altered sclerostin expression to changes in loading strongly support the validity of this model. However, recent findings of sclerostin-independent changes in bone formation following loading [24] have demonstrated that this model is somewhat over-simplified. Furthermore, the mechanisms by which loading-related stimuli initiate this process by down-regulating sclerostin have only been partially explored.
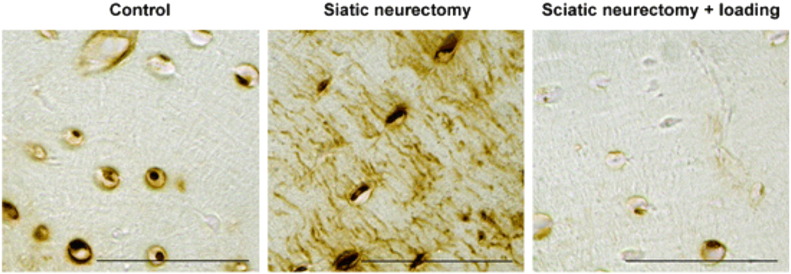
Fig. 1.
Mechanical loading decreases, whereas disuse increases sclerostin expression. Sclerostin immunolocalisation in tibial cortical bone osteocytes of control limbs subjected to normal cage activity, limbs subjected to disuse through sciatic neurectomy, and disused limbs subjected to exogenous osteogenic axial loading. Figure reproduced with permission from Moustafa et al. [19]. Scale bar = 50 μm.
Fig. 2.
Simplified model describing sclerostin's roles in bones' adaptation to loading-engendered strains. Strains greater than the minimum effective strain (MES, green) are association with low osteoclast activity and increased osteoblast activity, whereas the low strains experienced in disuse are associated with reduced osteoblast activity and increased osteoclast activity. The activity of these effector cells is coordinated by osteocytes at least in part through sclerostin (red) secretion. In low strain conditions, sclerostin inhibits osteoblast function and may indirectly promote resorption through Rankl [73]. Strains greater than the MES down-regulate sclerostin, allowing activation of osteoblasts at least in part through canonical Wnt signalling, which may indirectly inhibit resorption through Opg expression.
2. Loading-related changes in bone mass reflect sclerostin regulation
The model presented in Fig. 2 is largely based on the demonstration that the cross-sectional distribution of strains engendered within loading-responsive regions of mouse long bones spatially parallel the acute down-regulation of sclerostin protein (within 24 h following an episode of loading [18]) and subsequent increases in bone formation. As described above, this was first demonstrated in the mouse ulna subjected to non-invasive axial loading [16]. Axial loading of the mouse ulna generates different magnitudes of mechanical strain in the bone's proximal, middle and distal regions as well as in different cross-sectional sectors at the same longitudinal site. Strain magnitudes were found to correlate with both the increase in bone formation and the down-regulation of sclerostin within these regions. Conversely, the reduction in strain experienced through tail suspension-induced disuse increased Sost RNA expression in the mouse tibia. However, protein level analysis of sclerostin expression by immunohistochemistry following tail suspension did not detect changes in the proportion of osteocytes stained positive for sclerostin around the level of the tibia/fibula junction [16].
The lack of change in sclerostin expression around the mouse tibia/fibula junction during tail suspension is potentially consistent with the finding that this region appears to be the least affected by disuse, with the most significant bone loss occurring proximal and distal to this region [25]. In a later study, Moustafa et al. [19] mapped site-specific changes in sclerostin expression in the mouse tibia using immunohistochemistry following unilateral axial loading. In cross-sections from the highly load-responsive proximal tibia, the increase in bone formation and decrease in osteocyte sclerostin expression correlated with the mechanical strains predicted by finite element model analysis. In contrast, in the distal tibia below the tibia/fibula junction, sclerostin was not down-regulated and bone formation did not increase following loading. In the same study, disuse following sciatic neurectomy increased sclerostin expression in both the proximal and distal tibia, and additional loading after disuse significantly reduced sclerostin expression in both sites, although the magnitude of the effect was greater proximally. Similar site specificity was also observed in the trabecular compartment of the proximal tibia: loading reduced sclerostin expression and increased bone gain in the secondary spongiosa, but in the primary spongiosa no bone formation was observed nor any associated down regulation of sclerostin expression. These detailed analyses demonstrate that the spatial distribution of bone loss with disuse and of bone formation following loading closely follow the early changes in sclerostin expression. However, none of the studies published to date correlating changes in sclerostin expression with the spatial distribution of bone formation flowing loading have shown that the two are causally related. The relationship between sclerostin regulation and bone (re)modelling is clearly complex as both continuous (catabolic) and intermittent (anabolic) parathyroid hormone (PTH) treatments down-regulate Sost despite having opposite effects on bone mass [26], [27], [28].
