Abstract
Objective
To describe end-stage renal disease (ESRD) quality of care (receipt of pre-ESRD nephrology care, access to kidney transplantation, and placement of permanent vascular access for dialysis) in US patients with ESRD due to lupus nephritis (LN-ESRD) and to examine whether quality measures differ by patient sociodemographic characteristics or US region.
Methods
National surveillance data on patients in the US in whom treatment for LN-ESRD was initiated between July 2005 and September 2011 (n = 6,594) were analyzed. Odds ratios (ORs) and hazard ratios (HRs) with 95% confidence intervals (95% CIs) were determined for each quality measure, according to sociodemographic factors and US region.
Results
Overall, 71% of the patients received nephrology care prior to ESRD. Black and Hispanic patients were less likely than white patients to receive pre-ESRD care (OR 0.73 [95% CI 0.63–0.85] and OR 0.73 [95% CI 0.60–0.88], respectively) and to be placed on the kidney transplant waitlist within the first year after the start of ESRD (HR 0.78 [95% CI 0.68–0.91] and HR 0.82 [95% CI 0.68–0.98], respectively). Those with Medicaid (HR 0.51 [95% CI 0.44–0.58]) or no insurance (HR 0.36 [95% CI 0.29–0.44]) were less likely than those with private insurance to be placed on the waitlist. Only 24% had a permanent vascular access, and placement was even less likely among the uninsured (OR 0.62 [95% CI 0.49–0.79]). ESRD quality-of-care measures varied 2–3-fold across regions of the US, with patients in the Northeast and Northwest generally having higher probabilities of adequate care.
Conclusion
LN-ESRD patients have suboptimal ESRD care, particularly with regard to placement of dialysis vascular access. Minority race/ethnicity and lack of private insurance are associated with inadequate ESRD care. Further studies are warranted to examine multilevel barriers to, and develop targeted interventions to improve delivery of, care among patients with LN-ESRD.
The Centers for Medicare & Medicaid Services (CMS), which covers end-stage renal disease (ESRD) care for all eligible patients in the US, is highly invested in promoting quality of care, including mandated pay-for-performance (1). Through its 18 ESRD Networks (www.esrdnetworks.org), CMS regionally monitors ESRD care and facilitates quality improvement. Quality of ESRD care is also a priority of Healthy People 2020 (www.healthypeople.gov) (2), supported by evidence that receipt of pre-ESRD care (3–11), access to kidney transplantation (12–17), and permanent vascular accesses for dialysis, which include arteriovenous fistulae (AVFs) and grafts (18–25), are all associated with better patient outcomes and lower health care costs. Since 2005, CMS has collected information addressing these objectives on all patients with incident ESRD via the CMS Medical Evidence Report (CMS Form 2728), which is completed for all patients at the start of ESRD treatment. The data have been used to describe not only the translation of these quality-of-care measures, but also disparities in the success of this translation. In the overall ESRD population, black race, lower socioeconomic status, and US region (particularly, the Southeast) have all been associated with lower attainment of the goals of pre-ESRD care (26–28), being informed of the transplant option (29), placement on the national deceased donor kidney waitlist (17,30,31), and permanent vascular access (32,33).
With the exception of placement on the kidney transplant list (34,35), these markers of the quality of ESRD care remain relatively unexamined in patients with systemic lupus erythematosus (SLE) and ESRD secondary to lupus nephritis (LN-ESRD). Investigation into the translation of these measures in LN-ESRD patients is important because guidelines to address the preparation for ESRD are generally lacking for rheumatologists (36), who could partner with nephrologists and other providers to improve ESRD care among these patients. Identification of sociodemographic and geographic disparities in quality of ESRD care among SLE patients, as seen in LN care (37), could potentially guide the development of regional interventions targeted to patients most likely to receive inadequate ESRD care. Our aim in the present study was to describe the translation of ESRD quality-of-care measures among US patients with LN-ESRD and to estimate the associations of sociodemographic and geographic factors with successful translation of quality-of-care measures in these patients.
