Abstract
Aims
There are conflicting reports on the role of reactive oxygen species (ROS) i.e. beneficial vs. harmful, in vascular endothelium. Here, we aim to examine whether duration of exposure to ROS and/or subcellular ROS levels are responsible for the apparently paradoxical effects of oxidants on endothelium.
Methods and results
We have recently generated binary (Tet-ON/OFF) conditional transgenic mice (Tet-Nox2:VE-Cad-tTA) that can induce 1.8 ± 0.42-fold increase in NADPH oxidase (NOX)-derived ROS specifically in vascular endothelium upon withdrawal of tetracycline from the drinking water. Animals were divided in two groups: one exposed to high endogenous ROS levels for 8 weeks (short-term) and the other for 20 weeks (long-term). Using endothelial cells (EC) isolated from mouse hearts (MHEC), we demonstrate that both short-term and long-term increase in NOX-ROS induced AMPK-mediated activation of eNOS. Interestingly, although endothelium-dependent nitric oxide (NO)-mediated coronary vasodilation was significantly increased after short-term increase in NOX-ROS, coronary vasodilation was drastically reduced after long-term increase in ROS. We also show that short-term ROS increase induced proliferation in EC and angiogenic sprouting in the aorta. In contrast, long-term increase in cytosolic ROS resulted in nitrotyrosine-mediated inactivation of mitochondrial (mito) antioxidant MnSOD, increase in mito-ROS, loss of mitochondrial membrane potential (Δψm), decreased EC proliferation and angiogenesis.
Conclusion
The findings suggest that NOX-derived ROS results in increased mito-ROS. Whereas short-term increase in mito-ROS was counteracted by MnSOD, long-term increase in ROS resulted in nitrotyrosine-mediated inactivation of MnSOD, leading to unchecked increase in mito-ROS and loss of Δψm followed by inhibition of endothelial function and proliferation.
Keywords: Endothelium • Signal transduction • Nitric oxide • Reactive oxygen species • NADPH oxidase
1. Introduction
In endothelial cells (EC), intracellular reactive oxygen species (ROS) are generated from several different sources including NADPH oxidases, mitochondria, cytochrome P450 and xanthine oxidase.1–4 Since mitochondrial oxidative phosphorylation contributes very little to EC’s ATP synthesis, Rac1-dependent NADPH oxidase (NOX) acts as a major source of endothelial ROS. NADPH oxidase is a membrane-bound enzyme complex that contains gp91phox (Nox2) and p22phox subunits, and cytosolic p47phox, p67phox and Rac1.4–9 ECs also contain other NADPH oxidases such as Nox4, Nox1 and Nox5.10–14 In addition to the cell membrane, the Nox2 containing NADPH oxidase has been found in the perinuclear membrane and endoplasmic reticulum (ER).7,15–17
Although NADPH oxidase was first discovered as a phagocytic oxidase, it is noteworthy that several distinct differences between NADPH oxidase (NOX) in the phagocytic and endothelial cells (EC) necessitate careful studies of EC-NOX.18 Critical differences between EC-NOX and phagocytic-NOX are as follow (i) as mentioned above EC NOX, unlike phagocytic cells, is present in the cell membrane as well as in peri-nuclear and other intracellular membranes, (ii) whereas endothelial NOX is pre-assembled and generate ambient levels of ROS intracellularly, phagocytic NOX enzyme complex does not have any basal activity and is only formed upon stimulation to generate extracellular (within the phagosome) ROS, (iii) phagocytic ROS generation is fast (within seconds) and at the millimolar levels, but non-phagocytic NOX is slow to produce ROS at the micromollar levels . These critical differences should be taken into consideration while extrapolating non-endothelial NOX data to EC-NOX.
Increased levels of ROS are usually observed in pathological conditions involving vascular dysfunction, often seen in cardiovascular diseases (CVD). The major causes of morbidity and mortality in the Western world, coronary artery disease (CAD) and ischemic heart disease (IHD), both present with increased ROS.19–23 The notion that reduction in ROS levels in the vessel walls should improve vascular functions has been derived from these observations.24 Contrary to this notion are the findings of the recent interventional clinical trials using global antioxidants, e.g. HOPE, ATBC25–28; these trials produced negative results in reducing cardiovascular events.25,29,30 Recent overviews suggesting the importance of subcellular ROS and their differential roles in pathological vs. physiological processes, and the importance of targeting specific oxidant enzymes rather than global reduction in ROS help us better understand the reasons behind failure of the global antioxidants.31,32 Additionally, several recent studies demonstrated that ROS reduction did not improve endothelial and vascular functions. Interestingly, reduction in ROS resulted in inhibition of eNOS activation and NO synthesis in EC, and thus led to reduction in endothelial function and coronary vasodilatation.33,34 These results suggested that EC-ROS exert a critical positive effect on vascular endothelial function.
There are several reports suggesting a critical balance between sub-cellular ROS levels is critical for endothelial, and thus vascular, functions. ROS levels in different subcellular compartments such as mitochondria, cytosol and ER may play distinctly different roles in cellular functions. ROS in the cytosol may affect mitochondrial ROS and vice versa. However, very little is known about the communication between subcellular ROS compartments and whether endogenous ROS affect endothelial function differentially depending on their subcellular localization. In addition, there are no known systematic studies that examined the temporal effects of duration of ROS exposure to vascular endothelium in an animal model in vivo.
In the current study, using our recently generated binary (Tet-ON/OFF) conditional transgenic mouse that induces EC-specific increase in NADPH oxidase-derived endogenous ROS,35 we aimed to examine whether duration of exposure to increased endogenous ROS determines the apparently paradoxical redox effects (beneficial vs. harmful) on vascular endothelium. We also address whether increased levels of endogenous NOX-ROS modulate other subcellular sources of ROS such as mitochondrial ROS (mito-ROS). We divided these transgenic animals in two groups: one group was exposed to high ROS levels for 8 weeks (short-term) and another group was exposed to high ROS for 20 weeks (long-term). Using isolated mouse heart EC (MHEC), coronary microvessel and aortae from these two groups of animals, we report that the beneficial vs. deleterious effects of oxidants on vascular endothelium depends on the duration of ROS exposure as it perturbs the critical balance between subcellular ROS levels, i.e. mitochondrial vs. cytosolic ROS.
2. Methods
See online Supplemental data file for experimental details of the following methods.
2.1 Generation of binary Tet-NOX2: VE-Cadherin-tTA mice
All animal experiments were approved by the Lifespan Institutional Animal Care and Use Committee. The transgenic animal was generated as previously described.35 Detailed transgenic development has been described in the Supplementary material online, Supplemental data file.
2.2 Immunofluorescence and immunohistochemistry assays
Immunofluoresence and immunohistochemistry assays were performed as described.36
2.3 Ex vivo coronary microvessel relaxation studies
After cardiac harvest from animals that were Tet-ON (control) or Tet-OFF Tet-Nox2:VE-Cad-tTA (NVF) for 8 or 20 weeks, coronary arterioles (diameter, 80–120 μM; length, 2 mM) were dissected from the surrounding tissue. Microvessel studies [6 mice from each group (Tet-ON and Tet-OFF)] were performed using ex vivo organ bath videomicroscopy, as previously described.33
2.4 Mouse heart EC isolation and culture
Mouse heart ECs (MHECs) were isolated from the heart specimens of Tet-ON and Tet-OFF animals, as previously described.35 For each experiment, primary cultures of Tet-ON and Tet-OFF were started simultaneously (a pool of three hearts from each group). For cell culture experiments, for each time point per group we used in vitro assays in triplicate with an n = ≥ 5 independent replicate experiments (each using three animal hearts per batch of EC isolation per group or time points). Experiments using animal or animal organs such as coronary vessels, aortae, n = 6–8 were used unless otherwise stated.
2.5 Citrulline assay as a measure for nitric oxide synthesis
MHEC from Tet-ON and Tet-OFF animals were subject to citrulline assays as described33 (and in the Supplemental materials).