Evidence that the spatial correlation between loading-related sclerostin regulation and changes in bone (re)modelling may be causal is provided by loading studies using different genetically modified mouse models. Sclerostin knockout mice do not show bone loss in response to disuse induced by hind limb unloading [29] or botulinum toxin injection [24], suggesting that sclerostin up-regulation is necessary for disuse-induced bone loss. To determine whether sclerostin down-regulation following increased loading is necessary for subsequent bone formation, transgenic mice harbouring the human SOST gene driven by an 8 Kb Dmp1 promoter (SostTg) were generated [20]. Ulna axial loading down-regulates endogenous, but not human, Sost expression in these mice. Further supporting evidence that sclerostin down-regulation is required for loading-induced bone formation, was the observation that loading induced significantly greater bone formation in wild type than SostTg mice. These independent studies specifically test the roles of sclerostin in bone's adaptation to loading and as such provide strong evidence that both loading-related bone gain and disuse-associated bone loss require changes in sclerostin expression, at least in young mice.
Evidence supporting the potential importance of Sost down-regulation in bones' osteogenic response to loading also comes from studies utilising mice with genetic modifications in mechano-responsive pathways which result in altered Sost regulation following loading. For example, increased basal sclerostin expression, abrogation of sclerostin down-regulation with loading and reduced load-related bone formation is observed in periostin knockout (Postn−/−) mice [22]. Similarly, four point tibial bending of mice lacking osteocytic Igf1 expression does not result in Sost down-regulation and triggers a diminished osteogenic response to loading compared with wild type controls [23]. In contrast, deletion of the androgen receptor in male androgen receptor (AR) knockout mice is associated with greater sclerostin down-regulation and enhanced bone formation following loading compared with wild type controls [21]. Taken together, these studies provide examples of situations in which changes in sclerostin regulation are associated with altered adaptive responses to loading.
3. Mechanisms underlying sclerostin down-regulation by loading
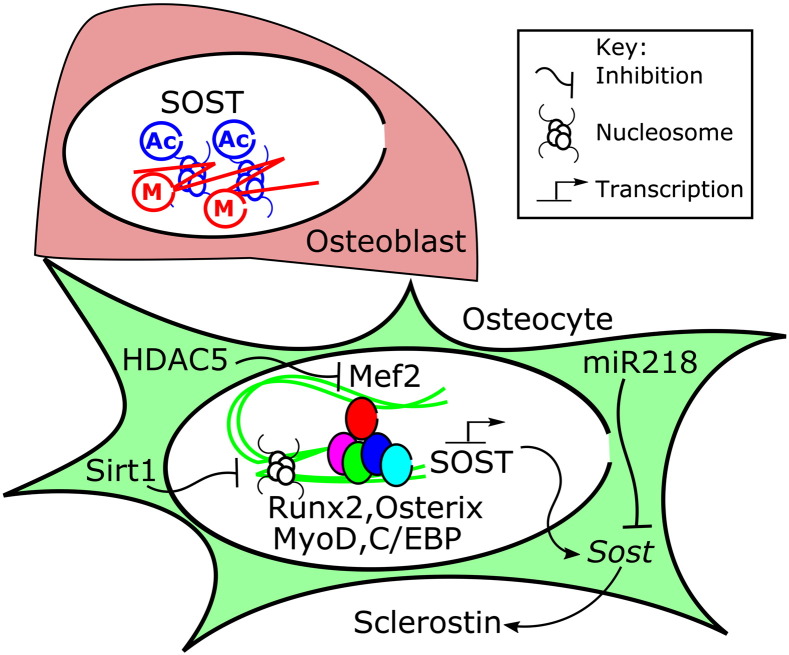
The above in vivo studies describing altered basal sclerostin expression and changes in the load-related regulation of sclerostin in genetically modified mice, while informative, provide limited insight into the molecular mechanisms by which osteocytes regulate sclerostin expression. Instead in vitro studies using a variety of model systems have been required to address this. These studies have shown that the basal rate of sclerostin expression is under both transcriptional and broader epigenetic control (Fig. 3). Its restricted expression in osteocytes is achieved through an epigenetic mechanism; the SOST promoter is DNA methylated in osteoblasts but becomes demethylated during the osteoblast to osteocyte transition, allowing initiation of gene expression [30]. Transcription factors known to bind elements in the demethylated SOST promoter include the bone-specific transcription factors Runx2 and Osterix [31], [32]. Bone non-specific transcription factors such as MyoD and C/EBP also bind the SOST promoter in human Saos-2 cells [31]. The ability of these various factors to regulate Sost expression is epigenetically determined by histone deacetylase (HDAC) enzymes such as Sirt1 and HDAC5 [33], [34], and once expressed Sost RNA stability is influenced by micro-RNAs such as miR-218 [35].
Fig. 3.
Simplified schematic representation of transcriptional and epigenetic regulators of basal Sost expression. In osteoblasts, the SOST gene is epigenetically repressed through DNA methylation (M) and potentially histone acetylation (Ac). HDACs also fine tune SOST promoter and Mef2-dependant enhancer activity in cells which express Sost. Transcriptional regulators able to bind the SOST promoter include osteoblast-specific (Runx2, osterix) and non-bone-specific (MyoD, C/EBP) transcription factors. Once expressed, Sost RNA stability is influenced by micro-RNAs including miR218.