PATIENTS AND METHODS
Study population and data sources
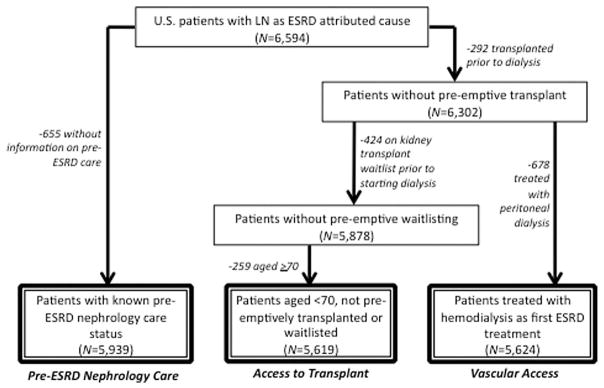
The primary data source for this study was the United States Renal Data System (USRDS). Data from the CMS-2728, completed on all treated US patients with incident ESRD, were obtained from the USRDS (17). A total of 6,594 patients with incident LN-ESRD in whom treatment was initiated between July 1, 2005 and September 30, 2011 and for whom there were data from the most recent (2005) version of the CMS-2728 were identified via a listing of “lupus erythematosus (SLE nephritis)” (International Classification of Diseases, Ninth Revision code 710.0) as the primary attributed cause of ESRD on the CMS-2728; this is the method of identification used in most recent population studies of LN-ESRD incidence (34,35). Of these patients, 655 (9.9%) had unknown pre-ESRD nephrology care status and were excluded from that analysis (Figure 1). For analysis of measures of access to transplantation (informed of transplant options, placement on the kidney transplant waitlist), patients who underwent preemptive transplantation (n = 292 [4.4%]) or were preemptively placed on the waitlist (n = 424 [6.4%]) or who were age ≥70 years (n = 259 [3.9%]) were excluded from the 6,594 LN-ESRD patients, leaving 5,619 (85.2%) for analysis. For analyses of permanent vascular access, those with preemptive transplants (n = 292 [4.4%]) and those treated with peritoneal dialysis (n = 678 [10.3%]) were excluded, leaving 5,624 (85.3%) (Figure 1).
Figure 1.
Selection of study populations for assessment of quality measures related to pre–end-stage renal disease (ESRD) nephrology care, access to transplant (informed of transplant options, time to placement on the kidney transplant waitlist), and presence of a permanent vascular access for dialysis, among US patients in whom treatment was being initiated for ESRD attributed to lupus nephritis (LN), July 1, 2005–September 30, 2011.
Data on primary attributed cause of ESRD, quality-of-care measures (nephrology care prior to ESRD, informed of transplant options, and vascular access at the time of first dialysis), race/ethnicity, insurance status, and clinical factors were all obtained from the CMS-2728 through the USRDS. The United Network for Organ Sharing (UNOS) maintains the national deceased donor kidney waitlist and provides these data to the USRDS. Data on characteristics of the patients’ residential neighborhoods, as defined by patient 5-digit ZIP code tabulation area (ZCTA), were obtained from the 2007–2011 American Community Survey (www.census.gov/acs/www/) via the Minnesota Population Center (www.nghis.org) (38) and linked by patient ZIP code at the start of ESRD to the USRDS data.
Study variables
Sociodemographics and geography
Individual sociodemographic exposures of interest included race/ethnicity (defined as white, black, Hispanic, and other) and insurance prior to ESRD (defined as private, Medicaid, none, or other). Due to the relative lack of individual-level information on socioeconomic status and the potential for neighborhood effects independent of individual socioeconomic status, we also examined aggregate residential ZCTA-level data on the percentage of residents reporting black race, the percentage of residents reporting Hispanic ethnicity, the percentage of residents who had dropped out of high school (residents age ≥25 years without a high school degree or equivalent), and the percentage of residents who were poor (households living below 100% of the federal poverty threshold) from the American Community Survey. Finally, due to the regional implementation of CMS quality-of-care measures via the 18 ESRD Networks, the ESRD Network in which the patient received treatment was included as a geographic exposure of interest.