2.6 Aortic sprouting angiogenesis assay
Aortae from Tet-ON and Tet-OFF mice were dissected and 1 mm-long pieces were incubated with 500 μl EGM-2 medium (Clonetics, Lonza, Walkersville, MD) with (Tet-ON, control) or without (Tet-OFF) tetracycline as previously described.37 The sprouting area, vessel density and branching index of each ring were quantified using Angiotool software.38 Immunostaining of the sprouts to identify EC was carried out on the frozen sections as follow. Aortic sprouts were taken out from the embedded matrigel and placed in OCT. Cryosections of 6–10 μM were subject to primary antibody (1:50 dilution of anti-CD31 antibody, Abcam, Cambridge, MA) followed by secondary antibody (488-alexa Fluor Rabbit, ratio 1:200, Thermofisher Scientific, Waltham, MA) and DAPI in the mounting media. Pictures were taken using 40× and 60× upright fluorescent microscope.
2.7 Western blots
Cell lysates were prepared from MHEC grown on 0.1% gelatin-coated plates to 80–90% confluence. Western blot (WB) analyses using MHEC protein lysates were performed as previously described.35 Quantitative densitometric analysis of the Western blots was carried out using NIH Image J (NIH, Bethesda, MD) as described.33 Phosphorylated moiety/bands of the protein such as p-eNOS and p-AMPK were normalized against total eNOS and AMPK protein levels, respectively.
2.8 NADPH oxidase assay
NADPH oxidase activity assay was performed on homogenized MHEC using a Dounce homogenizer on ice in a buffer containing 20 mM KH2PO4 (pH 7.0), 1× protease mixture inhibitor (Sigma, Chicago, IL), 1 mM EGTA, 10 μg/ml aprotinin, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, 0.5 mM phenylmethylsulfonyl fluoride.39,40 Concentration of NADPH used in the assay was 100 µmol/L.
2.9 ROS measurement
DCF fluorescence assays—Total intracellular levels of ROS were determined by oxidative conversion of cell-permeable 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Molecular Probes Inc., Eugene, OR) to fluorescent dichloro-fluorescein as described previously.35 HYPER fluorescence ratiometric assay—To measure intracellular H2O2, MHEC were transfected with pHyPer-2 (Addgene, Cambridge, MA), which encodes a derivative of hydrogen peroxide-specific sensor protein Hyper.41,42 Ratiomatric analysis of 500/420 nm was carried out as described by Belousov and Bilan.42
2.10 Quantitative real-time PCR
Real-time PCR was carried out as described previously.43 Briefly, RNA was extracted from MHEC using the RNeasy RNA extraction kit (Qiagen, Valencia, CA). Primers were designed using the Primer Express oligo design software and synthesized by Integrated DNA Technologies (Coralville, IA) as described.35 Mitochondrially encoded mt-Co1 gene expression was normalized against the 18 S rRNA described35.
2.11 Mitochondrial membrane potential (Δψm)
To analyse the Δψm, Tet-ON and Tet-OFF NVF MHEC were grown for 24 h in a 96-well black bottom plate.44 Data points were plotted against respective Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) (maximum depolarization) value as per the protocol of the manufacturer.
2.12 Mitochondrial ROS
MHEC were loaded with 1 μmol/L MitoSox and 100 nmol/L MitoTracker Green FM (Invitrogen, Carlsbad, CA) as described in the protocol of the manufacturer.45,46
2.13 SOD activity assay
MnSOD activity was determined using SOD activity kit as per protocol of the manufacturer (Enzo Life Sciences, Plymouth Meeting, PA). This assay determines SOD activity in the cell lysates by the percent inhibition of superoxide ion concentration in the reaction mix generated during the conversion of xanthine and oxygen to uric acid and hydrogen peroxide by xanthine oxidase (supplied in the kit). Each sample was loaded in a 96-well plate to the final amount of 10 μg/well. Percent inhibition of the rate of WST-1-formazan formation was carried out using a readout at 450 nm. In order to determine MnSOD (SOD2) activity, we preincubated cell lysates on ice with 2 mM potassium cyanide for 1 h to inhibit SOD1 and SOD3 (CuZnSODs). MnSOD activity per microgram of protein was measured from each triplicate separately and was plotted in a bar graph where the Y-axis represented units of SOD/µg of protein. MnSOD activity assay was performed using n = 5 MHEC cell cultures. Each batch of MHEC was prepared using pulled hearts from three transgenic animals/group per group per time point.
2.14 Cardiomyocyte isolation
Cardiomyocytes were isolated following published protocols. In brief, mice were anesthetized using the approved institutional IACUC protocol and hearts were subject to digestion buffer containing 30 mL perfusion buffer containing 400 U/ml collagenase II (Worthington Bioproducts, Lakewood, NJ) and 0.2% BSA for 20 min. Cells were allowed to pellet, the supernatant was removed and cells were resuspended in increasing concentrations of Ca2+.47,48
2.15 Statistical analyses
All values are presented as mean ± SEM where appropriate. A value of P < 0.05 between experimental groups was considered to represent a significant difference (*). Non-linear regression modelling utilizing the extra sum-of-squares F test to compare slopes (Prism 5. Graph Pad Software, San Deigo, CA) was used for vessel relaxation assays. ANOVA and Tukey's post-hoc test were carried out for comparison between groups. Significance of experimental data involving two or more factors was determined using two-way ANOVA.
3. Results
3.1 Effects of short-term (8 weeks) vs. long-term (20 weeks) overexpression of NOX2 on ROS levels in coronary endothelial cells
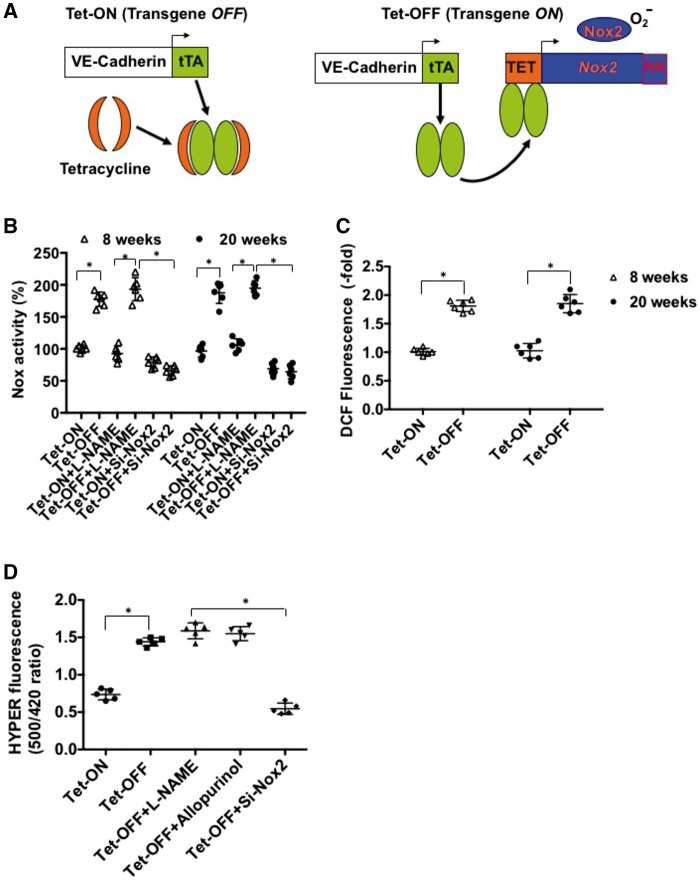