SOST promoter activity is enhanced by Mef2 binding to a distal enhancer element and inhibition of this binding is one of the mechanisms by which Sost is down-regulated by PTH [34], [36], [37]. Similar mechanistic studies into Sost regulation by strain have been hindered by the limited availability of cellular models. Primary osteoblasts do not express readily detectable levels of Sost until they form mineralised matrix, which precludes their use for in vitro strain studies. Mouse osteocytic MLO cell lines do not reliably produce readily detectable levels of Sost [38] and their expression of the constitutively active SV40 antigen [39] impacts PI3K/AKT signalling, which is a stain-responsive pathway [40]. The more recently developed IDG-SW3 cell line promises to circumvent this limitation, but these cells only express Sost after prolonged periods of differentiation [41]. Not surprisingly few osteoblastic cell lines express detectable Sost. However, rat UMR-106 osteosarcoma cells do respond to strain [40] and express very high levels of Sost in a manner akin to them having a constitutively active gene [38], but this makes the physiological relevance of this model questionable. In contrast, human Saos-2 osteosarcoma cells are also mechanoresponsive, but only confluent cultures express readily detectable Sost RNA and sclerostin protein [41], [42], [43] which is why we have used this model system. Subjecting subconfluent cultures of Saos-2 cells to in vitro strain by four point bending increases their proliferation [43], [44], whereas confluent cultures up-regulate osteocalcin and down-regulate Sost over a time course which parallels that seen in rodent bones following in vivo mechanical loading [43], [45].
Using the Saos-2 model we initially reported that Sost down-regulation by strain involves Cox2-initiated PGE2 signalling through an EP4/ERK pathway [45], consistent with a previous report that selective treatment with an EP4 agonist enhances the osteogenic responses to mechanical loading in vivo [46]. The importance of this pathway in the mechanical regulation of Sost expression is further demonstrated by the recent report that Cox inhibition with carprofen prevents sclerostin down-regulation in the ulnae of mice subjected to axial loading [47]. Cox2 upregulation in mechanically-stimulated osteoblastic cells is abrogated by inhibition of nitric oxide (NO)/protein kinase G (PKG) signalling down-stream of calcium signalling [48]. Inhibition of the NO synthase (Nos) enzyme also abrogates fluid shear-induced Sost down-regulation in osteoblastic cells [49], whereas long bone derived osteoblastic cells from AR knockout mice, which show enhanced sclerostin down-regulation in vivo, produced higher levels of NO when subjected to fluid shear in vitro [21].
AR, NO and PGE2 signalling pathways are all influenced by estrogen receptors (ERs), which also interact with canonical Wnt pathway components in mechanically strained osteoblastic cells [50], [51]. Our group and others have shown that the ERs, particularly ERα, are mediators of bone's adaption to loading (as reviewed in [50]). Global deletion of ERα greatly diminishes cortical osteogenic responses to loading [52] thus we were surprised to observe that blockade of ERα does not prevent Sost down-regulation by strain in Saos-2 cells, rather ERα inhibition in vitro or global deletion in vivo reduces basal Sost levels [43]. However, this observation is consistent with the subsequent demonstration that deletion of ERα in mature osteoblasts and osteocytes does not impair the adaptive response to axial tibial loading in female mice [53], [54]. In contrast, ERβ blockade does not alter basal Sost levels, but prevents strain-induced Sost down-regulation in Saos-2 cells [43]. Although the role of ERβ in bone's adaptation to loading has not been extensively investigated, it is worth noting that ERβ enhances Cox2 up-regulation [55] and ERK activation [56] following mechanical stimulation in different in vitro models.
Both these roles of ERβ are consistent with a down-stream Cox-2/PGE2/ERK pathway mediating Sost down-regulation following strain, although ERβ may also act down-stream of PGE2 signalling as PGE2 treatment increases estrogen response element activation in osteoblastic cells [57]. Interestingly ERβ knockdown prevents periostin up-regulation by estradiol in periodontal ligament cells [58] and given periostin knockout mice do not show significant sclerostin down-regulation [22], ERβ's role in sclerostin regulation may be through periostin as well as through ERK activation. Activation of ERK could be either up-stream of periostin action and/or down-stream of its binding to integrin receptors, including integrin αV [3], [59], [60] and deletion of integrin αV in the osteoblast lineage prevents Sost down-regulation in the ulnae of mice subjected to axial loading [61]. Integrin αV directly interacts with and facilitates Igf1/Igf1R signalling [62], [63], which is potentially consistent with the report that osteocyte Igf1 deletion also abrogates loading-induced Sost down-regulation [23]. Intriguingly, integrin αV also facilitates opening of connexin (Cx)43 hemichannels and Cx43 facilitates the release of PGE2, which is involved in the rapid activation of β-catenin in osteoblastic cells subjected to mechanical stimulation in vitro [64], [65]. However, integrin αV expression is not required for ERK activation in calvarial osteoblastic cells subjected to fluid shear [61].