Quality-of-care measures
The outcomes of interest were ESRD quality-of-care measures, specifically 1) pre-ESRD nephrology care, 2) access to transplant, including being informed of transplant options at the start of ESRD and being placed on the national deceased donor kidney transplant waitlist (“kidney transplant waitlisting”), and 3) permanent vascular access placement prior to the start of dialysis. Pre-ESRD nephrology care was defined by an answer of “yes” to item 18b on the CMS-2728: “Prior to ESRD therapy was the patient under the care of a nephrologist?” Whether patients were informed of transplant option was defined by a “yes” answer to CMS-2728 item 26: “Has patient been informed of kidney transplant options?” Date of placement on the deceased donor transplant waitlist was determined from UNOS data and used to calculate time to transplant waitlisting (from date of first ESRD service to date of placement on the waitlist). Censoring occurred at death (of 3,552 patients who were not waitlisted, 1,093 [30.8%] died, 562 [15.8%] within the first year) or at the end of followup (September 30, 2011; median followup 1.3 years). Finally, vascular access was determined from CMS-2728 item 18d: “What access was used on first outpatient dialysis?” with possible responses of “AVF,” “graft,” “catheter,” and “other” and 2 additional prompts for maturing permanent accesses in place (“Is maturing AVF present?” and “Is maturing graft present?”). Permanent vascular access was defined as AVF or graft used or in place on first dialysis.
Other variables
Data on sex and age at incident LN-ESRD were obtained from the USRDS patient demographics file. Smoking status, body mass index, presence of comorbid conditions, and serum albumin and hemoglobin levels at the start of ESRD were obtained from the CMS-2728.
Statistical analysis
Patient characteristics including sociodemographics and ESRD Network were summarized for all LN-ESRD patients, and quality-of-care measures were summarized and compared across sociodemographic characteristics and region within appropriate study populations. Odds ratios (ORs) and 95% confidence intervals (95% CIs) for the associations between dichotomous outcomes (pre-ESRD nephrology care, informed of transplant options, and permanent vascular access placement) were estimated using multivariable logistic regression models. For transplant waitlisting, incidence rates were calculated as the number of patients placed on the kidney transplant waitlist per person-time, which included all time contributed by all patients from start of ESRD to waitlisting, death, or last date of followup. Violations of Cox proportional hazards assumptions were assessed by tests of Schoenfeld residuals and examination of log–log plots. Hazard ratios (HRs) and 95% CIs were obtained using multivariable Cox proportional hazards models run over the entire followup period as well as using Heaviside functions to split the followup time. To avoid the arbitrary choice of referent group among US regions, adjusted probabilities and incidence rates were also estimated using marginal post-estimation of logistic and Poisson models and mapped using approximate quartiles or medians of the distribution, as appropriate. Factors that were associated with both sociodemographic predictors and quality-of-care measures and were not thought a priori to be mediators of the association were considered potential confounders.
Clustering at the ZCTA level (multiple LN-ESRD patients in a ZCTA) was minimal, with 85% of patients living in ZCTAs with only 1 patient (46%), 2 patients (25%), or 3 patients (14%). Sensitivity analyses at 2 extremes—i.e., with all missing values on pre-ESRD care either assigned “yes” or assigned “no”—were performed and the results compared to the primary results, to determine how much the estimates might be biased if missing data were differential with respect to delivery of pre-ESRD care. Stata version 13 (StataCorp) was used for all analyses, and the threshold for statistical significance was set at α = 0.05. Mapping was performed using ArcGIS version 10.1 (Esri).
RESULTS
Characteristics of the study population
The mean age of the 6,594 US patients with incident LN-ESRD was 40 years. Most were female (although, disproportionate to the overall SLE population, nearly 19% were male), and half were black (Table 1). One-third of these patients had Medicaid and >10% were uninsured at the start of ESRD. In patients’ residential ZCTAs, the median percentages of residents who were black, who had dropped out of high school, and who were living in poverty were 14%, 17%, and 17%, respectively (Table 1). The most common comorbidity among these young patients was hypertension, followed by cardiovascular disease including pericarditis (Table 1). The percentages of all LN-ESRD patients treated within ESRD Networks ranged from ~2% (Network 16, Northwest) to >10% (Networks 6 and 14, Southeast and Texas). The group of 655 patients with missing information on pre-ESRD care (excluded from pre-ESRD care analyses) was similar to those included in terms of most patient characteristics, except that those with missing information were more likely to be black (53.1% versus 49.3%) or Hispanic (20.3% versus 17.4%; P = 0.003) and to have Medicaid (36.3% versus 32.4%) or no insurance (15.6% versus 11.1%; P < 0.001) and less likely to have a body mass index of ≥35 kg/m2 (9.7% versus 12.9%; P = 0.02).