A novel binary transgenic NVF (Tet-Nox2:VE-Cad-tTA) animal model was established, which upon withdrawal of tetracycline from the drinking water (Tet-OFF) for 2 weeks induces the expression of the transgene Nox2/gp91phox (a major component of NADPH oxidase complex) in endothelium-specific manner under the guidance of VE-Cadherin promoter (Figure 1A). Tet-OFF animals (without tetracycline) induced Nox2 specifically in the endothelium of the transgenic mice for up to 20 weeks as previously reported35 (see Supplementary material online, Figure S1 shows Tet-OFF for 8 weeks). In order to examine the effects of Nox2 overexpression on endogenous ROS levels at different time points in ECs (for 8 and 20 weeks), mouse heart endothelial cells (MHEC) were isolated from two independent binary transgenic mouse lines as described.49 MHECs isolated from Tet-ON (control) and Tet-OFF animals were grown in medium containing tetracycline (2 μg/mL) and without tetracycline, respectively. To determine whether EC-specific Nox2 overexpression increased NADPH oxidase activity and total cellular ROS levels, low-concentration lucigenin-based NADPH oxidase assay50 and DCF-DA fluorescence assays35 were performed, respectively. Tet-OFF MHEC from animals that were without tetracycline for 8 (short-term) and 20 (long-term) weeks showed 1.8 ± 0.34-fold and 1.76 ± 0.28-fold increase in NADPH oxidase activity, respectively, compared with their Tet-ON (control) counterparts (Figure 1B). Corresponding increase in total cellular ROS levels were measured by H2-DCFDA as 2.0 ± 0.44-fold and 1.88 ± 0.36-fold in 8-week and 20-week Tet-OFF MHEC, respectively (Figure 1C). H2O2-sensitive intracellular fluorescence sensor HYPER41,51 demonstrated a significant increase in ROS in Tet-OFF (at 8 and 20 weeks) compared with Tet-ON control MHECs (Figure 1D). It may be noted that as measured by DCF-DA, a two-fold increase in fluorescence may not actually reflect two-fold increase in ROS in these cells. However, an increase in ratiometric analysis of ROS measurement by pC-Hyper adds additional support to an increase in ROS in our Tet-OFF animals as pC-Hyper is considered to be a more reliable measure for ROS levels. To demonstrate specificity, siRNA against Nox2 but not L-NAME (NO inhibitor) or allopurinol (xanthine oxidase inhibitor) was shown to inhibit ROS levels (fluorescence) in Tet-OFF MHEC (Figure 1D). These results suggest that overexpression of Nox2 for short term and long term resulted in similarly increased NADPH oxidase activity and ROS levels in ECs. Of note, there were no significant changes in the expression levels of other components of NADPH oxidase complex tested such p47phox and p22phox in Tet-OFF MHEC (data not shown). Together, the findings suggest that short-term (8 weeks) and long-term (20 weeks) overexpression of endothelium-specific Nox2 in the binary transgenic mouse (NVF, Tet-OFF) increases comparable levels of endogenous ROS in MHEC.
Figure 1.
Endothelium-specific overexpression of Nox2 and maintenance of increased ROS generation in coronary ECs for 8 (short-term)–20 (long-term) weeks. (A) Schematic presentation of conditional binary transgenic mice (NVF) generation. Tet-ON, tetracycline was added to the drinking water to suppress expression of the Tet-Nox2 transgene (left panel); Tet-OFF, withdrawal of tetracycline to induce the transgene (right panel). (B) NADPH oxidase activity in ECs isolated from mouse hearts (MHEC) was measured using low-concentration lucigenin (5 μM) and 100 µM NADPH. Tet-OFF animals were without tetracycline for eight or 20 weeks as indicated. MHEC were transfected with siRNA against Nox2 or pre-incubated with NOS-inhibitor l-NAME (500 μM), where indicated. (C) H2DCF-DA fluorescence assay as a measure of total intracellular ROS in MHECs. (D) H2O2-sensitive Hyper-2 probe was used to measure intracellular ROS in MHECs as indicated. Where indicated, MHEC Tet-OFF for 20 weeks were pre-incubated with L-NAME (NO synthase inhibitor; 500 µM), allopurinol (xanthine oxidase inhibitor; 100 μM) or transfeted with si-Nox2 for specificity of the probe. Tet-ON MHECs were grown in medium containing tetracycline 2 μg/mL. All assays were carried out in triplicate. Scatter plot data shown are of triplicate experiments using independent replicates of n = 5 or more batches of ECs as indicated. *P < 0.05 (N ≥ 5/time point/group).
3.2 Shot-term and long-term increase in NOX-ROS resulted in activation of AMPK and eNOS
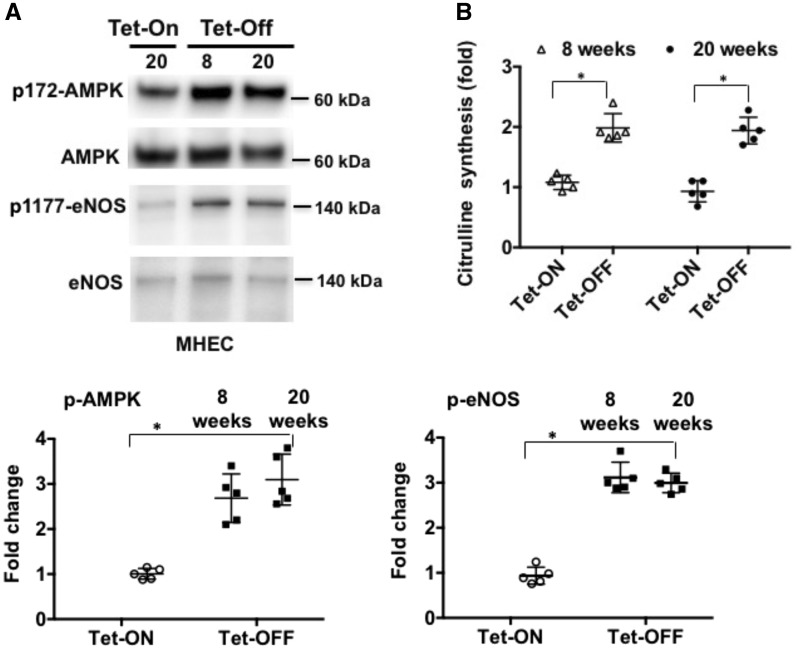
We have previously shown that increased ROS due to endothelium-specific overexpression of Nox2 for short-term induced AMPK-mediated activation of eNOS resulting in increased levels of nitric oxide (NO) in ECs.35 To compare the effects of short-term vs. long-term exposure of ECs to increased levels of NOX-ROS, protein lysates from MHEC derived from Tet-ON and Tet-OFF (8 weeks or 20 weeks with and without tetracycline, respectively) transgenic animals were subject to Western blot analysis. MHECs from 8-week Tet-OFF and 20-week Tet-OFF animals demonstrated increased phosphorylation of AMPK and eNOS (Figure 2A) compared with MHEC from Tet-ON (control) animals. There were no significant differences in the phosphorylation levels of AMPK and eNOS in MHEC from NVF Tet-OFF 8 weeks vs. 20 weeks animals, suggesting that ROS induce activation of AMPK and eNOS irrespective of the duration of increased redox levels in ECs. A corresponding increase in NO was also observed in MHEC from Tet-OFF animals (both 8 and 20 weeks Tet-OFF) compared with Tet-ON (control) animals (Figure 2B and see Supplementary material online, Figure S2A). This ROS-induced phosphorylation of eNOS occurs through AMPK in ECs (see Supplementary material online, Figure 2B).
Figure 2.
Both short (8 weeks)- and long (20 weeks)-term increase in endogenous ROS levels activate AMPK and eNOS in ECs. (A) Western blots (WB) of MHEC protein lysates from NVF Tet-ON (20 weeks) and Tet-OFF (8 and 20 weeks) mice were performed as indicated. WB was carried out using anti-phospho 172-AMPK (p-AMPK), anti-p-eNOS (ser1179) and anti-eNOS and anti-AMPK antibodies. Tet-ON MHECs were grown in culture medium containing tetracycline 2µg/mL. Lower panels demonstrate quantitative analyses of p-AMPK and p-eNOS activation in n = 5 independent batches of isolated MHECs performed in triplicates (n= 3 hearts pooled from thre animals per batch of MHEC isolation) and NIH Image J. Densitometry data of p-eNOS and p-AMPK were normalized against total eNOS and total AMPK levels, respectively. *P < 0.05. (B) Nitric oxide (NO) synthesis, as measured using l-NAME (500 µM)-inhibitable citrulline formation assay as described in the Methods section, in 8-week and 20-week Tet-OFF MHEC. Experiments were performed in triplicates using independent batches of MHECs (n = 5) per group and per time points. *P < 0.05.
