Abstract
Aims
Impaired Ca2 + cycling and myocyte contractility are a hallmark of heart failure triggered by pathological stress such as hemodynamic overload. The A-Kinase anchoring protein AKAP150 has been shown to coordinate key aspects of adrenergic regulation of Ca2+ cycling and excitation–contraction in cardiomyocytes. However, the role of the AKAP150 signalling complexes in the pathogenesis of heart failure has not been investigated.
Methods and results
Here we examined how AKAP150 signalling complexes impact Ca2+ cycling, myocyte contractility, and heart failure susceptibility following pathological stress. We detected a significant reduction of AKAP150 expression in the failing mouse heart induced by pressure overload. Importantly, cardiac-specific AKAP150 knockout mice were predisposed to develop dilated cardiomyopathy with severe cardiac dysfunction and fibrosis after pressure overload. Loss of AKAP150 also promoted pathological remodelling and heart failure progression following myocardial infarction. However, ablation of AKAP150 did not affect calcineurin-nuclear factor of activated T cells signalling in cardiomyocytes or pressure overload- or agonist-induced cardiac hypertrophy. Immunoprecipitation studies showed that AKAP150 was associated with SERCA2, phospholamban, and ryanodine receptor-2, providing a targeted control of sarcoplasmic reticulum Ca2+ regulatory proteins. Mechanistically, loss of AKAP150 led to impaired Ca2+ cycling and reduced myocyte contractility reserve following adrenergic stimulation or pressure overload.
Conclusions
These findings define a critical role for AKAP150 in regulating Ca2+ cycling and myocardial ionotropy following pathological stress, suggesting the AKAP150 signalling pathway may serve as a novel therapeutic target for heart failure.
Keywords: Cardiac hypertrophy , Pathological remodelling , Heart failure , Contractility , AKAP150
1. Introduction
Coordinated Ca2+ handling in cardiac myocytes is essential for efficient excitation–contraction (E–C) coupling in the heart. Sympathetic stimulation of the heart through β-adrenergic receptors (β-ARs) increases the rate and force of cardiac muscle contraction and relaxation as part of the ‘fight or flight’ response.1 In this pathway, activation of β-ARs leads to the production of cAMP, activation of protein kinase A (PKA), and phosphorylation of Ca2+ regulatory proteins, including the L-type Ca2+ channel (LTCC), phospholamban (PLN), ryanodine receptors (RYRs), troponin I, and myosin binding protein C.2,3
The specificity of cAMP-PKA signalling is achieved by the binding of PKA to A-kinase anchoring proteins (AKAPs), which provides spatial/temporal control of PKA signalling.4 AKAPs compose a growing list of diverse but functionally related scaffold proteins defined by their ability to bind to the regulatory subunit of PKA.5 Multiple AKAPs have been identified in the adult cardiac myocytes. For example, mAKAP (AKAP6) binds to RYR to mediate PKA-dependent RYR phosphorylation.6 AKAP18α (AKAP7α) targets LTCC to regulate Ca2+ influx in response to β-adrenergic stimulation.7,8 AKAP18δ (AKAP7δ) interacts with SERCA-PLN to regulate SR Ca2+ reuptake.9 Murine AKAP150 (also referred to as AKAP5) or human AKAP79 has been shown to interact with key signalling enzymes such as PKA, PKC, and calcineurin and their potential substrates, including LTCC, β-ARs, caveolin 3, adenylyl cyclase.10–17 Importantly, deletion of AKAP150 resulted in the loss of β-adrenergic stimulated Ca2+ transient and phosphorylation of RYR and PLN.15 Moreover, loss of AKAP150 also disrupted intracellular trafficking of β-ARs in cardiac myocytes.18 These results suggest that AKAP150 plays a critical role in regulating Ca2+ cycling and E–C coupling in the heart. However, the functional relevance of the AKAP150 signalling complex in response to pathological stress remains unknown.
In addition to targeting PKA, AKAP150 also binds to calcineurin, a protein phosphatase that has been implicated as a central regulator of cardiac hypertrophy.19,20 Calcineurin is activated in response to increased intracellular Ca2+, which in turn dephospholylates the transcription factor NFAT (nuclear factor of activated T cells), permitting its translocation from cytoplasm to nucleus to induce hypertrophic gene expression.19,20 In neurons, AKAP150 mediates targeted activation of the calcineurin-NFAT signalling, which serves as an important mechanism coupling LTCC Ca2+ influx to gene expression.21–23 Intriguingly, disruption of the binding of calcineurin to AKAP150 prevented LTCC-mediated NFAT nuclear translocation, suggesting a highly localized calcineurin-NFAT activation through AKAP150/LTCC docking.21 Similarly, it has been shown that a subgroup of LTCC in the localized caveolin-3 containing signalling domains provides a source of Ca2+ to activate calcineurin-NFAT signalling in cardiomyocytes.24 AKAP150, which binds to both LTCC and calcineurin, is also part of the caveolin-3 containing microdomains.15 However, the potential role of AKAP150 in mediating calcineurin-NFAT signalling and hypertrophic response in cardiomyocytes has not been investigated.
In this study, we found that AKAP150 expression was significantly decreased in the failing mouse heart after pressure overload. We generated a conditional cardiomyocyte-specific AKAP150 knockout mouse model to examine the role of AKAP150 in regulating cardiac Ca2+ cycling, contractility, and myocardial remodelling following pathological stress. Importantly, ablation of AKAP150 in the heart promoted adverse pathological remodelling and functional deterioration following pressure overload or myocardial infarction (MI), suggesting an important cardio-protective role of AKAP150 in myocardial stress response. Mechanistically, AKAP150 is essential in regulating Ca2+ cycling and cardiac ionotropy through targeted regulation of SR Ca2+ handling proteins in response to stress. However, AKAP150-deficient mice displayed normal calcineurin-NFAT activation and hypertrophic response, suggesting that AKAP150 is dispensable in cardiac hypertrophic signalling.
2. Methods
Detailed methods are available in Supplementary material online.
2.1 Animal models
The generation of the AKAP150-/- mouse and AKAP150 loxP-targeted (fl) mouse was described previously.25,26 AKAP150 fl/fl mice were crossed with αMHC-Cre mice27 to generate cardiac-specific AKAP150-deficient mice. The NFAT-luciferase reporter transgenic mouse, also described previously,28 was crossed with the AKAP150-/- mouse. All experimental procedures with animals were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of University of Washington and National Institute of Health.
2.2 Echocardiography, transverse aortic constriction, MI, ischemia/reperfusion, and isoproterenol infusion
Mice were anesthetized with 2% isoflurane by inhalation. Echocardiographic imaging was performed with a VisualSonics Vevo 2100 imaging system as described previously.29 Transverse aortic constriction (TAC), MI, ischemia/reperfusion (IR), and isoproterenol infusion were performed as previously described.30,31 Mice were euthanized by CO2 asphyxiation.
2.3 Histological analysis, cell size measurement, terminal deoxynucleotidyl transferase dUTP nick end labelling, and luciferase reporter assays
Histological analysis of fibrosis and cell surface area measurements were described previously.29 Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) from paraffin sections was performed with a TMR Red In Situ Death Detection Kit (Roche Diagnostics) according to the manufacturer’s instructions as described previously.29 Luciferase reporter assays in mouse hearts or cultured cadiomyocytes were performed as described.7,28,30
2.4 Cell cultures, [Ca2+]i measurements, and cell shortening
Ventricular myocytes were isolated using a Langendorff perfusion apparatus from adult mice euthanized with pentobarbital (100 mg/kg given IP) as previously described.32 Primary neonatal rat cardiomyocytes were prepared from hearts of 1- to 2-day-old Sprague-Dawley rat pups anesthetized by isloflurane inhalation followed by decapitation.30 Ventricular myocytes were loaded with the membrane-permeable acetoxymethyl-ester form of Fluo-4 (Fluo-4 AM) for measurement of [Ca2+]i as previously described.33 SR Ca2+ load was assayed by measuring the caffeine-induced Ca2+ transient evoked by rapid application of 20 mM caffeine to the cells via a picospritzer.32 These experiments were also performed in Tyrode’s solution containing 100 nM isoproterenol.