Table 1.
Characteristics at the time of incident ESRD in US patients in whom treatment for ESRD attributed to lupus nephritis was initiated between July 1, 2005 and September 30, 2011*
| Patient factors | |
| Age, mean ± SD years (n = 6,594) | 39.6 ± 15.4 |
| Sex (n = 6,594) | |
| Female | 81.1 |
| Male | 18.9 |
| Race/ethnicity (n = 6,594) | |
| White | 24.7 |
| Black | 49.7 |
| Hispanic | 17.7 |
| Other | 7.9 |
| Insurance (n = 6,594) | |
| Private | 37.4 |
| Medicare/other† | 18.4 |
| Medicaid | 32.8 |
| None | 11.5 |
| Smoking (n = 6,594) | |
| Yes | 4.3 |
| No | 95.7 |
| BMI, mean ± SD kg/m2 (n = 6,522) | 26.9 ± 7.4 |
| BMI ≥35 kg/m2 (n = 6,522) | |
| Yes | 12.6 |
| No | 87.4 |
| No. of comorbidities (n = 6,594) | |
| 0 | 10.9 |
| 1 | 56.0 |
| ≥2 | 33.1 |
| Hypertension (n = 6,594) | |
| Yes | 83.6 |
| No | 16.4 |
| CVD (n = 6,594) | |
| Yes | 18.6 |
| No | 81.4 |
| Serum albumin, mean ± SD gm/dl (n = 5,201) | 2.9 ± 0.8 |
| Serum hemoglobin, mean ± SD gm/dl (n = 6,124) | 9.5 ±1.7 |
| ESRD Network (n = 6,549) | |
| 1 (CT, ME, MA, NH, RI, VT) | 2.5 |
| 2 (NY) | 7.5 |
| 3 (NJ) | 3.9 |
| 4 (DE, PA) | 3.5 |
| 5 (DC, MD, VA, WV) | 5.6 |
| 6 (GA, NC, SC) | 10.3 |
| 7 (FL) | 7.0 |
| 8 (AL, MS, TN) | 6.3 |
| 9 (IN, KY, OH) | 5.1 |
| 10 (IL) | 4.6 |
| 11 (MI, MN, ND, SD, WI) | 6.2 |
| 12 (IA, KS, MO, NE) | 2.7 |
| 13 (AR, LA, OK) | 4.1 |
| 14 (TX) | 10.6 |
| 15 (AZ, CO, NM, NV, UT, WY) | 5.1 |
| 16 (AK, ID, MT, OR, WA) | 2.3 |
| 17 (HI, Northern CA) | 5.1 |
| 18 (Southern CA) | 7.6 |
| Patient residential neighborhood (ZCTA) factors, median (IQR) % | |
| Black race | 14.1 (3.7–41.4) |
| Hispanic ethnicity | 9.9 (3.4–29.9) |
| Dropped out of high school | 16.7 (10.1–24.4) |
| Income below poverty level | 16.5 (9.8–24.9) |
Except where indicated otherwise, values are the percent of patients. ESRD = end-stage renal disease; BMI = body mass index; CVD = cardiovascular disease (including pericarditis); ZCTA = ZIP code tabulation area; IQR = interquartile range.
Includes Medicare (n = 681), VA (n = 47), and other (n = 483).
Association of social predictors with quality-of-care measures
Pre-ESRD care
Overall, 71.1% of the US patients with LN-ESRD received pre-ESRD nephrology care (Table 2), and the percentage did not differ by incidence year (P = 0.47 [data not shown]). After adjustment for potential confounders, black and Hispanic LN-ESRD patients were found to be 27% less likely to have received pre-ESRD care than their white counterparts, and those with Medicaid and those with no insurance prior to ESRD development were 36% and 74% less likely to receive this care, relative to those with private insurance (Table 2). Results were not substantially different when models were further adjusted for hemoglobin or albumin levels (data not shown). Of the 5,939 patients with information on pre-ESRD care, 26 (0.4%) were potentially misclassified in that the patient either had a preemptive transplant or was preemptively waitlisted; redefining these individuals as having pre-ESRD care did not change the results (data not shown).