To date, no in vivo studies have been published that have systematically investigated the roles of different mechano-responsive signalling pathways in sclerostin regulation following loading. The majority of available studies are based on in vitro observations in osteoblastic cell lines subjected to defined mechanical stimuli which cannot fully replicate the effects of in vivo loading on the heterogeneous cell populations residing in and on bone. Currently, only Cox2/prostaglandin signalling has been demonstrated to acutely regulate sclerostin expression in vitro, suggesting a direct effect, and to also facilitate sclerostin down-regulation following loading in vivo. Furthermore, the mechanisms by which unloading results in sclerostin up-regulation have not been investigated and cannot be assumed to be the same as those which result in its down-regulation following increased loading. Nonetheless, putting the available jigsaw pieces together it is possible to propose a linear pathway which links early strain-related signalling events to ultimate down-regulation of Sost expression (Fig. 4). The sequence of events proposed in Fig. 4 is potentially consistent with the timing of gene expression changes seen following loading; Cox2 is up-regulated within 1–2 h [66] followed by Postn up-regulation around 6 h [22] and eventually Sost down-regulation 8–24 h after loading [47], [67]. However, the direct mechanisms by which loading-related stimuli decrease Sost promoter activity and/or reduce Sost RNA stability remain unknown and merit further study. The proposed model is also limited in assuming that all of the reported mediators of Sost down-regulation are involved in osteocytes' acute and immediate responses to strain. Bones' ability to respond to acute changes in loading is context dependent and multiple factors, local and systemic, are likely to influence the way Sost expression is regulated by loading; e.g. in a bone which has adapted its mass and architecture to the customary loads placed upon it, osteocytes and/or adjacent osteoblasts are likely to express factors which may limit or enhance strain-related Sost down-regulation.
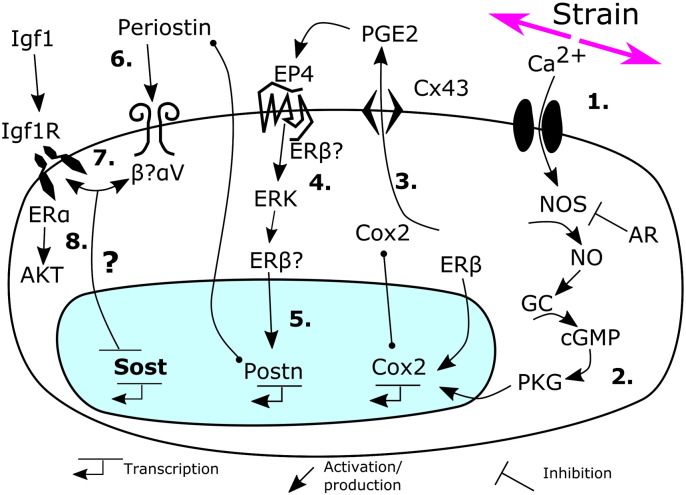
Fig. 4.
Schematic representation of pathways implicated in sclerostin down-regulation by mechanical stimulation. (1) Strain rapidly activates Ca signalling and down-stream (2) NO/guanylate cyclase (GC)/PKG signalling leading to up-regulation of Cox2, which also involves ERβ. Cox2 produces PGE2 (3) which is released at least in part through Cx43 hemichannels to activate EP receptors including EP4. EP4 activates ERK (4) signalling. Strain-induced ERK activation also involves ERβ and ER transcriptional activity is in turn increased in osteoblastic cells by PGE2. Activated ERβ can up-regulate periostin (Postn) expression (5). Periostin acts through integrins (6) including integrin αV, which interacts with the Igf1 receptor (7). Responses down-stream of Igf1R include ERα-mediated activation of AKT (8), however, the mechanisms by which the signalling cascades described inhibit Sost expression following exposure to strain remain unknown.
4. Sclerostin itself influences the osteogenic context in which loading acts
Sclerostin itself is one such modulator of the osteogenic context; e.g. in vitro, its presence inhibits recruitment of Saos-2 cells to the cell cycle following mechanical strain or Wnt3a treatment, but not following treatment with estradiol [43], [44]. Sclerostin has also been shown to reduce proliferation and increase apoptosis in the absence of mechanical stimulation in other models [68], [69]. In addition, sclerostin has the potential to influence multiple signalling pathways that regulate various stages of the osteoblast lineage. Reported effects of sclerostin treatment on osteoblastic cells in vitro include inhibition of differentiation [70], [71], [72], inhibition of mineralisation [71], induction of RANKL expression [73], and promotion of osteocytic osteolysis [74]. Short term treatment of osteoblastic cells with recombinant sclerostin alters (predominantly down-regulates) the expression of a large number of genes, many of which are components of the Wnt signalling pathway [75]. This is consistent with sclerostin acting primarily as a canonical Wnt signalling inhibitor, although potential interactions with BMP and platelet derived growth factor (PDGF) cascades have also been reported [9], [72]. In the context of bone's response to loading, transgenic mice deficient for canonical Wnt co-receptors or the intra-cellular secondary signalling molecule β-catenin show diminished responses to mechanical loading [47], [76], [77]. β-Catenin is rapidly activated in osteocytes subjected to mechanical loading, but this response is diminished in osteocytes of mice unable to down-regulate sclerostin [20]. Taken together, these studies provide strong evidence that sclerostin acts as a canonical Wnt pathway inhibitor and that its down-regulation facilitates activation of this pathway following loading, but whether sclerostin directly or indirectly modulates other pathways following loading remains unknown.