2.5 Immunoprecipitation and Western blotting
Protein extraction from mouse heart or cultured cardiomyocytes and subsequent immunocprecipitation and Western blotting were performed as previously described.29,30
2.6 Statistics
Sample size was estimated by conducting pilot experiments and power analysis. Results are presented as means ± SEM. Mann–Whitney U-test or Kruskal–Wallis test with post-hoc Mann–Whitney U-test and Bonferroni’s correction was used for studies with small sample sizes. Two-way nested ANOVA with Tukey’s post-hoc test was used for calcium and contractility data. P < 0.05 was considered statistically significant.
3. Results
3.1 Generation of cardiac-specific AKAP150 knockout mice
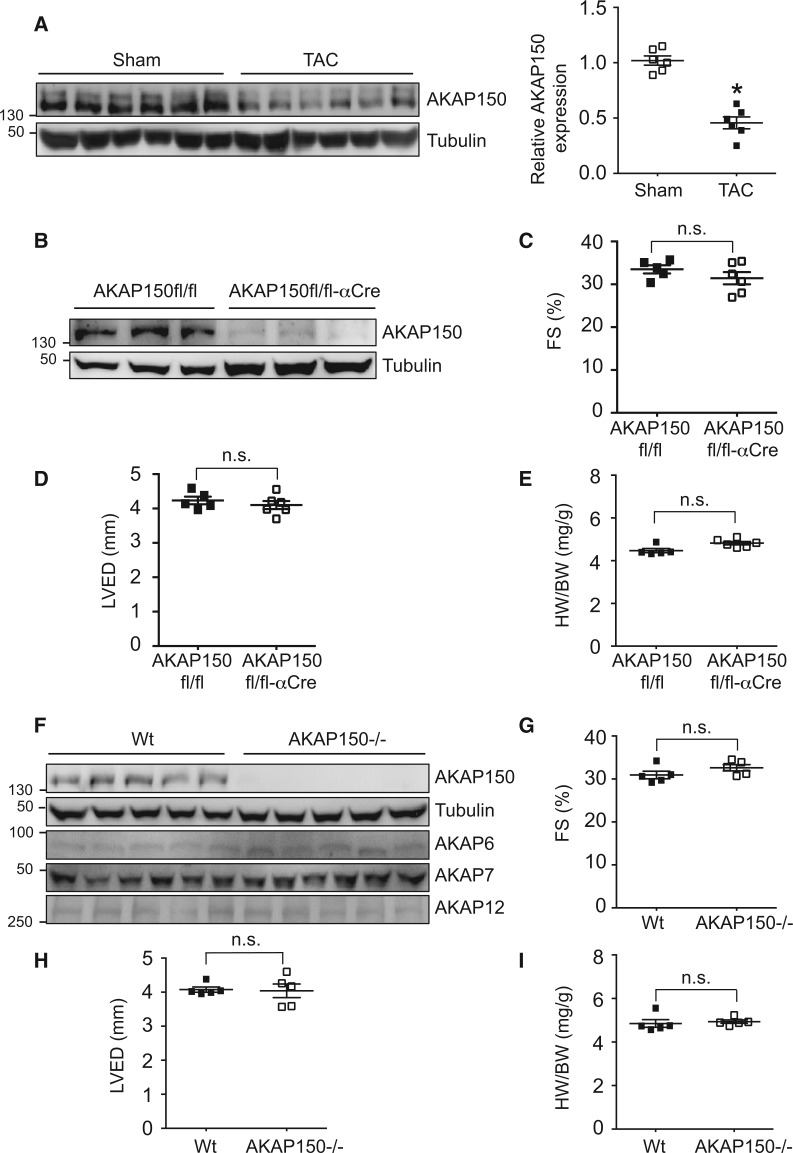
To study the function of AKAP150 in the heart in response to pathological stress, we first measured cardiac AKAP150 expression in mice subjected to pressure overload by TAC (27-gauge) for 4 weeks, which was used to induce pathological cardiac remodelling and heart failure (Supplementary material online, Figure S1). Importantly, AKAP150 expression was markedly decreased in the failing heart induced by TAC (Figure 1A, Supplementary material online, Figure S1B), suggesting a potential role for AKAP150 in regulating myocardial response to pathological stress. This prompted us to generate a cardiomyocyte-specific AKAP150 null mouse model using a Cre/LoxP-dependent conditional gene targeting approach. Mice homozygous for the AKAP150-LoxP targeted allele (AKAP150fl/fl)26 were crossed with cardiomyocyte-specific αMHC-Cre transgenic mice27, which drives Cre recombinase expression in neonatal heart proceeding to adulthood. Western blot analysis showed efficient deletion of AKAP150 in the heart as well as in isolated cardiomyocytes from the AKAP150fl/fl-αMHC-Cre mice (Figure 1B, Supplementary material online, Figure S1D). The AKAP150fl/fl-αMHC-Cre mice were generated at the predicted Mendelian ratios and were overly normal well into the adulthood, with no changes in cardiac performance as assessed by echocardiography and no signs of cardiac hypertrophy as measured by heart weight/body weight ratio (HW/BW) at 2 months of age (Figure 1C–E). Similarly, mice with global deletion of AKAP150 showed no detectable basal phenotype (Figure 1F–I). The expression of other AKAP isoforms in the heart was not altered in AKAP150-deficient mice (Figure 1F).
Figure 1.
Assessment of AKAP150 expression after TAC and characterization of cardiac-specific and global AKAP150 knockout mice. (A) Western blotting and quantification of AKAP150 expression in cardiac extracts from wild-type mice subjected to TAC (27-gauge) or sham surgery for 4 weeks. n = 6 for each group. P < 0.05. (B) Western blots for the indicated proteins in cardiac extracts from AKAP150fl/fl and AKAP150fl/fl-αCre mice at two months of age. (C), (D), and (E) Echocardiographic assessment of FS, LVED, and HW/BW ratio from AKAP150fl/fl or AKAP150fl/fl-αCre mice at two months of age. n = 5 for each group. n.s. indicates non-significance. (F) Western blots for the indicated proteins in cardiac extracts from wild-type (Wt) and AKAP150-/- mice at two months of age. (G), (H), and (I) Assessment of FS, LVED, and HW/BW from Wt or AKAP150-/- mice at two months of age. n = 5 for each group. n.s. indicates non-significance. Mann–Whitney U-test was used.