Table 2.
Crude and adjusted odds ratios for dichotomous ESRD quality-of-care indicators (pre-ESRD care, knowledge of transplant options, and permanent hemodialysis vascular access) by sociodemographic factor, among US patients in whom treatment for ESRD attributed to lupus nephritis was initiated between July 1, 2005 and September 30, 2011*
| Patient received pre-ESRD nephrology care | Patient informed of transplant options | Fistula/graft used or in place at first dialysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Crude % | Odds ratio (95% CI) | Crude % | Odds ratio (95% CI) | Crude % | Odds ratio (95% CI) | ||||
|
|
|
|
|||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | ||||
| Overall | 71.1 | – | – | 84.8 | – | – | 24.4 | – | – |
| Individual sociodemographic factors† | |||||||||
| Race/ethnicity, % | |||||||||
| White | 76.7 | 1.00 (ref.) | 1.00 (ref.) | 84.0 | 1.00 (ref.) | 1.00 (ref.) | 28.2 | 1.00 (ref.) | 1.00 (ref.) |
| Black | 69.1 | 0.68 (0.59–0.79) | 0.73 (0.63–0.85) | 85.4 | 1.21 (0.99–1.48) | 1.07 (0.88–1.30) | 24.1 | 0.81 (0.70–0.94) | 0.96 (0.81–1.12) |
| Hispanic | 67.1 | 0.61 (0.52–0.73) | 0.73 (0.60–0.88) | 83.9 | 1.02 (0.80–1.31) | 0.95 (0.75–1.20) | 20.5 | 0.65 (0.54–0.80) | 0.85 (0.69–1.05) |
| Other | 74.8 | 0.90 (0.70–1.14) | 0.95 (0.74–1.22) | 84.8 | 1.15 (0.83–1.61) | 0.99 (0.72–1.35) | 24.4 | 0.83 (0.64–1.07) | 1.06 (0.81–1.38) |
| Insurance, % | |||||||||
| Private | 78.4 | 1.00 (ref.) | 1.00 (ref.) | 87.6 | 1.00 (ref.) | 1.00 (ref.) | 25.3 | 1.00 (ref.) | 1.00 (ref.) |
| Medicare/other‡ | 73.4 | 0.77 (0.65–0.91) | 0.76 (0.63–0.90) | 82.8 | 0.66 (0.51–0.85) | 0.61 (0.47–0.80) | 28.5 | 1.18 (0.99–1.10) | 1.03 (0.86–1.23) |
| Medicaid | 69.3 | 0.62 (0.54–0.72) | 0.64 (0.56–0.74) | 83.4 | 0.71 (0.59–0.87) | 0.66 (0.54–0.80) | 24.4 | 0.95 (0.82–1.10) | 1.05 (0.91–1.22) |
| None | 47.5 | 0.25 (0.21–0.30) | 0.26 (0.21–0.31) | 83.4 | 0.65 (0.51–0.83) | 0.68 (0.54–0.87) | 16.0 | 0.55 (0.43–0.69) | 0.62 (0.49–0.79) |
| Residential neighborhood (ZCTA) sociodemographic factors§ | |||||||||
| % black | |||||||||
| Below median | 73.4 | 1.00 (ref.) | 1.00 (ref.) | 84.2 | 1.00 (ref.) | 1.00 (ref.) | 24.6 | 1.00 (ref.) | 1.00 (ref.) |
| Above median | 68.9 | 0.81 (0.72–0.91) | 0.95 (0.82–1.09) | 85.4 | 1.09 (0.94–1.26) | 1.04 (0.87–1.25) | 24.2 | 0.97 (0.86–1.10) | 1.01 (0.86–1.18) |
| % Hispanic | |||||||||
| Below median | 73.4 | 1.00 (ref.) | 1.00 (ref.) | 84.5 | 1.00 (ref.) | 1.00 (ref.) | 25.2 | 1.00 (ref.) | 1.00 (ref.) |
| Above median | 68.9 | 0.81 (0.72–0.91) | 0.83 (0.73–0.95) | 85.2 | 1.06 (0.91–1.23) | 1.05 (0.91–1.23) | 23.6 | 0.92 (0.81–1.04) | 0.99 (0.87–1.12) |
| % dropped out of high school | |||||||||
| Below median | 74.0 | 1.00 (ref.) | 1.00 (ref.) | 85.8 | 1.00 (ref.) | 1.00 (ref.) | 25.0 | 1.00 (ref.) | 1.00 (ref.) |