3.3 Differential effects of short-term vs. long-term ROS increase on coronary endothelial function
Next, we wanted to determine the effects of short vs. long exposure of ECs to increased levels of endogenous ROS on coronary endothelial function. Coronary microvessels were isolated from the hearts of Tet-ON (control) and Tet-OFF (high ROS) animals for microvessel reactivity assay as a measure of endothelium-dependent coronary vasodilatation. Endothelium-dependent vasodilatation of coronary blood vessels from age- and sex-matched Tet-Nox2:VE-Cad-tTA Tet-ON (n = 6) and Tet-OFF (Tet-OFF for 8 and 20 weeks; n = 6/age group) mice were analysed as described.33 Acetylcholine (Ach) and VEGF were used as EC agonists, and NO-donor sodium–nitroprusside (SNP) was used as control for vascular smooth muscle function. Coronary microvessels from transgenic animals that were Tet-OFF for 8 weeks (i.e. with high NOX-ROS) demonstrated 24±4.6% increase in endothelium-dependent vasorelaxation compared with their control littermates (Figure 3A and B). Interestingly, coronary microvessels from animals that were Tet-OFF for 20 weeks demonstrated 44±6.2% reduction in vasorelaxation in response to Ach and VEGF as compared with Tet-ON littermates (Figure 3C and D), whereas SNP response was similar in both groups (see Supplementary material online, Figure S3). Together, these data suggest that whereas short-term increase in endothelium-specific NOX-derived ROS enhances endothelium-dependent vasorelaxation, sustained elevation in NOX-ROS (beyond 20 weeks) results in a significant decrease in coronary vasorelaxation. Since increased superoxide and NO are known to generate peroxynitrite (OONO-), next we examined whether sustained (long-term) elevation in endogenous ROS in Tet-OFF animals increased OONO− levels in EC. Using Nitrotyrosine assay kit (Cell Biolabs, San Diego, CA), MHEC that were exposed to long-term increase in ROS demonstrated more than two-fold increase in OONO- compared to MHEC from short-term (8 weeks) Tet-OFF animals (Supplementary material online, Figure S4). Together, these findings suggest that whereas short-term ROS increase results in NO-mediated coronary vasorelaxation, long-term increase in ROS induce OONO− formation in EC resulting in the net outcome of inhibition of coronary endothelial function.
Figure 3.
Short-term increase in endogenous Nox-ROS improves coronary vasodilatation but long-term increase in Nox-ROS does not. (A) Endothelium-dependent dilation of coronary arterioles from Tet-ON (n = 8 animals per group per time points) and Tet-OFF (for 8 weeks) (n = 8 animals per group per time points) NVF mice in response to VEGF. (B) Endothelium-dependent dilation of coronary arterioles from Tet-ON (n = 8) and Tet-OFF (for 8 weeks) (n = 8) NVF mice in response to acetylcholine (Ach). (C) Endothelium-dependent dilation of coronary arterioles from Tet-ON (n = 6) and Tet-OFF (for 20 weeks) (n = 6) NVF mice in response to VEGF. (D) Endothelium-dependent dilation of coronary arterioles from Tet-ON (n = 6) and Tet-OFF (for 20 weeks) (n = 6) NVF mice in response to acetylcholine (Ach). All coronary vessels were pre-constricted ex vivo using U4669 prior to the addition of VEGF or Ach as indicated.
Next we wanted to examine whether long-term exposure to NOX-derived ROS resulted in uncoupling of eNOS. To that end, we performed experiments to determine tyrosine phosphorylation (at Y657 residue) or S-glutathionylation of eNOS in MHECs. Interestingly, MHECs from animals that were exposed to short-term or long-term EC-ROS did not show any significant increase in Y657 phosphorylation and S-glutathionylation (Supplementary material online, Figure S5), suggesting that EC-specific increase (∼2-fold) in NOX-ROS does not uncouple eNOS via Y657 phosphorylation or S-glutathionylation in this transgenic animal model.
3.4 Sustained increase in NOX-ROS results in decreased endothelial proliferation and angiogenic sprouting compared with short-term elevation in ROS
Since sustained exposure to ROS affected endothelium-dependent vasorelaxation, we wanted to examine whether cell proliferation, another critical function of EC, was differentially regulated by short-term vs. long-term exposure to ROS. MHEC from Tet-ON and Tet-OFF mice (8 weeks and 20 weeks Tet-OFF) were subject to proliferation assay using Click-iT EdU Kit (ThermoFisher Scientific, Waltham, MA). Exposure to increased ROS for 8 weeks resulted in increased EC proliferation. In contrast, increased ROS for 20 weeks inhibited EC proliferation (Figure 4A).
Figure 4.
Cell proliferation increased in ECs that were exposed to high levels of endogenous ROS for 8 weeks but not 20 weeks. (A) MHEC from Tet-ON and Tet-OFF mice (for 8 and 20 weeks, n = 6/group per time points) as indicated was subject to proliferation assay using incorporation of EdU in the DNA of ECs. *P < 0.05. (B) Aortic sprouting assays were carried out ex vivo using aortic ring from Tet-ON and Tet-OFF (for 8 and 20 weeks as indicated) transgenic animals (n = 6 aortae per group per time points, using n ≥ 4 pieces from each aorta). Lower panel shows quantitative analysis of the sprouting area, sprouting density and branching index using Angiotool software as described in the Methods section. Experiments were carried using n = 6 animals per group per time point. *P < 0.05.
Next, we wanted to determine whether duration of ROS exposure has differential effects on sprouting angiogenesis. To that end, we performed ex vivo aortic ring sprouting assays using aortae from Tet-ON and Tet-OFF animals. Increased endothelium-specific ROS for 8 weeks significantly increased sprouting area and vessel density, but aorta with increased ROS for 20 weeks did not (Figure 4B). Together with the EC proliferation data, these findings suggest that whereas short-term ROS increase improves EC proliferation and angiogenic sprouting, long-term ROS exposure does not.
3.5 Sustained elevation in NOX-ROS increases mitochondrial ROS and reduces mitochondrial membrane potential (Δψm)
We consistently observed that increase in endothelium-specific NADPH oxidase-derived ROS in our transgenic animal resulted in overexpression of mitochondrial antioxidant MnSOD (Supplementary material online, Figure S6A), suggesting plausible modulation of subcellular ROS levels in the mitochondria of these ECs. Therefore, we wanted to determine mitochondrial (mito)-ROS levels in ECs of NVF Tet-OFF animals. Mito-ROS levels were increased in ECs from both short-term and long-term Tet-OFF NVF animals. However, short-term Tet-OFF ECs demonstrated a moderately increased (1.98±0.26) and long-term Tet-OFF showed a very high (4.2±0.83) level of mito-ROS (Figure 5A). Changes in mitochondrial ROS are known to have effects on mitochondrial activity. Depolarization of the membrane is a measure of inhibition of mitochondrial activity. In order to examine whether mitochondrial membrane potential (Δψm) was affected, we performed TMRE-based membrane potential assay using ECs isolated from the transgenic animals that were Tet-ON or Tet-OFF for 8 weeks and 20 weeks. Although there were no significant differences in Δψm in ECs from animals that were exposed to high ROS for 8 weeks compared with their Tet-ON counterparts, there was significant reduction in Δψm in ECs from animals that were Tet-OFF for 20 weeks (Figure 5B), suggesting that sustained increase in NOX-ROS results in reduction in mitochondrial membrane potential. Next, we wanted to determine whether increased mito-ROS had any effect on mitochondrial DNA (mtDNA) content, another measure of mitochondrial number and function in cells. RT-PCR assay for mitochondrially encoded gene, mt-Co1 (cytochrome c oxidase I), was analysed against 18S rRNA to determine mt-DNA content. RT-PCR demonstrated a significant reduction in mitochondrial DNA in MHEC exposed to higher redox content for long-term (20 weeks) but not for short-term (Figure 5C). These results suggest that higher levels of NADPH oxidase-derived ROS initially (for up to 8 weeks) increase mito-ROS, expression of antioxidant MnSOD and mt-DNA content but it does not have any effect on Δψm in ECs. In contrast, increased cytosolic ROS for 20 weeks results in very high levels of mito-ROS as well as decrease in Δψm and DNA content, suggesting adverse effects of sustained or chronic increase in NADPH oxidase-derived ROS on mitochondrial function in ECs.