3.2 AKAP150 deficient mice are predisposed to adverse cardiac remodelling and dysfunction following pressure overload
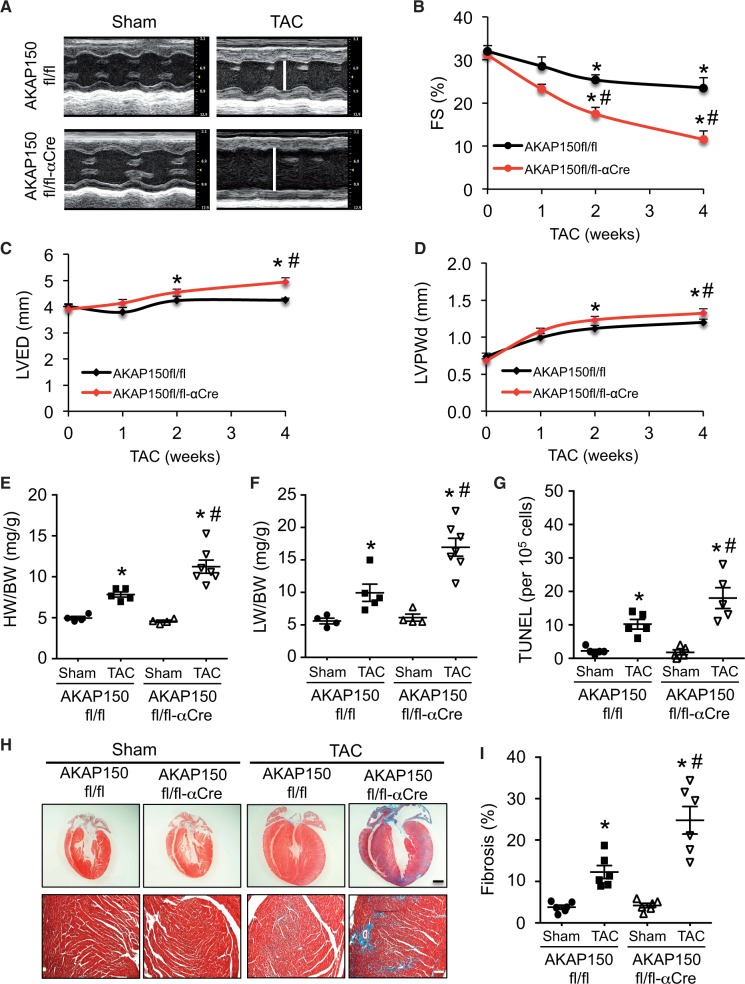
Next, we investigated the role of AKAP150 in regulating pathological stress response and heart failure propensity. The AKAP150fl/fl-αMHC-Cre mice and littermate controls were subjected to chronic pressure overload by TAC (27-gauge) for 0–4 weeks to analyse their susceptibility to heart failure. The AKAP150-deficient mice developed more prominent ventricular dilation and cardiac dysfunction compared with AKAP150fl/fl control mice 4 weeks after TAC, as indicated by significantly increased left ventricular dimension (LVED), decreased fractional shortening (FS), and increased ventricular wall thickness (Figure 2A–D). Cardiomyocytes from AKAP150-deficient mice also showed more prominent elongation after TAC (Supplementary material online, Figure S2). This was associated with enhanced pathological hypertrophy as assessed by heart weights normalized to body weights (HW/BW), as well as increased pulmonary oedema as measured by lung weights normalized to body weights (LW/BW; Figure 2E and F), indicating more severe heart failure and exacerbated pathological remodelling in the AKAP150-deficient mice. Moreover, hearts from the AKAP150-deficient mice showed increased apoptosis and fibrosis after TAC compared with control mice, as assessed by TUNEL and Masson’s trichrome staining of cardiac sections (Figure 2G–I). No significant difference in cardiac function or histology was detected in αMHC-Cre only mice (data no shown). Thus, ablation of AKAP150 predisposed the heart to dilated cardiomyopathy with severe cardiac dysfunction and pathological remodelling, suggesting a critical role for AKAP150 in regulating myocardial homeostasis and heart failure susceptibility following pathological stress.
Figure 2.
AKAP150-deficient mice are predisposed to adverse cardiac remodelling and dysfunction following pressure overload. (A) Representative echocardiographic M-mode images from AKAP150fl/fl and AKAP150fl/fl-αCre mice subjected to TAC (27-gauge) or a sham procedure for 4 weeks. The vertical white lines indicate LVED. (B), (C), and (D) FS, LVED, and left ventricular posterior wall thickness (LVPWd) from mice indicated in A. *P < 0.05 vs. corresponding Sham. #P < 0.05 vs. AKAP150fl/fl TAC. (E) and (F) HW/BW ratio and LW/BW ratio from mice indicated in A. *P < 0.05 vs. corresponding Sham. #P < 0.01 vs. Con TAC. (G) Quantification of TUNEL-positive nuclei in cardiac sections from mice indicated in A. (H) Masson trichrome-stained heart sections from the indicated mice subjected to sham surgery or TAC. Scale bars: top, 1 mm; bottom, 50 μm. (I) Quantitation of cardiac fibrotic area of cardiac sections from mice indicated in G. *P < 0.01 vs. corresponding Sham. #P < 0.05 vs. AKAP150fl/fl TAC. n = 4 for AKAP150fl/fl Sham; n = 5 AKAP150fl/fl TAC; n = 4 for AKAP150fl/fl-αCre Sham; n = 7 for AKAP150fl/fl-αCre TAC. Kruskal–Wallis test was used to compare each parameter. Post-hoc Mann–Whitney U-test with Bonferroni’s correction was then performed.
3.3 Loss of AKAP150 promotes pathological remodelling and heart failure progression after MI
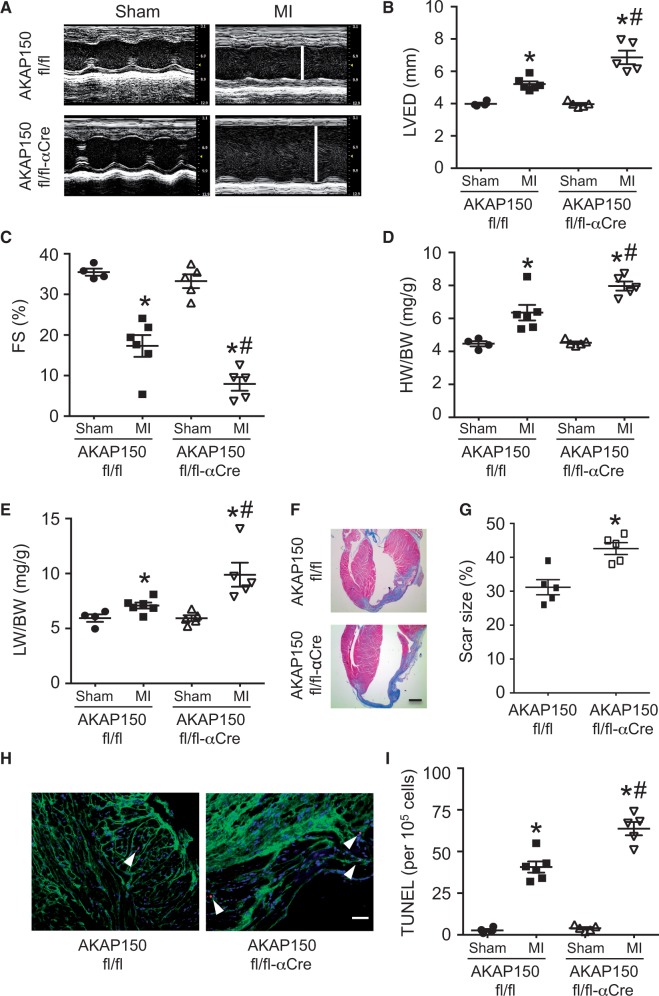
We investigated the role of AKAP150 signalling in another experimental model of heart failure induced by MI. Here we subjected the AKAP150fl/fl-αMHC-Cre mice and littermate controls to 2 weeks of MI to assess pathological remodelling and heart failure propensity. Echocardiographic analysis showed more pronounced ventricular dilation with wall thinning in the AKAP150-deficient mice compared with controls after 2 weeks of MI (Figure 3A and B). The AKAP150fl/fl-αMHC-Cre mice also showed worse ventricular performance than control mice after MI (Figure 3C). Intriguingly, AKAP150fl/fl-αMHC-Cre mice developed exaggerated cardiac hypertrophy (increased HW/BW) as well as pulmonary edema (increased LW/BW), an indicator of heart failure (Figure 3D and E). Masson’s trichrome staining of cardiac sections showed more expansive and thinner scars with increased chamber dilation in the AKAP150fl/fl-αMHC-Cre mice compared with controls (Figure 3F and G). Interestingly, an increase in TUNEL positive cardiomycytes, corresponding to increased apoptotic cell death, was also detected in the AKAP150-deficient hearts (Figure 3H and I). However, there was no significant difference in infarct area normalized to area at risk (IA/AAR) between AKAP150-deficient mice and controls after 1 h ischemia plus 24 h reperfusion (I/R, Supplementary material online, Figure S3), suggesting that AKAP150 doesn’t regulate acute myocardial injury. These data indicate that ablation of AKAP150 in the heart promoted pathological remodelling, functional decomposition, and heart failure progression following MI, further suggesting a cardioprotective role of AKAP150 upon pathological stress.