| Above median | 68.3 | 0.76 (0.68–0.85) | 0.89 (0.79–1.01) | 83.9 | 0.85 (0.73–0.98) | 0.87 (0.74–1.02) | 23.9 | 0.93 (0.83–1.06) | 1.01 (0.88–1.15) |
| % with income below poverty level | |||||||||
| Below median | 74.6 | 1.00 (ref.) | 1.00 (ref.) | 85.3 | 1.00 (ref.) | 1.00 (ref.) | 25.9 | 1.00 (ref.) | 1.00 (ref.) |
| Above median | 67.7 | 0.72 (0.64–0.81) | 0.84 (0.74–0.95) | 84.3 | 0.91 (0.79–1.06) | 0.93 (0.79–1.09) | 23.0 | 0.85 (0.75–0.96) | 0.88 (0.77–1.00) |
Adjusted models (complete case analysis) include age, race/ethnicity, insurance, body mass index (≥35 kg/m2 versus <35 kg/m2), hypertension, and cardiovascular disease. ref. = referent; ZCTA = ZIP code tabulation area.
Numbers of patients in the assessment of crude percent and in the assessment of odds ratio (95% confidence interval [95% CI]), respectively, were 5,939 and 5,875 for pre–end-stage renal disease (pre-ESRD) nephrology care, 5,619 and 5,558 for patient informed of transplant options, and 5,624 and 5,562 for fistula/graft used or in place at first dialysis.
Includes Medicare, VA, and other.
Numbers of patients in the assessment of crude percent and in the assessment of odds ratio (95% CI), respectively, were 5,810 and 5,746 for pre-ESRD nephrology care, 5,492 and 5,431 for patient informed of transplant options, and 5,500 and 5,438 for fistula/graft used or in place at first dialysis.
After adjustment, LN-ESRD patients living in ZCTAs in which above-median percentages of residents were black, Hispanic, high school dropouts, and poor were 5%, 17%, 11%, and 16% less likely, respectively, to have had pre-ESRD care, although only the association with poverty was statistically significant (Table 2). Sensitivity analyses showed that associations with imputed missing values of pre-ESRD care were similar in terms of magnitude and statistical significance, ranging from 0.72 to 0.77 for black versus white race, 0.70 to 0.78 for Hispanic versus white, 0.72 to 0.80 for Medicaid versus private insurance, and 0.28 to 0.31 for no insurance versus private insurance.
Access to transplant
Overall, 84.8% of LN-ESRD patients had been informed of transplant options at the start of ESRD, with 83.6% and 87.8% being informed in incidence years 2006 and 2010, respectively (P = 0.05 for time trend). Relative to having private insurance at the start of ESRD, having Medicaid, no insurance, or other types of insurance was associated with a 32–39% decreased likelihood of having been informed of transplant options, after adjustment for potential confounders (Table 2). Race/ethnicity and ZCTA-level proportion of residents of black race or living in poverty were not associated with having been informed of transplant options. ZCTA-level education (percentage who dropped out of high school) was associated with a 13% decreased likelihood of being informed of transplant options, although the association was not statistically significant (Table 2).