Figure 5.
NADPH oxidase-derived cytosolic Nox-ROS induce increase in mitochondrial ROS and decrease in mitochondrial membrane potential (Δψm) in MHEC. (A) Mitochondrial ROS assay. MHEC isolated from Tet-ON control (20 weeks) and Tet-OFF (8 and 20 weeks) were loaded with mitochondrial marker mitotracker (green, Lifetechnologies) and marker for mitochondrial ROS mito-Sox (1 μM, red, Lifetechnologies). Upper panels demonstrate green-red superimposition of MHEC as indicated. Lower panel shows bar graph obtained using red immunofluorescence, normalized against EC number, and expressed as fold-changes. 20 weeks of increased NADPH oxidase-derived ROS induced almost two-fold increase in mitochondrial ROS as compared to 8 weeks of increased cytosolic ROS. *P < 0.05. n = 5 experiments each using independent batches of MHEC per group per time point, and each using triplicate samples. (B) Mitochondrial membrane potential (Δψm) assay. Tet-ON and Tet-OFF NVF MHEC with high NADPH oxidase-derived ROS for 8 and 20 weeks were grown for 24 h in a 96-well black bottom plate. Cells were washed with PBS and loaded with 20 nM TMRE (Invitrogen, Carlsbad, CA) for 10 min at 37 °C and fluorescence was measured using a microplate reader. Data points were plotted against respective FCCP (maximum depolarization) value as per manufacturer’s protocol. n = 5 experiments each using independent batches of MHEC per group per time point, and each using triplicate samples. *P < 0.05. (C) Mitochondrial DNA content measurement. RT-PCR was carried out using total RNA prepared from MHEC as indicated from Tet-ON and Tet-OFF (8 and 20 weeks) transgenic animals. Mitochondrial gene, mt-Co1, was analysed against 18S rRNA to determine mt-DNA content. n = 5 experiments each using independent batches of MHEC per group per time point, and each using triplicate samples. *P < 0.05.
These findings were intriguing as there were no significant differences in mitochondrial antioxidant protein MnSOD levels between ECs from short-term and long-term Tet-OFF animals (Supplementary material online, Figure S6A, compare Tet-OFF 8 weeks vs. 20 weeks). This led us to determine whether there were any changes in the functional activity of MnSOD in these two groups of ECs. Since sustained (long-term) increase in ROS resulted in increased peroxynitrite in ECs compared to ECs with short-term ROS exposure (Supplementary material online, Figure S4), and nitration of the tyrosine residue 34 (Tyr-34) at MnSOD is known to have inhibitory effects on the protein’s activity,52 we performed assays to determine nitro-tyrosine modification of MnSOD in endothelial cell lysates. Immunoprecipitation with anti-nitrotyrosine antibody followed by immunoblot using anti-MnSOD antibody showed significantly increased levels of nitration modification of Tyr-34 at MnSOD in ECs from NVF animals that were exposed to sustained increase (20 weeks) in NADPH oxidase-derived ROS compared with ECs with short-term (8 weeks) ROS exposure (Supplementary material online, Figure S6B). Corresponding MnSOD activity assay confirmed significant inhibition of MnSOD activity in ECs from animals that were exposed to long-term NADPH oxidase-derived ROS (Supplementary material online, Figure S6C). Taken together, these findings suggest that, in contrast to short-term increase in cytosolic ROS, sustained or long-term increase in NADPH oxidase-derived ROS exerts adverse effects on mitochondrial function through tyrosine inactivation of MnSOD in ECs.
Inner membrane mitochondrial Permeability Transition Pores (mPTP) are known to be affected by increased ROS. In order to determine whether increased NOX-derived ROS resulted in changes in mPTPs, isolated mitochondria from MHECs from Tet-ON and Tet-OFF mice that were exposed to short-term and long-term ROS were examined for decrease in light scattering at 525 nM for mitochondrial swelling as an indicator of mPTP opening. MHEC exposed to long-term EC-ROS demonstrated significant decrease in light scattering compared to short-term EC-ROS (Supplementary material online, Figure S7), suggesting mitochondrial swelling in long-term ROS MHEC. mPTP inhibitor cyclosporine A (CsA) blocked mitochondrial swelling in these MHEC, further suggesting an important role for mPTP in NOX-ROS-derived effects on EC mitochondria.
3.6 Reduction in mito-ROS partially restores EC proliferation and sprouting angiogenesis in the aorta of animals with high NOX-ROS
In order to examine whether NOX-ROS-induced increase in mito-ROS is responsible for inhibition of EC proliferation, Tet-OFF MHEC were pre-incubated with 100 nM mito-TEMPO (an inhibitor of mito-ROS) and were subject to EC proliferation assay as described in Methods section. Mito-TEMPO-treated MHEC from long-term NOX-ROS animals resulted in a significant increase in EC proliferation (Figure 6A), suggesting that an increase in mito-ROS plays a role, at least in part, in the inhibition of proliferation in ECs that are exposed to increased levels of NOX-ROS for 20 weeks.
Figure 6.
Reduction in mito-ROS partially reverses the adverse effects of long-term Nox-ROS exposure on ECs. (A) MHEC were pre-treated with mitochondrial ROS inhibitor mito-TEMPO (100 nM) and were then subject to EC proliferation assay as indicated. *P < 0.05. n = 6 experiments each using independent batches of MHEC per group per time point, and each using triplicate samples. (B) Aortic sprouting assay using mito-TEMPO as described in the Methods section. n = 6 aortae per group per time points. Lower panel shows quantitative analysis of the sprouting area using Angiotool software as described in the Methods section. Experiments were carried using n = 6 animals per group per time point using n ≥ 4 pieces from each aorta. *P < 0.05.
We next wanted to examine the effects of reduction in mito-ROS on sprouting angiogenesis assay using aorta from the NVF Tet-OFF (long-term) animals. Incubation with mito-TEMPO partially restored sprouting angiogenesis in aorta from the transgenic animals that were exposed to NOX-ROS for 20 weeks (Figure 6B), suggesting that higher levels of mito-ROS, at least in part, result in reduced aortic sprouting angiogenesis. Furthermore, in order to confirm that majority of cells in aortic sprouts are ECs, we performed immunofluorescence studies on the frozen sections of the aortic sprouts using anti-CD31 antibody (Supplementary material online, Figure S8).
4. Discussion
We demonstrate that above-physiological levels of EC-specific NADPH oxidase-derived ROS in vivo exert distinct beneficial and adverse effects on vascular endothelium depending on the duration of the ROS exposure and on subcellular ROS levels in mitochondria. Whereas short-term ROS (up to 8 weeks) induced activation of AMPK and eNOS resulting in an increased endothelium-dependent coronary vasorelaxation, longer-term ROS exposure (20 weeks) resulted in oxidative damages to EC by increasing ONOO− formation, inactivation of mitochondrial antioxidant MnSOD, increased mitochondrial ROS and decreased mitochondrial membrane potential (Supplementary material online, Figure S7). An increase in peroxynitrite and mitochondrial dysfunction due to sustained (long-term) elevation in endogenous ROS in the cytosol of EC may have resulted in decreased endothelium-dependent vasorelaxation and EC proliferation.