Figure 3.
Loss of AKAP150 promotes pathological remodelling and heart failure progression after MI. (A) Representative echocardiographic M-mode images from AKAP150fl/fl and AKAP150fl/fl-αCre mice subjected to MI or a sham procedure for 2 weeks. The vertical white lines indicate left ventricular end-diastolic dimension (LVED). (B) and (C) Echocardiographic analysis of LVED and FS in AKAP150fl/fl and AKAP150fl/fl-αCre mice subjected to sham or MI surgical procedure for 2 weeks. *P < 0.05 vs. corresponding Sham. #P < 0.05 vs. AKAP150fl/fl MI. (D) and (E) HW/BW and LW/BW from mice indicated in B. *P < 0.05 vs. corresponding Sham. #P < 0.05 vs. AKAP150fl/fl MI. (F) and (G) Masson’s trichrome-stained cardiac sections and quantitation of infarct size from AKAP150fl/fl and AKAP150fl/fl-αCre mice subjected to MI or sham procedure for 2 weeks. Scale bars, 1 mm. *P < 0.05 vs. AKAP150fl/fl. (H) and (I) Representative images and quantification of TUNEL-positive nuclei from peri-infarct areas in cardiac sections from AKAP150fl/fl or AKAP150fl/fl-αCre mice subjected to 2 weeks of MI. Arrow head indicates TUNEL-positive nucleus (red). Scale bars, 50 μm. *P < 0.01 vs. corresponding Sham. #P < 0.05 vs. AKAP150fl/fl MI. n = 4 for AKAP150fl/fl Sham; n = 6 AKAP150fl/fl MI; n = 5 for AKAP150fl/fl-αCre Sham; n = 5 for AKAP150fl/fl-αCre MI. Kruskal-Wallis test followed by Post-hoc Mann–Whitney U-test with Bonferroni’s correction was used.
3.4 AKAP150 is a critical regulator of cardiac calcium handling proteins in response to adrenergic stimulation or pressure overload
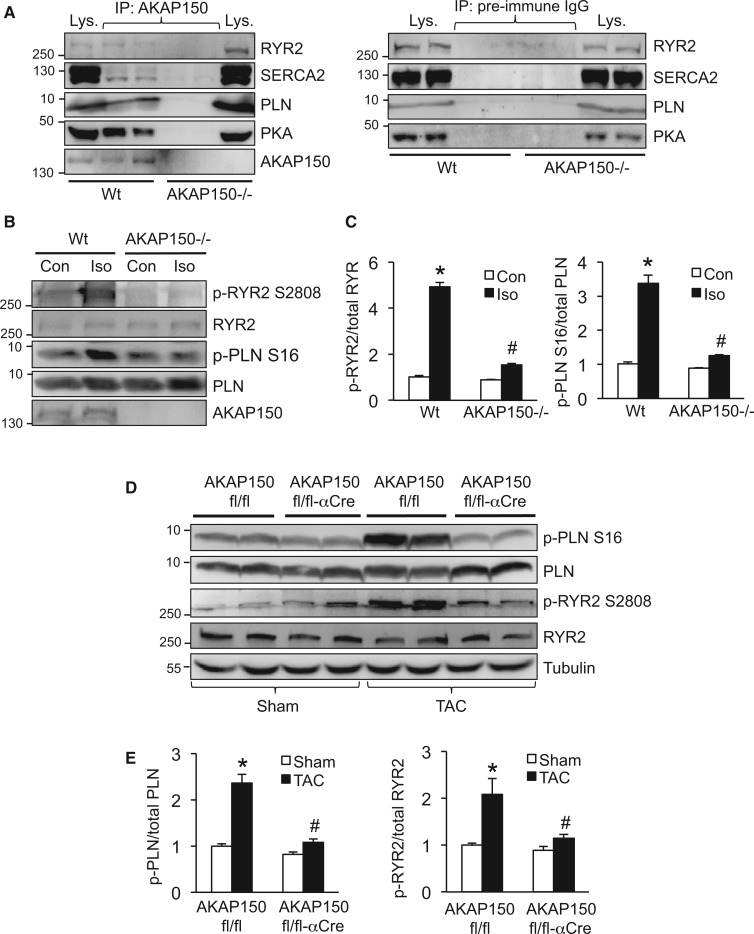
AKAP150 has been shown to play an important role in regulating Ca2+ cycling and E–C coupling in cardiomyocytes by interacting with multiple signalling proteins.11–15 Here we examined the role of AKAP150 in regulating Ca2+ regulatory proteins in cardiomyocytes under basal conditions as well as after pathological stimulation. First, we performed a co-immunoprecipitation experiment to determine potential interactions between AKAP150 and Ca2+ regulatory proteins in the heart. AKAP150 was immunoprecipitated in cardiac extracts from wild-type or AKAP150-/- mice, followed by Western blot analysis with the indicated antibodies (Figure 4A). We observed that three key SR Ca2+ regulatory proteins, RYR2, SERCA2, and PLN, interacted with AKAP150 to form a signalling complex in wild-type hearts (Figure 4A). Immunoblot detection of one of the known AKAP150 binding partners PKA served as an internal control (Figure 4A). These proteins were not detected from the immunoprecipitates obtained from AKAP150-/- hearts. As a negative control, no specific proteins were immunoprecipitated with normal pre-immune IgG (Figure 4A). Importantly, pressure overload further increased the interaction of AKAP150 with PKA, as well as with RYR2 (Supplementary material online, Figure S4). No significant changes in the association between AKAP150 and SERCA2 or PLN were observed. Next, we examined phosphorylation status of SR Ca2+ regulatory proteins in cardiomyocytes isolated from wild-type and AKAP150-/- mice, after stimulation with the adrenergic agonist isoproterenol. Consistent with the previous observation,15 increased phosphorylation of PLN and RYR2 was induced by isoproterenol in wild-type cells, and this effect was largely blocked in AKAP150-/- cells, suggesting AKAP150 is a critical regulator of SR Ca2+ regulatory proteins (Figure 4B and C). We then determined the role of AKAP150 in regulating SR Ca2+ regulatory proteins in response to pathological stimulation. We assessed phosphorylation of PLN and RYR2 from cardiac extracts of AKAP150fl/fl-αMHC-Cre and AKAP150fl/fl mice subjected to TAC (27-gauge) for 1 week. TAC stimulation induced an increase in PLN and RYR2 phosphorylation in AKAP150fl/fl mice, and this effect was again blunted in AKAP150fl/fl-αMHC-Cre mice (Figure 4D and E). These data therefore suggest a critical role for AKAP150 in regulating Ca2+ handling proteins in the heart upon pathological stimulation.
Figure 4.