5. Sclerostin down-regulation is not sufficient for load-related osteogenesis
The findings discussed thus far suggest that altered sclerostin expression is a critical osteocyte response to changes in mechanical loading and that sclerostin regulation permits/facilitates both adaptive osteogenesis when loads are increased and net resorption when they are decreased as in disuse. However, while it is clear that osteocytes, and sclerostin, are important for mediating bone's adaptive responses, it is wrong to assume that bone's responses to disuse and loading are regulated by the same mechanisms. This was suggested several years ago by a microarray study which showed that the genes and pathways regulated by loading are not all the same as those regulated by disuse [67]. Putting it another way; just because a cell or signalling pathway plays a critical role in the context of disuse, it does not mean that it will also be as important in regulating the bone formation response following loading. This is illustrated in an experiment which targeted ablation of osteocytes using diphtheria toxin [78]. Osteocyte ablated mice do not lose bone during unloading induced by tail suspension, however, osteocyte ablation does not prevent bone restoration caused by return to normal activity following a period of disuse. This suggests that either tail suspension induces bone loss through mechanisms unrelated to loading, such as increased glucocorticoid production [79], or that the responses of other cells to changes in loading are sufficient for normal bone gain following loading in the absence of osteocytes (and therefore sclerostin).
This latter interpretation is consistent with the recent report that Sost knockout mice do not lose bone due to unloading, but still show osteogenic responses to increased loading [24]. In fact, when loaded so as to generate equivalent strains, Sost−/− mice show greater bone formation than wild-type controls. Thus, while viable osteocytes able to up-regulate sclerostin expression appear to be an absolute requirement for bone loss in disuse, down-regulation of sclerostin following loading does not appear to be so critical for the subsequent osteogenic response. That osteocytes are not the only cell involved in the adaptive response to loading should not come as a surprise given that numerous studies have shown that osteoblast-like cells are also mechano-sensitive. Well-established responses of osteoblast-like cells to strain include enhanced osteoblastic differentiation of marrow stromal cells (MSCs) as well as resumption of proliferation of cortical long bone derived osteoblastic cells [44], [52], [80], [81], [82]. Furthermore, the study which, to the authors' knowledge, was the first to demonstrate that osteocytes respond rapidly to changes in mechanical loading showed equally rapid responses (within 6 min) in adjacent periosteal cells [2].
The ability of osteoblasts to sense and respond to strain in vitro is clearly demonstrated by their ability to very rapidly enter into the cell cycle after strain exposure in the absence of sclerostin [18], [43], [44], [52]. In vivo, an increase in the number of osteoblasts on the periosteal surface is seen within 24 h following loading [18], although the location and nature of the proliferative osteoblast population remains undefined. A recent study on the effect of age on the loading response provides further evidence that down-regulation of sclerostin in osteocytes does not necessarily translate into an appropriate bone formation response. We hypothesised that in old mice loading would not down regulate sclerostin, but instead found that loading down-regulated sclerostin in 19-month-old mice to the same extent as in young (17-week-old) mice [18], even though the osteogenic response to non-invasive axial tibial loading was lower in old than in young animals. Interestingly this study showed that in old mice it was the ability of osteoblasts to proliferate that was compromised; osteoblast progression through the cell cycle following strain exposure in vitro and the increase in the number of periosteal osteoblasts following loading in vivo were impaired. These deficiencies in osteoblast function that occur with age may not only limit bone's adaptive responses to loading but also the beneficial effect of sclerostin neutralising therapies [83].
The finding that osteocytes in tibiae of old mice remain able to sense changes in mechanical loading and acutely respond by down-regulating Sost has recently been independently replicated by Holguin et al. [84]. In the Holguin study, a single bout of axial tibial loading effectively down-regulated Sost in 5-month-old as well as 12-month-old and 22-month-old mice, although the bone formation response was blunted with age. A possible explanation is that Sost RNA down-regulation is more transient in bones from 22-month-old than 5-month-old mice and others have shown changes in Wnt pathway-related gene transcripts and blunting of β-catenin activity in the old [84], [85], [86]. Intriguingly, Holguin et al. found that while repeated bouts of loading on subsequent days repeatedly down-regulate Sost in young mice, only the first bout of loading results in Sost down-regulation in the old. This suggests that old bone cells become refractory to repeated bouts of increased loading. However, we have recently reported that prior and concurrent disuse enhances the osteogenic response to repeated bouts of axial tibial loading in aged mice [87]. Whether this “rescue” of bone's response to loading in old mice is associated with the restoration of cells' ability to down-regulate sclerostin after each bout of loading needs to be determined. Nonetheless these studies demonstrate bone's “strain memory” influences subsequent responsiveness and that this relationship becomes less effective in the elderly. The relevance of these findings from rodent studies to elderly humans remains to be established.