The data presented in this study were obtained using conditional increase (1.8–2-fold) in NAPDH oxidase-derived ROS in a vascular endothelium-specific manner for 8–20 weeks. There were no changes observed in gross phenotype including blood pressure in these animals. These finding are in accordance with the findings of the Channon and the Shah groups; Tie2 promoter-based constitutive overexpression of Nox2 did not modulate basal blood pressure in their transgenic animals.53,54 The absence of any adverse effects on endothelium during the short-term ROS exposure was very interesting. These findings point to the existence of an endothelial compensatory mechanism(s) that prevents oxidative damages to the vasculature during the short-term (8 weeks) exposure to ROS. Increased expression of antioxidant MnSOD that protects mitochondria from oxidative insults, and increased synthesis of NO, appears to play critical roles in resetting endothelial signalling pathways during short-term increase in oxidant levels. It is thus plausible that the differential effects of short- and long-term ROS exposure on EC may have emanated from the compensatory mechanism of the mitochondria. At a later time point (20 weeks), this protective mechanism probably fails to protect ECs as tyrosine nitration and inactivation of MnSOD results in several fold increases in mito-ROS and loss of membrane potential (Figure 7, proposed model). It is plausible that increased mito-ROS disrupts the electron transport chain (ETC) in the mitochondria, which in turn reduces mitochondrial membrane potential. However, although it is known that mitochondrial dysfunction reduces dNTP synthesis,55 the data presented in this study do not establish a direct link between mitochondrial dysfunction and loss of EC proliferation in animals exposed to sustained high levels of ROS. Further studies are required to address this issue.
Figure 7.
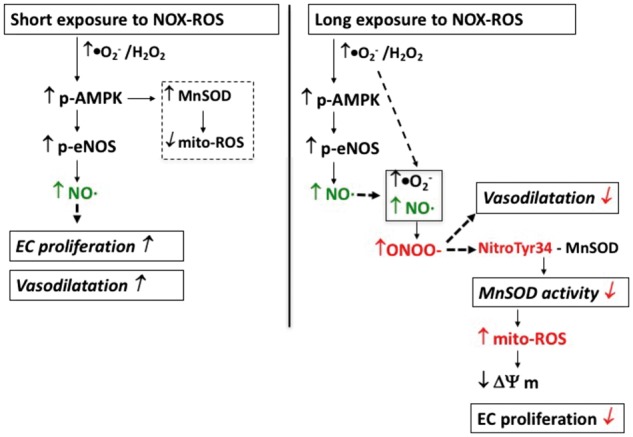
Schematic presentation showing the effects of short-term and long-term increase in endogenous NADPH oxidase-derived ROS increase on vascular endothelium. Left panel: Nox2-induced short-term increase in EC-specific cytosolic ROS activates AMPK-eNOS axis, which in turn, induces NO-mediated vasodilatation and proliferation. Short-term ROS also induce MnSOD expression via activation of AMPK, which in turn reduces increased mitochondrial ROS in proliferating EC. Right panel: long-term increase in endogenous cytosolic ROS induces peroxynitrite-mediated inhibition of vasodilatation and nitro-tyrosine-mediated inactivation of mitochondrial antioxidant MnSOD resulting in increased mito-ROS and decreased Δψm. Mitochondrial dysfunction then leads to inhibition of proliferation and oxidative damage to EC.
The finding that overexpression of Nox2 was not accompanied with an increase in p22phox is interesting as there are reports of 1:1 stoichiometry between Nox2 and p22phox. However, majority of the previous stoichiometric studies were performed using neutrophil or phagocytic cells where the authors showed p22phox:gp91phox (Nox2) ratio to be 1: 1.156 or 1: 157 in membrane-bound cytochrome b558 subunit of the NADPH oxidase complex. It may be noted that there are several distinct differences between NADPH oxidase (NOX) in the phagocytic and non-phagocytic cells including endothelial cells (EC)18 such as (i) unlike phagocytic cells, non-phagocytic NOX is present, in addition to cell membrane, in perinuclear and other intracellular membranes e.g. endoplasmic reticulum, (ii) whereas non-phagocytic NOX is pre-assembled and generate ambient levels of ROS intracellularly, phagocytic NOX enzyme complex does not have any basal activity and is only formed upon stimulation to generate extracellular (within the phagosome) ROS, (iii) phagocytic ROS generation is fast (within seconds) and at the millimolar levels but non-phagocytic NOX is slow to produce ROS at the micromollar levels. Thus, there may be some stoichiometric differences between the ratio of p22phox and gp92phox (Nox2) in EC and phagocytic NADPH oxidase. Careful structural studies are required to address this issue in future.
The data presented in this study showing an increased level of OONO- in the 20-week model as compared to the 8-week model (Supplementary material online, Figure S4) suggest an increase in peroxynitrite accumulation (and thus decrease in NO bioavailability) as a reason, at least in part, for the loss of coronary vasorelaxation in the 20-week animal model. However, our data do not exclude the possibility of an altered rate of peroxynitrite formation and thus decreased bioavailability of NO as a possible mechanism of inhibition of endothelial function in the 20-week animals. Our proposed model suggests that prolonged ROS exposure, even in the presence of increased eNOS activity (and NO synthesis), may give rise to increased accumulation of OONO- in ECs (Figure 7).
We have also shown differential effects of short-term vs. long-term ROS exposure on EC proliferation. Our in vitro EC proliferation data and ex vivo aortic sprouting angiogenesis assay demonstrated significant differences between transgenic animals that were exposed to increased EC-ROS for 8 and 20 weeks. Together, these findings demonstrated that an increase in ROS for short-term (8 weeks) but not long-term (20 weeks) induces EC proliferation and sprouting angiogenesis.