AKAP150 is a critical regulator of cardiac calcium handling proteins in response to adrenergic stimulation or pressure overload. (A) Western blots for the indicated proteins following immunoprecipitation (IP) with an AKAP150 antibody (left panel) or pre-immune IgG (right panel) from cardiac extracts of wild-type (Wt) or AKAP150-/- mice. (B) Western blots for the indicated proteins from Wt or AKAP150-/- cardiomyocytes treated with isopreterenol (Iso, 100 nmol/l) or vehicle control for 5 min. (C) Quantification of phosphorylation level of RYR2 (left) and PLN (right) from mice indicated in B. *P < 0.01 vs. Con. #P < 0.05 vs. Wt Iso. Data were obtained from three independent experiments. (D) Western blots for the indicated proteins from cardiac extracts of AKAP150fl/fl or AKAP150fl/fl-αCre mice subjected to TAC (27-gauge) or a sham procedure for 1 week. (E) Quantification of phosphorylation level of PLN (left) and RYR2 (right) from mice indicated in D. *P < 0.05 vs. Sham. #P < 0.05 vs. AKAP150fl/fl TAC. Data were obtained from three independent experiments. Kruskal–Wallis test followed by post-hoc Mann–Whitney U-test was used.
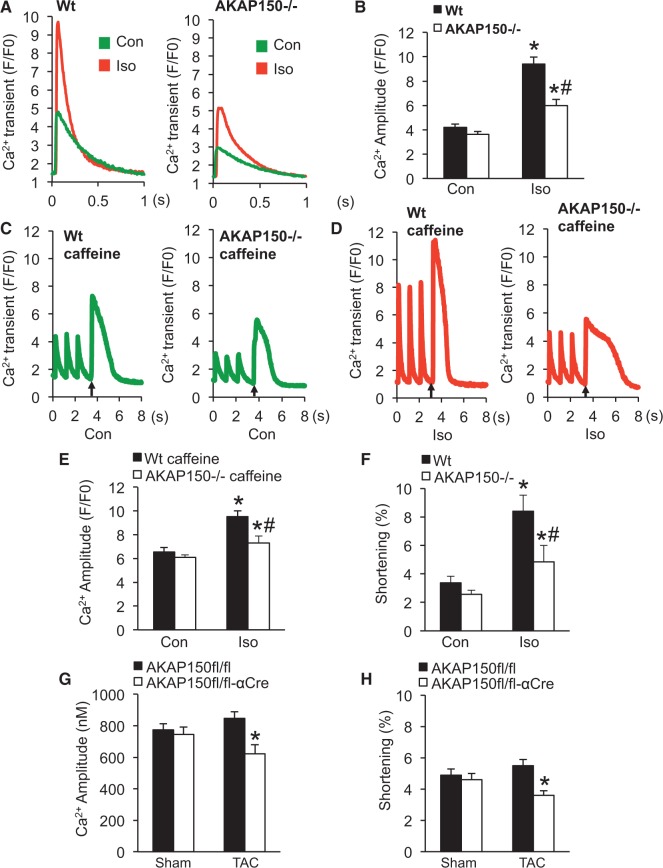
3.5 Loss of AKAP150 decreases the amplitude of [Ca2+]i transient, sarcoplasmic reticulum Ca2+ load, and contractility following adrenergic stimulation or pressure overload
Next, we directly assessed the role of AKAP150 in regulating Ca2+ cycling and myocyte contractility at basal conditions and following pathological stimulation. The Ca2+ amplitude of the steady-state action potential-evoked (1 Hz) [Ca2+]i transients was measured from Wt and AKAP150-/- cells (Figure 5A and B). Stimulation with isoproterenol induced a significant increase in Ca2+ amplitude in Wt cells, which was significantly inhibited in the AKAP150-/- cells (Figure 5A and B). Based on the observation that AKAP150 regulates phosphorylation of PLN and RYR2, we hypothesized that AKAP150 may directly influence SR Ca2+ load. Similar levels of SR Ca2+ load were detected in AKAP150-/- and Wt cells at basal conditions (Figure 5C–E). Adrenergic stimulation with isoproterenol induced a significant increase in SR Ca2+ load in Wt cells, but only a slight increase was detected in AKAP150-/- cells (Figure 5C–E). Consistent with these observations, adrenergic stimulation of myocyte contractility was also largely inhibited in AKAP150-/- cells, indicating reduced contractility reserve (Figure 5F). Lastly, we determined the effect of AKAP150 deletion on Ca2 + cycling and myocyte contractility in mice subjected to pressure overload by TAC. Compared with control cells, AKAP150-deficient cardiomyocytes showed impaired Ca2+ cycling and myocyte contractility at early stage of pressure overload by 1-week TAC (Figure 5G and H). These data indicate that AKAP150 is an important regulator of Ca2+ cycling and myocyte contractility following pathological stimulation, thus revealing a critical role for AKAP150 in maintaining Ca2+ homeostasis and myocyte ionotropy.
Figure 5.
Loss of AKAP150 decreases the amplitude of the [Ca2+]i transient, SR Ca2+ load, and contractility following adrenergic stimulation or pressure overload. (A) Representative tracings of field stimulation-induced Ca2+ transient from wild-type (Wt) and AKAP150-/- cardiomyocytes in the presence or absence of isoproterenol (Iso). (B) Quantification of Ca2+ amplitude as a change in fluorescence (F/F0) from Wt and AKAP150-/- cardiomyocytes in the presence or absence of Iso. (C) Representative tracings of caffeine-induced Ca2+ transient in Wt and AKAP150-/- cardiomyocytes treated with vehicle control. Three field stimulations were performed to ensure a steady state of SR Ca2+ load followed by caffeine stimulation indicated by arrow. (D) Representative tracings of caffeine-induced Ca2+ transients in Wt and AKAP150-/- cardiomyocytes treated with Iso. (E) Quantification of the amplitude of the caffeine induced Ca2+ transient in Wt or AKAP150-/- cardiomyocytes treated with vehicle control or Iso. *P < 0.05 vs. corresponding Con. #P < 0.05 vs. Wt Iso. (F) Cell shortening of Wt and AKAP150-/- cardiomyocytes treated with vehicle control or Iso. *P < 0.05 vs. corresponding Con. #P < 0.05 vs. Wt Iso. (G) and (H) Ca2+ amplitude (nM) and cell shortening in cardiomyocytes isolated from AKAP150fl/fl or AKAP150fl/fl-αCre mice subjected to TAC (27-gauge) or a sham procedure for 1 week. *P < 0.05 vs. AKAP150fl/fl TAC. n ≥ 50 adult myocytes were analysed from four separate mice in each group. Two-way nested ANOVA with Tukey’s post-hoc analysis was used to determine statistical significance in these experiments.