6. Conclusions
Numerous studies have demonstrated that sclerostin plays a role in the effective working of the mechanisms associated with regulation of bone mass and architecture in relation to mechanical loading (the mechanostat). Sclerostin expression increases following unloading with the consequent inhibition of Wnt signalling and associated bone loss. Down-regulation of sclerostin is permissive for osteogenesis in response to loading, at least in part by relieving inhibition of canonical Wnt signalling. This is consistent with the potently osteogenic responses observed in humans treated with sclerostin-inhibiting antibodies now in advanced stages of clinical development [88]. However, sclerostin down-regulation in osteocytes is not the only process linking cellular mechanically related responses to functional remodelling as evidenced by mice lacking Sost having an enhanced response to loading. This is consistent with the emerging narrative that there is not a single linear pathway regulating bone's adaptive responses to loading, rather multiple pathways in which osteoblasts as well as osteocytes play important roles [50], [89], [90]. Elucidating the complex cellular mechanisms involved in mechano-responsiveness remains important because it could lead to the development of ‘smart’ novel therapeutic targets able to augment bones' specific physiological adaptive responses to loading-engendered stimuli rather than relying on non-specific, and largely ineffective, therapies to prevent or reverse loss of bone mass.
Acknowledgements
All authors declare they have no conflicts of interest. GLG is supported by a Postdoctoral Training Fellowship for Clinicians from the Wellcome Trust [107474/Z/15/Z].
References
- 1.Rubin C.T., Lanyon L.E. Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J. Orthop. Res. 1987;5(2):300–310. doi: 10.1002/jor.1100050217. [DOI] [PubMed] [Google Scholar]
- 2.Skerry T.M. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J. Bone Miner. Res. 1989;4(5):783–788. doi: 10.1002/jbmr.5650040519. [DOI] [PubMed] [Google Scholar]
- 3.Sugiyama A. Periostin promotes hepatic fibrosis in mice by modulating hepatic stellate cell activation via alpha integrin interaction. J. Gastroenterol. 2016 doi: 10.1007/s00535-016-1206-0. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 4.Frost H.M. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2(2):73–85. [PubMed] [Google Scholar]
- 5.Skerry T.M. One mechanostat or many? Modifications of the site-specific response of bone to mechanical loading by nature and nurture. J. Musculoskelet. Neuronal Interact. 2006;6(2):122–127. [PubMed] [Google Scholar]
- 6.Galli C., Passeri G., Macaluso G.M. Osteocytes and WNT: the mechanical control of bone formation. J. Dent. Res. 2010;89(4):331–343. doi: 10.1177/0022034510363963. [DOI] [PubMed] [Google Scholar]
- 7.Schaffler M.B. Osteocytes: master orchestrators of bone. Calcif. Tissue Int. 2014;94(1):5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q. Ex vivo 3D osteocyte network construction with primary murine bone cells. Bone Res. 2015;3:15026. doi: 10.1038/boneres.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler D.G. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakashima T. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17(10):1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy O.D. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012;50(5):1115–1122. doi: 10.1016/j.bone.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer I. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 2010;30(12):3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonewald L.F., Johnson M.L. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palazzini S. Stromal cell structure and relationships in perimedullary spaces of chick embryo shaft bones. Anat. Embryol. (Berl.) 1998;197(5):349–357. doi: 10.1007/s004290050145. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara Y. Ex vivo real-time observation of Ca(2 +) signaling in living bone in response to shear stress applied on the bone surface. Bone. 2013;53(1):204–215. doi: 10.1016/j.bone.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Robling A.G. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008;283(9):5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 17.Sinnesael M. Androgens inhibit the osteogenic response to mechanical loading in adult male mice. Endocrinology. 2015;156(4):1343–1353. doi: 10.1210/en.2014-1673. [DOI] [PubMed] [Google Scholar]
- 18.Meakin L.B. Age-related impairment of bones’ adaptive response to loading in mice is associated with sex-related deficiencies in osteoblasts but no change in osteocytes. J. Bone Miner. Res. 2014;29(8):1859–1871. doi: 10.1002/jbmr.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moustafa A. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos. Int. 2012;23(4):1225–1234. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tu X. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50(1):209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callewaert F. Androgen receptor disruption increases the osteogenic response to mechanical loading in male mice. J. Bone Miner. Res. 2010;25(1):124–131. doi: 10.1359/jbmr.091001. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet N. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J. Biol. Chem. 2009;284(51):35939–35950. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau K.H. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am. J. Physiol. Endocrinol. Metab. 2013;305(2):E271–E281. doi: 10.1152/ajpendo.00092.2013. [DOI] [PubMed] [Google Scholar]