Rates of transplant waitlisting were 206 per 1,000 patient-years overall, ranging from 177 to 263 per 1,000 patient-years in 2006 and 2010, respectively (P < 0.001 for time trend). Tests of the proportional hazards assumption indicated potential violations (P < 0.05 for all sociodemographic predictors). Examination of log–log plots did not reveal substantial departures from parallel except at around the end of the first year, when data were sparse but suggested potential crossing of the curves; thus, followup split at 1 year as well as overall was utilized. Adjusted hazards of kidney transplant waitlisting during followup were substantially lower among LN-ESRD patients with Medicaid, no insurance, or other insurance, relative to those with private insurance at ESRD development (48%, 51%, and 30% lower, respectively); results were similar in the first year of ESRD and after the first year of ESRD (Table 3). Black and Hispanic LN-ESRD patients, respectively, were 22% and 18% less likely to be waitlisted than white LN-ESRD patients, but only within the first year of ESRD. LN-ESRD patients living in ZCTAs with lower educational attainment and more poverty were less likely to be waitlisted (25% and 35%, respectively), but this was also the case only within the first year of ESRD (Table 3).
Table 3.
Crude and adjusted hazard ratios for time to kidney transplant waitlisting by sociodemographic factor, among US patients in whom treatment for ESRD attributed to lupus nephritis was initiated between July 1, 2005 and September 30, 2011*
| Events/1,000 patient-years | Hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Entire followup | In first year after start of ESRD treatment | After first year of ESRD treatment | |||||
|
|
|
|
|||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Overall | 206 | – | – | – | – | – | – |
| Individual sociodemographic factors† | |||||||
| Race/ethnicity, % | |||||||
| White | 215 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Black | 195 | 0.91 (0.81–1.02) | 0.91 (0.81–1.03) | 0.79 (0.68–0.91) | 0.78 (0.68–0.91) | 1.13 (0.94–1.36) | 1.15 (0.95–1.38) |
| Hispanic | 208 | 0.99 (0.86–1.13) | 0.97 (0.84–1.11) | 0.84 (0.70–1.01) | 0.82 (0.68–0.98) | 1.23 (1.00–1.53) | 1.22 (0.98–1.52) |
| Other | 261 | 1.22 (1.03–1.45) | 1.09 (0.87–1.30) | 1.29 (1.04–1.60) | 1.16 (0.93–1.43) | 1.12 (0.83–1.50) | 1.00 (0.75–1.34) |
| Insurance, % | |||||||
| Private | 293 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Medicare/other‡ | 194 | 0.67 (0.59–0.77) | 0.70 (0.62–0.80) | 0.63 (0.53–0.74) | 0.65 (0.55–0.77) | 0.74 (0.61–0.91) | 0.78 (0.64–0.95) |
| Medicaid | 158 | 0.55 (0.50–0.61) | 0.52 (0.47–0.58) | 0.54 (0.47–0.62) | 0.51 (0.44–0.58) | 0.58 (0.50–0.68) | 0.55 (0.47–0.65) |
| None | 159 | 0.55 (0.47–0.63) | 0.49 (0.43–0.57) | 0.39 (0.32–0.49) | 0.36 (0.29–0.44) | 0.77 (0.63–0.94) | 0.69 (0.57–0.84) |
| Residential neighborhood (ZCTA) sociodemographic factors§ | |||||||
| % black | |||||||
| Below median | 225 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Above median | 168 | 0.84 (0.77–0.92) | 0.88 (0.79–0.98) | 0.76 (0.68–0.86) | 0.96 (0.84–1.10) | 0.80 (0.70–0.91) | 1.00 (0.86–1.16) |
| % Hispanic | |||||||
| Below median | 201 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Above median | 213 | 1.07 (0.98–1.17) | 1.03 (0.93–1.13) | 1.02 (0.91–1.15) | 0.98 (0.86–1.10) | 1.14 (0.99–1.30) | 1.10 (0.95–1.26) |
| % dropped out of high school | |||||||
| Below median | 239 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Above median | 180 | 0.77 (0.70–0.84) | 0.82 (0.74–0.90) | 0.70 (0.63–0.79) | 0.75 (0.67–0.85) | 0.86 (0.75–0.98) | 0.91 (0.79–1.04) |
| % with income below poverty level | |||||||
| Below median | 250 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Above median | 172 | 0.70 (0.64–0.76) | 0.76 (0.69–0.83) | 0.60 (0.53–0.67) | 0.65 (0.58–0.73) | 0.87 (0.76–0.99) | 0.94 (0.82–1.07) |
Adjusted models (complete case analysis) include age, race/ethnicity, insurance, body mass index (≥35 kg/m2 versus <35 kg/m2), hypertension, and cardiovascular disease. ref. = referent; ZCTA = ZIP code tabulation area.