The findings that increased EC-ROS results in increased expression of mitochondrial antioxidant enzymes (e.g. SOD2) and activation of AMPK (an AMP:ATP sensor, mito-ROS sensor) enhances the probability of altered mitochondrial function and/or ROS/Δψm levels.58–65 Future studies to measure mitochondrial oxygen consumption (as a marker for oxidative phosphorylation),66,67 and lactic acid formation (marker for glycolysis)68 will examine the functional role of mitochondria in NOX-ROS-induced phenotypic changes in ECs. However, we do not expect to see any significant changes in mitochondrial oxygen consumption/demand in Tet-ON and Tet-OFF (8 weeks) MHEC as ECs do not depend on mitochondrial oxidative phosphorylation for energy (ATP) production.69
The findings in the current study that eNOS is phosphorylated and activated by pAMPK are not novel. There are reports of activation of eNOS and NO generation by several different non-Akt kinases such as AMPK and PKA in ECs and cardiovascular cells.70–75 Interestingly, in the current study, the levels of activation of the survival kinase AMPK and eNOS remained same throughout the duration (8–20 weeks) of the exposure of EC to increased levels of endogenous ROS. The findings reported in this article suggest that NADPH oxidase-derived increase in endothelium-specific ROS initially induces protective mechanisms in coronary endothelium by activating AMPK, eNOS, and inducing MnSOD expression. This protective role of ROS is reversed with time, i.e. with longer exposure of EC to ROS, because of the accumulation of ONOO− that results in nitrotyrosine-mediated inhibition of MnSOD. This, in turn, results in the loss of mitochondrial protection from higher oxidant levels in EC. The data presented in this study point to a critical role for mitochondrial redox content in shifting the balance of cytosolic ROS from being beneficial to harmful. Further studies are required to elucidate the mechanisms by which cytosolic ROS modulate mitochondrial ROS levels and function. There are reports showing that the interaction between NOX-ROS and mitochondria occurs through several different pathways including mPTP and ion channels in mitochondria.76–80 Using animal models treated with mitoTEMPO and with global overexpression of MnSOD, the Dikalov group demonstrated that mitochondria play a critical role in Ang-II-induced hypertension and vascular oxidative stress77. The Daiber group showed that mito-ROS activates NADPH oxidase in phagocytes and cardiovascular tissue resulting in vascular dysfunction; they have also demonstrated that reduction in MnSOD activity and increase in mito-ROS exacerbated endothelial dysfunction and uncoupling of eNOS through mPTP.76 Our preliminary findings presented in this study support a role for mPTP through which NOX-ROS may affect EC mitochondria. However, further studies are required in future to determine the precise mechanisms involved in NOX-mito-ROS crosstalk in ECs with endothelium-specific temporal increase in NOX-ROS. Interestingly, increased NOX-derived EC ROS in our binary conditional animal model did not demonstrate evidence of eNOS uncoupling as examined by Y657 phosphorylation (inhibitory phosphorylation site) and S-glutathionylation of eNOS using MHEC. Although it may appear in apparent contradiction with the reports that demonstrated ROS inhibited/uncoupled eNOS by phosphorylation of its Y657 residue through a PYK2-dependent manner,81 the apparent discrepancies between the findings of these studies may be explained by the following. Whereas the other studies used exogenous H2O2 and agonist-induced (Ang-II) increase in ROS in animal and cell culture models, the current study uses a genetic model that results in increase (∼2-fold) in NOX-ROS in a vascular endothelium-specific manner. It is plausible that eNOS activation (or uncoupling) depends on the type of agonists and/or subcellular localization/sources of ROS; for example, PYK2 plays a central positive role in vascular endothelial growth factor (VEGF)-induced activation of Akt-eNOS in ECs and in a hind-limb ischemia model.82
These findings also raise several important translational questions to be studied in future: (i) Does cytosolic-mitochondrial ROS axis modulate endothelial cell growth and proliferation in vivo, such as angiogenesis in post-infarct or chronically ischemic myocardium that often has higher ROS levels? (ii) What effects do increased ROS levels have on AMPK-regulated signalling intermediates and transcription factors in vascular endothelium, namely PGC-1α, HIF-1α and FOXO1, which are known to be involved in mitochondrial biogenesis and MnSOD expression? (iii) Does higher redox state alter metabolism in ECs including mitochondrial oxidative phosphorylation, oxygen consumption and dNTP synthesis? (iv) Can targeting subcellular source-specific ROS by antioxidants revert pathological changes that have been caused by the prolonged increase in oxidants (such as in diabetes, chronic myocardial ischemia)? Ongoing studies in our lab are addressing these important questions of pathophysiological significance.
Finally, the finding that tyrosine nitration modification of the mitochondrial antioxidant MnSOD depends on the duration of endothelial exposure to NADPH oxidase-derived ROS advances our current understanding of temporal roles of ROS in the pathological processes. The current study suggests that the mitochondrial ROS balance is critical in exerting the differential effects (good vs. bad) of NADPH oxidase-derived ROS on vascular endothelium and will have great impact on the future development of subcellular compartment-specific therapeutic modalities in cardiovascular disease.
Supplementary Material
Acknowledgements
We would like to thank the Microscope Core Facility of Rhode Island Hospital and the Cardiopulmonary Vascular Biology (CPVB) COBRE Cell Signaling and Organ Function Core (P20 GM103652).
Conflict of interest: none declared.
Funding
This work was supported by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20 GM103652, Project# 3 to M.R.A.) and American Heart Association (AHA) Grant-in-Aid 14GRNT20460291 (to M.R.A.); Brazilian government grants 2011/14550-7 and 2012/09130-1 (to A.T. and K.R.); National Heart, Lung, and Blood Institute (NHLBI) Grant HL46716 (to FWS); and NHLBI Grant R25 HL088992 (supported V.R. to E.O.H.).
References
- 1.San Martin A, Griendling KK. NADPH oxidases: progress and opportunities. Antioxid Redox Signal 2014;20:2692–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol 2003;91:7A–11A. [DOI] [PubMed] [Google Scholar]
- 3.Irani K. Oxidant signaling in vascular cell growth, death, and survival : a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res 2000;87:179–183. [DOI] [PubMed] [Google Scholar]
- 4.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J 2001;15:2548–2550. [DOI] [PubMed] [Google Scholar]
- 5.Moldovan L, Irani K, Moldovan NI, Finkel T, Goldschmidt-Clermont PJ. The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxid Redox Signal 1999;1:29–43. [DOI] [PubMed] [Google Scholar]
- 6.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 2002;91:1160–1167. [DOI] [PubMed] [Google Scholar]
- 7.Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol 2000;20:1903–1911. [DOI] [PubMed] [Google Scholar]
- 8.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 2003;107:1383–1389. [DOI] [PubMed] [Google Scholar]
- 9.Alami R, Greally JM, Tanimoto K, Hwang S, Feng YQ, Engel JD, Fiering S, Bouhassira EE. Beta-globin YAC transgenes exhibit uniform expression levels but position effect variegation in mice. Hum Mol Genet 2000;9:631–636. [DOI] [PubMed] [Google Scholar]
- 10.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A 2002;99:715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 2002;90:E58–E65. [DOI] [PubMed] [Google Scholar]
- 12.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res 2003;93:767–775. [DOI] [PubMed] [Google Scholar]
- 13.Brandes RP. Role of NADPH oxidases in the control of vascular gene expression. Antioxid Redox Signal 2003;5:803–811. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi S, Nojima Y, Shibuya M, Maru Y. Nox1 regulates apoptosis and potentially stimulates branching morphogenesis in sinusoidal endothelial cells. Exp Cell Res 2004;300:455–462. [DOI] [PubMed] [Google Scholar]
- 15.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 2006;8:1473–1484. [DOI] [PubMed] [Google Scholar]
- 16.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 2005;7:308–317. [DOI] [PubMed] [Google Scholar]
- 17.McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler Thromb Vasc Biol 2005;25:1623–1628. [DOI] [PubMed] [Google Scholar]
- 18.Li JM, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol 2003;14:S221–S226. [DOI] [PubMed] [Google Scholar]
- 19.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 2010;30:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 2010;56:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, Wilcox CS. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension 2010;56:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 2011;15:1583–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 2009;11:791–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 2011;10:453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol 2008;101:75D–86D. [DOI] [PubMed] [Google Scholar]
- 26.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003;361:2017–2023. [DOI] [PubMed] [Google Scholar]
- 27.Virtamo J, Rapola JM, Ripatti S, Heinonen OP, Taylor PR, Albanes D, Huttunen JK. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med 1998;158:668–675. [DOI] [PubMed] [Google Scholar]