- 24.Morse A. Mechanical load increases in bone formation via a sclerostin-independent pathway. J. Bone Miner. Res. 2014;29(11):2456–2467. doi: 10.1002/jbmr.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galea G.L. Quantification of alterations in cortical bone geometry using site specificity software in mouse models of aging and the responses to ovariectomy and altered loading. Front Endocrinol. (Lausanne) 2015;6:52. doi: 10.3389/fendo.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellido T. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 27.Keller H., Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37(2):148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Jilka R.L. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. J. Bone Miner. Res. 2010;25(11):2427–2437. doi: 10.1002/jbmr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin C. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J. Bone Miner. Res. 2009;24(10):1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 30.Delgado-Calle J. DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J. Bone Miner. Res. 2012;27(4):926–937. doi: 10.1002/jbmr.1491. [DOI] [PubMed] [Google Scholar]
- 31.Sevetson B., Taylor S., Pan Y. Cbfa1/RUNX2 directs specific expression of the sclerosteosis gene (SOST) J. Biol. Chem. 2004;279(14):13849–13858. doi: 10.1074/jbc.M306249200. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Campo F.M. Osterix and RUNX2 are transcriptional regulators of sclerostin in human bone. Calcif. Tissue Int. 2016;99(3):302–309. doi: 10.1007/s00223-016-0144-4. [DOI] [PubMed] [Google Scholar]
- 33.Cohen-Kfir E. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152(12):4514–4524. doi: 10.1210/en.2011-1128. [DOI] [PubMed] [Google Scholar]
- 34.Baertschi S. Class I and IIa histone deacetylases have opposite effects on sclerostin gene regulation. J. Biol. Chem. 2014;289(36):24995–25009. doi: 10.1074/jbc.M114.564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan M.Q. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 2012;287(50):42084–42092. doi: 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leupin O. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J. Bone Miner. Res. 2007;22(12):1957–1967. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wein M.N. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J. Bone Miner. Res. 2015;30(3):400–411. doi: 10.1002/jbmr.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu L. Sclerostin expression is induced by BMPs in human Saos-2 osteosarcoma cells but not via direct effects on the sclerostin gene promoter or ECR5 element. Bone. 2011;49(6):1131–1140. doi: 10.1016/j.bone.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Kato Y. Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 1997;12(12):2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 40.Sunters A. Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to Ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J. Biol. Chem. 2010;285(12):8743–8758. doi: 10.1074/jbc.M109.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo S.M. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J. Bone Miner. Res. 2011;26(11):2634–2646. doi: 10.1002/jbmr.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Campo F.M. A Sclerostin super-producer cell line derived from the human cell line SaOS-2: a new tool for the study of the molecular mechanisms driving Sclerostin expression. Calcif. Tissue Int. 2014;95(2):194–199. doi: 10.1007/s00223-014-9880-5. [DOI] [PubMed] [Google Scholar]
- 43.Galea G.L. Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J. Biol. Chem. 2013;288(13):9035–9048. doi: 10.1074/jbc.M112.405456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galea G.L. Planar cell polarity aligns osteoblast division in response to substrate strain. J. Bone Miner. Res. 2015;30(3):423–435. doi: 10.1002/jbmr.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galea G.L. Sost down-regulation by mechanical strain in human osteoblastic cells involves PGE2 signaling via EP4. FEBS Lett. 2011;585(15):2450–2454. doi: 10.1016/j.febslet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagino H. Effect of a selective agonist for prostaglandin E receptor subtype EP4 (ONO-4819) on the cortical bone response to mechanical loading. Bone. 2005;36(3):444–453. doi: 10.1016/j.bone.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Lara-Castillo N. In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone. 2015;76:58–66. doi: 10.1016/j.bone.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rangaswami H. Protein kinase G and focal adhesion kinase converge on Src/Akt/beta-catenin signaling module in osteoblast mechanotransduction. J. Biol. Chem. 2012;287(25):21509–21519. doi: 10.1074/jbc.M112.347245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delgado-Calle J., Riancho J.A., Klein-Nulend J. Nitric oxide is involved in the down-regulation of SOST expression induced by mechanical loading. Calcif. Tissue Int. 2014;94(4):414–422. doi: 10.1007/s00223-013-9821-8. [DOI] [PubMed] [Google Scholar]
- 50.Galea G.L., Price J.S., Lanyon L.E. Estrogen receptors’ roles in the control of mechanically adaptive bone (re)modeling. BoneKEy Rep. 2013;2:413. doi: 10.1038/bonekey.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armstrong V.J. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J. Biol. Chem. 2007;282(28):20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 52.Lee K. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424(6947):389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 53.Windahl S.H. Estrogen receptor-alpha in osteocytes is important for trabecular bone formation in male mice. Proc. Natl. Acad. Sci. U. S. A. 2013;110(6):2294–2299. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melville K.M. Effects of deletion of ERalpha in osteoblast-lineage cells on bone mass and adaptation to mechanical loading differ in female and male mice. J. Bone Miner. Res. 2015;30(8):1468–1480. doi: 10.1002/jbmr.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castillo A.B. Estrogen receptor-beta regulates mechanical signaling in primary osteoblasts. Am. J. Physiol. Endocrinol. Metab. 2014;306(8):E937–E944. doi: 10.1152/ajpendo.00458.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aguirre J.I. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J. Biol. Chem. 2007;282(35):25501–25508. doi: 10.1074/jbc.M702231200. [DOI] [PubMed] [Google Scholar]
- 57.Zaman G. Mechanical strain activates estrogen response elements in bone cells. Bone. 2000;27(2):233–239. doi: 10.1016/s8756-3282(00)00324-0. [DOI] [PubMed] [Google Scholar]