3.6 AKAP150 is dispensable for the activation of calcineurin-NFAT signalling in cardiomyocytes and cardiac hypertrophy response
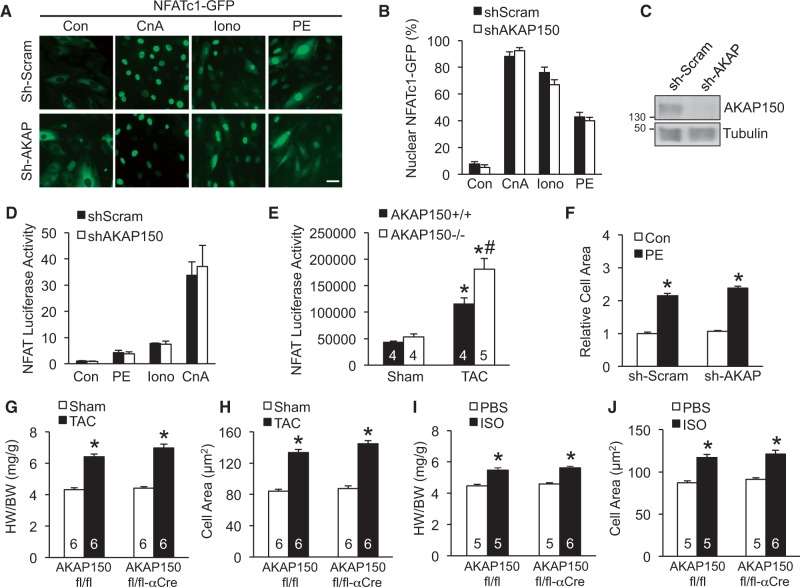
In addition to targeting PKA, AKAP150 has also been implicated in regulating calcineurin signalling,23,34 a key regulator of cardiac hypertrophy.19,20 Whether AKAP150 is required for the activation of calcineurin-NFAT signalling in cardiomyocytes and/or the cardiac hypertrophic response has not been investigated. First, we examined if AKAP150 regulates calcineurin-NFAT signalling in cardiomyocytes. Rat neonatal cardiomyocytes were infected with an adenovirus encoding NFATc1-GFP along with lentiviral vectors expressing shRNA for AKAP150 (shAKAP) or a scrambled sequence (shScram), followed by stimulation of hypertrophic agonists. Robust nuclear translocation of NFATc1 was induced in shScram control cells by overexpression of the constitutively active truncated calcineurin (ΔCnA) or stimulation with ionomyocin or phenylephrine (Figure 6A and B). shRNA-mediated gene silencing of AKAP150 had no significant effect on agonist-induced NFATc1 nuclear translocation (Figure 6A–C). This observation was confirmed using wild-type and AKAP150-/- MEFs (Supplementary material online, Figure S4). Next, cardiomyocytes were infected with adenoviral vectors expressing an NFAT-dependent luciferase reporter cassette to examine if AKAP150 regulates endogenous NFAT transcriptional activity. Luciferase activity was induced by hypertrophic agonists phenylephrine, ionomyocin, and active calcineurin, which was not affected by AKAP150 deletion (Figure 6D). To determine the effect of AKAP150 deletion on NFAT transcriptional activity in the heart in vivo, we crossed AKAP150-/- mice with the NFAT-luciferase reporter mice28. AKAP150-/- mice displayed similar NFAT activity as AKAP+/+ mice at baseline (Figure 6E). Surprisingly, deletion of AKAP150 mildly increased pressure overload-induced NFAT activity after TAC stimulation (Figure 6E). In addition, cardiomyocyte hypertrophic growth induced by phenylephrine was not altered by AKAP150 ablation (Figure 6F). Therefore, AKAP150 is not essential for calcineurin-NFAT activation in neonatal cardiomyocytes, nor is required for the cardiomyocyte hypertrophic response.
Figure 6.
AKAP150 is dispensable for cardiac hypertrophy response and the activation of calcineurin-NFAT signalling. (A) Fluorescent images from cardiomyocytes infected with lentiviruses expressing AKAP150 shRNA (shAKAP) or a scrambled sequence (shScram) along with adenoviruses encoding NFATc1-GFP (green), followed by stimulation with 1 μmol/l ionomycin (Iono), 50 μmol/l phenylephrine (PE), or vehicle control (Con) or infection with an adenovirus encoding active calcineurin (CnA). Scale bars, 10 μm. (B) Quantification of nuclear localized NFATc1 in cells treated as indicated in A. Data were from 3 independent experiments with ≥ 200 cells analysed. (C) Western blot for AKAP150 and tubulin from cardiomyocyte extracts indicated in A. (D) NFAT-luciferase activity in the indicated cardiomyocytes infected with shAKAP or shScram lentiviruses along with adenoviruses expressing NFAT-luciferase reporter, followed by stimulation with PE, Iono, or vehicle control, or infection with CnA adenoviruses. Data were from three independent experiments. (E) NFAT luciferase activity in relative fluorescence units from AKAP150+/+ and AKAP150-/- mice containing the NFAT luciferase reporter transgene after 2 weeks of TAC or a sham procedure. *P < 0.05 vs. Sham. #P < 0.05 vs. AKAP150+/+ TAC. (F) Surface areas of cardiomyocyte infected with shAKAP or shScram lentiviruses followed by stimulation with 50 μmol/l PE or vehicle control for 24 h. *P < 0.05 vs. corresponding Sham. Data were from 3 independent experiments with ≥ 200 cells analysed. (G) Assessment of HW/BW ratio from AKAP150fl/fl and AKAP150fl/fl-αMHC-Cre mice subjected to TAC (27-gauge) or sham procedure for 1 week. *P < 0.05 vs. corresponding Sham. (H) Myocyte surface area from cardiac sections of mice indicated in G. Surface areas of 500 cells per mouse were measured in random fields. *P < 0.05 vs. corresponding Sham. (I) HW/BW from AKAP150fl/fl and AKAP150fl/fl-αMHC-Cre mice subjected to isoproterenol (ISO) or phosphate-buffered saline (PBS) infusion for 2 weeks. *P < 0.05 vs. corresponding Sham. (J) Myocyte surface area from cardiac sections of mice indicated in I. The number of mice analysed is shown in the bars of each panel. *P < 0.05 vs. corresponding Sham. Data were analysed by Kruskal-Wallis test followed by Post-hoc Mann–Whitney U-test with Bonferroni’s correction.
To assess the role of AKAP150 in regulating cardiac hypertrophy in vivo, AKAP150fl/fl mice with or without αMHC-Cre were subjected to pressure overload by TAC stimulation (27-gauge) for 1 week. This short pressure overload protocol was used here because prolonged TAC stimulation predisposes the AKAP150-deficient mice to heart failure (Figure 2), which may secondarily affect the development of heart hypertrophy. Surprisingly, similar hypertrophic response was detected in the AKAP150-deficient mice and littermate controls, as assessed by HW/BW ratios and myocyte surface area measurements (Figure 6G and H). Chronic infusion of isoproterenol was used as another hypertrophic stimulus to extend and validate the results of pressure overload. Once again, the AKAP150fl/fl-αMHC-Cre mice showed no reduction in cardiac hypertrophic growth at the whole organ or cellular level compared with control mice after isoproterenol infusion (Figure 6I and J). Similar hypertrophic response was also observed in the AKAP150-/- mice compared with wild-type controls (Supplementary material online, Figure S5). These data clearly indicated that AKAP150 is not directly involved in cardiac hypertrophic response in vivo.
4. Discussion
This study reveals a critical role for AKAP150 in regulating myocardial remodelling and heart failure susceptibility in response to pathological stress. We show that loss of AKAP150 predisposed the heart to adverse remodelling, ventricular dysfunction, and heart failure following pressure overload or MI, revealing a cardioprotective role for AKAP150 upon pathological stimulation. However, AKAP150 seems dispensable for calcineurin-NFAT activation and cardiac hypertrophic response. Importantly, we identified three SR Ca2+ regulatory proteins, RYR, SERCA, and PLN, as new AKAP150 interacting proteins in addition to PKA, PKC, and calcineurin. This AKAP150 signalling complex provides a targeted control of PKA-dependent phosphorylation of SR Ca2+ regulatory proteins. Further, our results indicate that AKAP150 plays a cardio-protective role by regulating Ca2+ cycling and myocardial ionotropy in response to pathological stimulation.
Progressive cardiac hypertrophy and chamber dilation have been reported in global AKAP150 knockout mice.35 However, we observed no overt basal cardiac phenotype in our AKAP150-/- mice within 3 months of age, which were maintained on the C57BL/6 genetic background (Figure 1). In this study, we generated cardiac-specific AKAP150 knockout mice to examine cardiomyocyte autonomous role of AKAP150 in the heart, thereby circumventing the potential confounding changes associated with global AKAP150 deletion. Indeed, AKAP150 expressed in vascular smooth muscle cells mediates PKC-dependent regulation of vascular tone, and AKAP150-/- mice are naturally hypotensive and do not develop angiotensin II-induced hypertension.16 In addition, AKAP150 expressed in neurons regulates synaptic plasticity and behaviour, and also affects the activity of autonomic nervous system.26,36 Thus, our conditional targeting approach bypasses potential abnormalities observed with the conventional gene deletion and allows for functional assessment of AKAP150 in the heart under both physiological and pathological conditions.