- 28.Hegele RA. Angiotensin-converting enzyme (ACE) inhibition in the secondary prevention of vascular disease: the Heart Outcomes Prevention Evaluation (HOPE) Trial and its substudies. Curr Atheroscler Rep 2000;2:361–362. [DOI] [PubMed] [Google Scholar]
- 29.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007;297:842–857. [DOI] [PubMed] [Google Scholar]
- 30.Bagi Z, Feher A, Beleznai T. Preserved coronary arteriolar dilatation in patients with type 2 diabetes mellitus: implications for reactive oxygen species. Pharmacol Rep 2009;61:99–104. [DOI] [PubMed] [Google Scholar]
- 31.Dao VT, Casas AI, Maghzal GJ, Seredenina T, Kaludercic N, Robledinos-Anton N, Di Lisa F, Stocker R, Ghezzi P, Jaquet V, Cuadrado A, Schmidt HH. Pharmacology and clinical drug candidates in redox medicine. Antioxid Redox Signal 2015;23:1113–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt HHHW, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, Cuadrado A. Antioxidants in translational medicine. Antioxid Redox Signal 2015;23:1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng J, Damrauer SM, Lee M, Sellke FW, Ferran C, Abid R. Endothelium-dependent coronary vasodilatation requires NADPH oxidase – derived reactive oxygen species. Arterioscler Thromb Vasc Biol 2010;30:1703–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol 2008;28:1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafique E, Choy WC, Liu Y, Feng J, Cordeiro B, Lyra A, Arafah M, Yassin-Kassab A, Zanetti A V, Clements RT, Bianchi C, Benjamin LE, Sellke FW, Abid MR. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging (Albany NY) 2013;5:515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abid MR, Yano K, Guo S, Patel VI, Shrikhande G, Spokes KC, Ferran C, Aird WC. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J Biol Chem 2005;280:29864–29873. [DOI] [PubMed] [Google Scholar]
- 37.Dharaneeswaran H, Abid MR, Yuan L, Dupuis D, Beeler D, Spokes KC, Janes L, Sciuto T, Kang PM, Jaminet SC, Dvorak A, Grant MA, Regan ER, Aird WC. FOXO1-mediated activation of Akt plays a critical role in vascular homeostasis. Circ Res 2014;115:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One 2011;6:e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994;74:1141–1148. [DOI] [PubMed] [Google Scholar]
- 40.Abid MR, Schoots IG, Spokes KC, Wu SQ, Mawhinney C, Aird WC. Vascular endothelial growth factor-mediated induction of manganese superoxide dismutase occurs through redox-dependent regulation of forkhead and IkappaB/NF-kappaB. J Biol Chem 2004;279:44030–44038. [DOI] [PubMed] [Google Scholar]
- 41.Markvicheva KN, Bilan DS, Mishina NM, Gorokhovatsky AY, Vinokurov LM, Lukyanov S, Belousov VV. A genetically encoded sensor for H2O2 with expanded dynamic range. Bioorg Med Chem 2011;19:1079–1084. [DOI] [PubMed] [Google Scholar]
- 42.Bilan DS, Belousov VV. HyPer family probes: state of the art. Antioxid Redox Signal 2016;24:731–751. [DOI] [PubMed] [Google Scholar]
- 43.Abid MR, Shih S-C, Otu HH, Spokes KC, Okada Y, Curiel DT, Minami T, Aird WC. A novel class of vascular endothelial growth factor-responsive genes that require forkhead activity for expression. J Biol Chem 2006;281:35544–35553. [DOI] [PubMed] [Google Scholar]
- 44.Correa RM, Lafayette SS, Pereira GJ, Hirata H, Garcez-do-Carmo L, Smaili SS. Mitochondrial involvement in carbachol-induced intracellular Ca2+ mobilization and contraction in rat gastric smooth muscle. Life Sci 2011;89:757–764. [DOI] [PubMed] [Google Scholar]
- 45.Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Weihrauch D, Gutterman DD, Vita JA. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res 2010;156:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011;124:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Readnower RD, Brainard RE, Hill BG, Jones SP. Standardized bioenergetic profiling of adult mouse cardiomyocytes. Physiol Genomics 2012;44 :1208–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clements RT, Cordeiro B, Feng J, Bianchi C, Sellke FW. Rottlerin increases cardiac contractile performance and coronary perfusion through BKca ++ channel activation after cold cardioplegic arrest in isolated hearts. Circulation 2011;124: S55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 2006;10:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abid MR, Spokes KC, Shih S-C, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem 2007;282:35373–35385. [DOI] [PubMed] [Google Scholar]
- 51.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 2006;3:281–286. [DOI] [PubMed] [Google Scholar]
- 52.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys 1999;366:82–88. [DOI] [PubMed] [Google Scholar]
- 53.Douglas G, Bendall JK, Crabtree MJ, Tatham AL, Carter EE, Hale AB, Channon KM. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE(-)/(-) mice. Cardiovasc Res 2012;94:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol 2011;106:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desler C, Lykke A, Rasmussen LJ. The effect of mitochondrial dysfunction on cytosolic nucleotide metabolism. J Nucleic Acids 2010;2010:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang J, Hitt ND, Kleinberg ME. Stoichiometry of p22-phox and gp91-phox in phagocyte cytochrome b558. Biochemistry 1995;34:16753–16757. [DOI] [PubMed] [Google Scholar]
- 57.Wallach TM, Segal AW, Wallach TM, Segal AW. Stoichiometry of the subunits of flavocytochrome b558 of the NADPH oxidase of phagocytes. Biochem J 1996;320:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 2010;468:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca(2+). Am J Physiol Endocrinol Metab 2002;282:E1008–E1013. [DOI] [PubMed] [Google Scholar]
- 60.Wang XR, Zhang MW, Chen DD, Zhang Y, Chen AF. AMP-activated protein kinase rescues the angiogenic functions of endothelial progenitor cells via manganese superoxide dismutase induction in type 1 diabetes. Am J Physiol Endocrinol Metab 2011;300:E1135–E1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun L, Zhao M, Yu XJ, Wang H, He X, Liu JK, Zang WJ. Cardioprotection by acetylcholine: a novel mechanism via mitochondrial biogenesis and function involving the PGC-1alpha pathway. J Cell Physiol 2013;228:1238–1248. [DOI] [PubMed] [Google Scholar]
- 62.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 2012;13:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 2009;104:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L, Chen Y, Gibson SB. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal 2013;25:50–65. [DOI] [PubMed] [Google Scholar]
- 65.Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One 2011;6:e17234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Djafarzadeh S, Vuda M, Takala J, Jakob SM. Effect of remifentanil on mitochondrial oxygen consumption of cultured human hepatocytes. PLoS One 2012;7:e45195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Namasivayam M, Adji A, O’Rourke MF. Influence of aortic pressure wave components determined noninvasively on myocardial oxygen demand in men and women. Hypertension 2011;57:193–200. [DOI] [PubMed] [Google Scholar]
- 68.Frederich M, O’Rourke MR, Furey NB, Jost JA. AMP-activated protein kinase (AMPK) in the rock crab, Cancer irroratus: an early indicator of temperature stress. J Exp Biol 2009;212:722–730. [DOI] [PubMed] [Google Scholar]
- 69.Dromparis P, Michelakis ED. Mitochondria in vascular health and disease. Annu Rev Physiol 2013;75:95–126. [DOI] [PubMed] [Google Scholar]
- 70.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 1999;443:285–289. [DOI] [PubMed] [Google Scholar]
- 71.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 2002;277:3388–3396. [DOI] [PubMed] [Google Scholar]
- 72.Karnewar S, Vasamsetti SB, Gopoju R, Kanugula AK, Ganji SK, Prabhakar S, Rangaraj N, Tupperwar N, Kumar JM, Kotamraju S. Mitochondria-targeted esculetin alleviates mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3 regulation in endothelial cells: potential implications in atherosclerosis. Sci Rep 2016;6:24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han F, Zhang S, Hou N, Wang D, Sun X. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol 2015;309:H1501–H1508. [DOI] [PubMed] [Google Scholar]
- 74.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 2004;279:1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem 2009;284:1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kröller-Schön S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Münzel T, Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 2014;20:247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 2010;107:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dikalov SI, Li W, Doughan AK, Blanco RR, Zafari AM. Mitochondrial reactive oxygen species and calcium uptake regulate activation of phagocytic NADPH oxidase. Am J Physiol Regul Integr Comp Physiol 2012;302:R1134–R1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol 2015; doi:10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta – Bioenerg 2010;1797:897–906. [DOI] [PubMed] [Google Scholar]
- 81.Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med 2009;206:2889–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsui A, Okigaki M, Amano K, Adachi Y, Jin D, Takai S, Yamashita T, Kawashima S, Kurihara T, Miyazaki M, Tateishi K, Matsunaga S, Katsume A, Honshou S, Takahashi T, Matoba S, Kusaba T, Tatsumi T, Matsubara H. Central role of calcium-dependent tyrosine kinase PYK2 in endothelial nitric oxide synthase-mediated angiogenic response and vascular function. Circulation 2007;116:1041–1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.