- 58.Mamalis A. Oestrogen regulates proliferation, osteoblastic differentiation, collagen synthesis and periostin gene expression in human periodontal ligament cells through oestrogen receptor beta. Arch. Oral Biol. 2011;56(5):446–455. doi: 10.1016/j.archoralbio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Wallace D.P. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am. J. Physiol. Ren. Physiol. 2008;295(5):F1463–F1471. doi: 10.1152/ajprenal.90266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li G. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208(2):358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaneko K. Integrin alphav in the mechanical response of osteoblast lineage cells. Biochem. Biophys. Res. Commun. 2014;447(2):352–357. doi: 10.1016/j.bbrc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujita M., Takada Y.K., Takada Y. Insulin-like growth factor (IGF) signaling requires alphavbeta3-IGF1-IGF type 1 receptor (IGF1R) ternary complex formation in anchorage independence, and the complex formation does not require IGF1R and Src activation. J. Biol. Chem. 2013;288(5):3059–3069. doi: 10.1074/jbc.M112.412536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saegusa J. The direct binding of insulin-like growth factor-1 (IGF-1) to integrin alphavbeta3 is involved in IGF-1 signaling. J. Biol. Chem. 2009;284(36):24106–24114. doi: 10.1074/jbc.M109.013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batra N. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc. Natl. Acad. Sci. U. S. A. 2012;109(9):3359–3364. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitase Y. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J. Bone Miner. Res. 2010;25(12):2657–2668. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grimston S.K. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaman G. Loading-related regulation of gene expression in bone in the contexts of estrogen deficiency, lack of estrogen receptor alpha and disuse. Bone. 2010;46(3):628–642. doi: 10.1016/j.bone.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutherland M.K. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35(4):828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 69.Bao X. The effect on proliferation and differentiation of cementoblast by using sclerostin as inhibitor. Int. J. Mol. Sci. 2013;14(10):21140–21152. doi: 10.3390/ijms141021140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winkler D.G. Sclerostin inhibition of Wnt-3a-induced C3H10T1/2 cell differentiation is indirect and mediated by bone morphogenetic proteins. J. Biol. Chem. 2005;280(4):2498–2502. doi: 10.1074/jbc.M400524200. [DOI] [PubMed] [Google Scholar]
- 71.Atkins G.J. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J. Bone Miner. Res. 2011;26(7):1425–1436. doi: 10.1002/jbmr.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thouverey C., Caverzasio J. Sclerostin inhibits osteoblast differentiation without affecting BMP2/SMAD1/5 or Wnt3a/beta-catenin signaling but through activation of platelet-derived growth factor receptor signaling in vitro. BoneKEy Rep. 2015;4:757. doi: 10.1038/bonekey.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wijenayaka A.R. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kogawa M. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J. Bone Miner. Res. 2013;28(12):2436–2448. doi: 10.1002/jbmr.2003. [DOI] [PubMed] [Google Scholar]
- 75.van Bezooijen R.L. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J. Bone Miner. Res. 2007;22(1):19–28. doi: 10.1359/jbmr.061002. [DOI] [PubMed] [Google Scholar]
- 76.Kang K.S., Robling A.G. New insights into Wnt-Lrp5/6-beta-catenin signaling in mechanotransduction. Front Endocrinol. (Lausanne) 2014;5:246. doi: 10.3389/fendo.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saxon L.K. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone. 2011;49(2):184–193. doi: 10.1016/j.bone.2011.03.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tatsumi S. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Ide S. Reduced emotional and corticosterone responses to stress in mu-opioid receptor knockout mice. Neuropharmacology. 2010;58(1):241–247. doi: 10.1016/j.neuropharm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ott C.E. Promiscuous and depolarization-induced immediate-early response genes are induced by mechanical strain of osteoblasts. J. Bone Miner. Res. 2009;24(7):1247–1262. doi: 10.1359/jbmr.090206. [DOI] [PubMed] [Google Scholar]
- 81.Kamel M.A. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone. 2010;47(5):872–881. doi: 10.1016/j.bone.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sen B. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells. 2011;29(11):1829–1836. doi: 10.1002/stem.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ominsky M.S. Differential temporal effects of sclerostin antibody and parathyroid hormone on cancellous and cortical bone and quantitative differences in effects on the osteoblast lineage in young intact rats. Bone. 2015;81:380–391. doi: 10.1016/j.bone.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 84.Holguin N., Brodt M.D., Silva M.J. Activation of Wnt signaling by mechanical loading is impaired in the bone of old mice. J. Bone Miner. Res. 2016 doi: 10.1002/jbmr.2900. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farr J.N. Effects of age and estrogen on skeletal gene expression in humans as assessed by RNA sequencing. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almeida M. Aging mechanisms in bone. BoneKEy Rep. 2012;1 doi: 10.1038/bonekey.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meakin L.B. Disuse rescues the age-impaired adaptive response to external loading in mice. Osteoporos. Int. 2015;26(11):2703–2708. doi: 10.1007/s00198-015-3142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Recker R.R. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J. Bone Miner. Res. 2015;30(2):216–224. doi: 10.1002/jbmr.2351. [DOI] [PubMed] [Google Scholar]
- 89.Thompson W.R., Rubin C.T., Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503(2):179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liedert A. Mechanobiology of bone tissue and bone cells. In: Kamkin A., Kiseleva I., editors. Mechanosensitivity in Cells and Tissues. 2005. (Moscow) [Google Scholar]