We observed that AKAP150 expression was markedly decreased in the failing mouse heart after chronic pressure overload. Similarly, decreased AKAP150 expression was also observed in the lung from patient with chronic obstructive pulmonary disease.37 On the other hand, AKAP150 expression was increased in adipocytes from exercise trained rats.38 At present, the signalling mechanisms that regulate AKAP150 expression and processing remain unclear. In addition to contractile dysfunction, we showed that AKAP150 deficient mice developed exacerbated ventricular dilation, interstitial fibrosis, and myocyte hypertrophy following pressure overload or MI. Although we observe no changes in IA/AAR after acute IR, indicating AKAP150 has no direct role in acute ischemic myocardial injury, we detected a significantly increase in scar size in AKAP150-deficient mice after 2-week MI compared with control mice. The increased scar size indicates more severe pathological remodelling (fibrosis, scar expansion, and ventricular dilatation) in AKAP150-deficient mice after chronic MI. Therefore, ablation of AKAP150 promotes pathological cardiac remodelling after chronic MI, but not acute myocardial injury. Downregulation of AKAP150 following sustained pathological stress may contribute to disease progression and heart failure susceptibility. It has been shown that diminished myocardial contractility was sufficient to promote pathological myocardial remodelling, presumably by invoking an increase in adrenergic drive and reactive signalling.39 For example, decreased cardiac contractility by phospholamban overexpression40 or a dominant negative mutation41 can induce adverse myocardial remodelling and heart failure in an otherwise normal setting. On the other hand, enhanced cardiac contractility with phospholamban inhibition42 or SERCA2 overexpression43 prevented pathological remodelling and heart failure progression.
Loss of AKAP150 led to impairments in Ca2+ transient, SR Ca2+ load, and myocyte contractility following adrenergic stimulation or pressure overload, which were associated with the dysregulation of Ca2+ handling proteins. Defects in Ca2+ cycling and contractile function are key features of experimental and human heart failure. One of the most notable abnormalities is reduced SR Ca2+ load caused by decreased Ca2+ reuptake by SERCA or dysregulated Ca2+ release through RYR. Downregulation of AKAP150 may represent a new mechanism underlying impaired Ca2+ cycling in heart failure. Changes in expression of other AKAPs, such as AKAP15/189 and mAKAP44, could also potentially lead to defects in Ca2+ cycling. Genetic manipulations that improve Ca2+ cycling by adenoviral mediated SERCA overexpression or genetic ablation of PLN have been proven to be beneficial in attenuating heart failure.39 As a future direction, it will be important to determine if transgenic overexpression of AKAP150 in the heart could preserve Ca2+ cycling and contractile function in the setting of heart failure.
Our study provides new insights into the functional role of AKAP150 signalling in the heart under pathological conditions. During pathological stress such as TAC and MI, the myocardium adapts by increasing calcium cycling and myocyte contractility via adrenergic stimulation. This involves PKA-mediated phosphorylation of calcium handling proteins. Our data indicate that AKAP150 is required in this process by targeting PKA to key calcium regulatory proteins. Indeed, loss of AKAP150 led to impairments in phosphorylation of calcium regulatory proteins, calcium transient, SR calcium load, and myocyte contractility in response to stress. Mechanistically, AKAP150 co-immunoprecipitates with RYR2, SERCA2, and PLN to form a multimolecular signalling complex that also contains PKA and calcineurin. Ablation of AKAP150 inhibited phosphorylation of RYR2 and PLN, leading to decreased SR Ca2+ load and Ca2+ amplitude. This underlies the reduced contractility reserve and increased heart failure susceptibility in the AKAP-deficient heart following pathological stress. AKAP150 has also been shown to interact with LTCC (Cav1.2) at sarcolemma and T-tubules,11,15,17 which is located in close proximity with the Ca2+ handling proteins in the SR. This AKAP150-mediated clustering of Ca2+ entry (LTCC), SR Ca2+ reuptake (SERCA), and SR Ca2+ release (RYR) pathway could lead to increased efficiency of Ca2+ cycling. Indeed, it has been suggested that AKAP150 can function in a dynamic manner by cycling between distinct subcellular locations, in contrast to the previous notion that AKAPs are static anchors that position signalling proteins to fix targets.45 Our data do not preclude the potential roles of other AKAPs in regulating Ca2+ cycling. For example, it has been suggested that AKAP15/18 localizes PKA in SERCA-PLN complexes.9 However, Ca2+ cycling or PLN phosphorylation in cardiomyocytes was not affected by genetic deletion of AKAP15/18.46 In addition, mAKAP has been shown to form a complex with RYR2 and PKA in cardiomyocytes,47 although the functional relevance in vivo has not been determined. We speculate that different AKAPs signalling complexes may display redundant roles to sequester slightly different combinations of enzymes to specific targets. The utility of this mechanism provides a versatile means to respond to the continually changing signalling environment within myocytes. Future studies will address this possibility and functional roles of other AKAPs in the heart during physiological and pathological conditions.
Since AKAP150 also targets calcineurin, a central player in cardiac hypertrophy, we also investigated the potential role of AKAP150 in mediating calcineurin-NFAT signalling in cardiomyocytes and cardiac hypertrophy, although this was not a main focus of this study. Surprisingly, our data showed that AKAP150 is not necessary for calcineurin-NFAT signalling in cardiomyocytes in vitro, or pressure overload- or agonist-induced cardiac hypertrophic response in vivo. In contrast, AKAP150 has been shown as a calcineurin-anchoring protein in smooth muscle cells and neurons, where it plays an important role in coupling local Ca2+-dependent calcineurin activation to NFAT-mediated gene expression.16,17,22 Therefore, the role of AKAP150 seems to be cell type specific, and although AKAP150 anchors calcineurin, this doesn’t seem to be coupled to NFAT activation or hypertrophic response in cardiomyocytes. Intriguingly, the AKAP150-deficient mice developed greater cardiac hypertrophy that control mice following chronic pressure overload. This increased hypertrophic response was likely attributable to more severe pathological myocardial remodelling with cardiac dilation and fibrosis due to cardiac decompensation in the AKAP150-deficient mice. It is formally possible that other AKAP(s) might be involved in calcineurin-NFAT-mediated hypertrophic signalling or that AKAP150 manages the actions of other NFAT isoforms in the myocardium. Indeed, mAKAP has been reported as another calcineurin-anchoring protein in cardiomyocytes, and mAKAP depletion inhibited adrenergic-mediated NFATc1 translocation and hypertrophic growth in cultured myocytes.48 A recent study using cardiac-specific mAKAP knockout mice further demonstrated that mAKAP is important for cardiac hypertrophy induced by pressure overload, catecholamine, and exercise.44 Another cardiac AKAP, AKAP-lbc, mediates α-adrenergic receptor-mediated cardiomyocyte hypertrophy in vitro, possibly through its interaction with the PKD-HDAC5 signalling pathway.49,50
In conclusion, our study revealed an important role for the scaffold protein AKAP150 in regulating Ca2+ cycling and myocyte ionotropy following pathological stress, through targeted regulation of SR Ca2+ handling proteins. AKAP150 expression in the heart was markedly decreased after sustained pathological stimulation, and ablation of AKAP150 led to impaired Ca2+ cycling and reduced contractility reserve, which were associated with increased pathological remodelling and heart failure susceptibility. The finding that AKAP150 plays a cardio-protective role upon pathological stress suggests that AKAP150 could serve as a potential diagnostic or therapeutic target for heart failure. Further work will be necessary to determine how and to what extent the AKAP150 signalling complex can be targeted to regulate Ca2+ cycling and myocyte contractility in the setting of heart failure.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Acknowledgements
We thank Dr. Jeffery D. Molkentin at Cincinnati Children’s Hospital for providing the αMHC-Cre mice and NFAT-luciferase reporter transgenic mice.
Conflict of interest: none declared.
Funding
This work was supported by grants from the National Institutes of Health [R00HL0908076 and R01HL116507 to Q.L., R01DK105542 and R01DK54441 to J.D.S., R01HL085686 to L.F.S., and F30 HL126249-02 to B.D.].
References
- 1.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science 2003;300:1530–1532. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
- 3.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 2005;66:12–21. [DOI] [PubMed] [Google Scholar]
- 4.Scott JD, Santana LF. A-kinase anchoring proteins: getting to the heart of the matter. Circulation 2010;121:1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauban JR, O'Donnell M, Warrier S, Manni S, Bond M. AKAP-scaffolding proteins and regulation of cardiac physiology. Physiology 2009;24:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 2000;101:365–376. [DOI] [PubMed] [Google Scholar]
- 7.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem 1997;272:6297–6302. [DOI] [PubMed] [Google Scholar]
- 8.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. EMBO J 1998;17:2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, Taskén K, Klussmann E. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep 2007;8:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science 1996;271:1589–1592. [DOI] [PubMed] [Google Scholar]
- 11.Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron 1997;19:185–196. [DOI] [PubMed] [Google Scholar]
- 12.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, Scott JD. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell 2006;23:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem 2006;281:33537–33553. [DOI] [PubMed] [Google Scholar]
- 14.Shcherbakova OG, Hurt CM, Xiang Y, Dell'Acqua ML, Zhang Q, Tsien RW, Kobilka BK. Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol 2007;176:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res 2010;107:747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navedo MF, Nieves-Cintrón M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res 2008;102:e1–e11. [DOI] [PubMed] [Google Scholar]
- 17.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 2007;55:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Nooh MM, Bahouth SW. Role of AKAP79/150 protein in β1-adrenergic receptor trafficking and signaling in mammalian cells. J Biol Chem 2013;288:33797–33812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998;93:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol 2002;541:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy JG, Sanderson JL, Gorski JA, Scott JD, Catterall WA, Sather WA, Dell'Acqua ML. AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell Rep 2014;7:1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Pink MD, Murphy JG, Stein A, Dell'Acqua ML, Hogan PG. Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat Struct Mol Biol 2012;19:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Shapiro MS. Activity-dependent transcriptional regulation of M-Type (Kv7) K(+) channels by AKAP79/150-mediated NFAT actions. Neuron 2012;76:1133–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR. A caveolae-targeted L-type Ca2+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ Res 2012;110:669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry 2007;46:1635–1646. [DOI] [PubMed] [Google Scholar]
- 26.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, Raber J, Scott JD. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A 2008;105:12557–12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palermo J, Gulick J, Colbert M, Fewell J, Robbins J. Transgenic remodeling of the contractile apparatus in the mammalian heart. Circ Res 1996;78:504–509. [DOI] [PubMed] [Google Scholar]
- 28.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 2004;94:110–118. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Chen Y, Doan J, Murray J, Molkentin JD, Liu Q. Transforming growth factor β-activated kinase 1 signaling pathway critically regulates myocardial survival and remodeling. Circulation 2014;130:2162–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Chen Y, Auger-Messier M, Molkentin JD. Interaction between NFκB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res 2012;110:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q, Sargent MA, York AJ, Molkentin JD. ASK1 regulates cardiomyocyte death but not hypertrophy in transgenic mice. Circ Res 2009;105:1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drum BM, Dixon RE, Yuan C, Cheng EP, Santana LF. Cellular mechanisms of ventricular arrhythmias in a mouse model of Timothy syndrome (long QT syndrome 8). J Mol Cell Cardiol 2014;66:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol 2002;544:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nystoriak MA, Nieves-Cintrón M, Nygren PJ, Hinke SA, Nichols CB, Chen CY, Puglisi JL, Izu LT, Bers DM, Dell'acqua ML, Scott JD, Santana LF, Navedo MF. AKAP150 contributes to enhanced vascular tone by facilitating large-conductance Ca2+-activated K+ channel remodeling in hyperglycemia and diabetes mellitus. Circ Res 2014;114:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Matta SM, Sullivan RD, Bahouth SW. Carvedilol reverses cardiac insufficiency in AKAP5 knockout mice by normalizing the activities of calcineurin and CaMKII. Cardiovasc Res 2014;104:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisenhaus M, Allen ML, Yang L, Lu Y, Nichols CB, Su T, Hell JW, McKnight GS. Mutations in AKAP5 disrupt dendritic signaling complexes and lead to electrophysiological and behavioral phenotypes in mice. PLoS One 2010;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poppinga WJ, Heijink IH, Holtzer LJ, Skroblin P, Klussmann E, Halayko AJ, Timens W, Maarsingh H, Schmidt M. A-kinase-anchoring proteins coordinate inflammatory responses to cigarette smoke in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2015;308:L766–L775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nomura S, Kawanami H, Ueda H, Kizaki T, Ohno H, Izawa T. Possible mechanisms by which adipocyte lipolysis is enhanced in exercise-trained rats. Biochem Biophys Res Commun 2002;295:236–242. [DOI] [PubMed] [Google Scholar]
- 39.Dorn GW, 2nd, Molkentin JD. Manipulating cardiac contractility in heart failure: data from mice and men. Circulation 2004;109:150–158. [DOI] [PubMed] [Google Scholar]
- 40.Dash R, Kadambi V, Schmidt AG, Tepe NM, Biniakiewicz D, Gerst MJ, Canning AM, Abraham WT, Hoit BD, Liggett SB, Lorenz JN, Dorn GW, II, Kranias EG. Interactions between phospholamban and beta-adrenergic drive may lead to cardiomyopathy and early mortality. Circulation 2001;103:889–896. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 2003;299:1410–1413. [DOI] [PubMed] [Google Scholar]
- 42.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J., Jr. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest 2004;113:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayama H, Otsu K, Yamaguchi O, Nishida K, Date MO, Hongo K, Kusakari Y, Toyofuku T, Hikoso S, Kashiwase K, Takeda T, Matsumura Y, Kurihara S, Hori M, Tada M. Cardiac-specific overexpression of a high Ca2+ affinity mutant of SERCA2a attenuates in vivo pressure overload cardiac hypertrophy. FASEB J 2003;17:61–63. [DOI] [PubMed] [Google Scholar]
- 44.Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, Dayan J, Dodge-Kafka K, Kapiloff MS. The scaffold protein muscle A-kinase anchoring protein β orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ Heart Fail 2014;7:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langeberg LK, Scott JD. Signalling scaffolds and local organization of cellular behaviour. Nat Rev Mol Cell Biol 2015;16:232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones BW, Brunet S, Gilbert ML, Nichols CB, Su T, Westenbroek RE, Scott JD, Catterall WA, McKnight GS. Cardiomyocytes from AKAP7 knockout mice respond normally to adrenergic stimulation. Proc Natl Acad Sci U S A 2012;109: 17099–17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci 2001;114:3167–3176. [DOI] [PubMed] [Google Scholar]
- 48.Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci 2005;118:5637–5646. [DOI] [PubMed] [Google Scholar]
- 49.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A 2007;104:10140–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell 2008;32:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.