Number of patients in the assessment of events/1,000 patient-years was 5,619. Numbers of patients in the assessment of hazard ratio (95% confidence interval [95% CI]) were 5,558 for the entire followup, 5,558 for the first year after the start of treatment for end-stage renal disease (ESRD), and 3,203 for after the first year of ESRD treatment.
Includes Medicare, VA, and other.
Number of patients in the assessment of events/1,000 patient-years was 5,493. Numbers of patients in the assessment of hazard ratio (95% CI) were 5,432 for the entire followup, 5,432 for the first year after the development of ESRD, and 3,119 for after the first year of ESRD.
Findings of analyses including both waitlisting and living donor transplants without prior waitlisting (n = 69), with censoring at the time of transplant, were slightly further from the null but were not substantially different from results reported in Table 3 (data not shown). Results were similar when only the patients who survived 1 year were included, except that ZCTA-level black race remained statistically significantly associated with lower likelihood of waitlisting in the first year of ESRD, after adjustment (HR 0.79 [95% CI 0.69–0.90]).
Permanent hemodialysis vascular access
Only approximately one-quarter of LN-ESRD patients who received hemodialysis as the initial ESRD treatment had a fistula or graft used or in place on first dialysis (Table 2), with no differences over time (P = 0.45). The percentage of LN-ESRD patients with a permanent vascular access did not differ by early transplant status (27.2% of those undergoing transplant within 1 year versus 24.3% of those not undergoing transplant within 1 year; P = 0.32). The likelihood of having a permanent vascular access did not differ by race/ethnicity or ZCTA-level sociodemographics. The likelihood was also equivalent among those with private insurance, Medicaid, or other insurance, but having no insurance was associated with a 38% reduced likelihood of permanent vascular access among LN-ESRD patients (Table 2).
Association of ESRD Network with quality-of-care measures
Pre-ESRD care
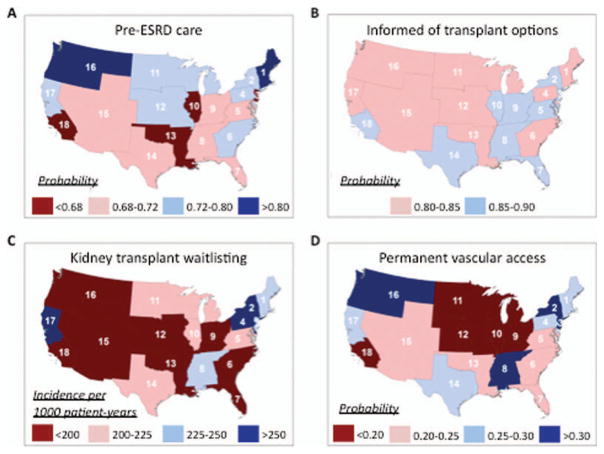
Receipt of pre-ESRD nephrology care among LN-ESRD patients differed substantially by ESRD Network (Table 4). Age-, sex-, race/ethnicity- and insurance-adjusted probabilities of pre-ESRD care ranged from 0.66 (95% CI 0.60–0.71) in Illinois (Network 10) to 0.81 (95% CI, 0.74–0.87) in New England (Network 1) (Figure 2A).
Table 4.
Age-, sex-, race/ethnicity-, and insurance-adjusted risk ratio estimates for quality-of-care indicators by ESRD Network, among US patients in whom treatment for ESRD attributed to lupus nephritis was initiated between July 1, 2005 and September 30, 2011*
| Network | Odds ratio (95% CI) for having received pre-ESRD nephrology care | Odds ratio (95% CI) for being informed of transplant options | Hazard ratio (95% CI) for kidney transplant waitlisting | Odds ratio (95% CI) for having fistula/graft in place at first dialysis | |
|---|---|---|---|---|---|
|
| |||||
| In first year after start of ESRD treatment | After first year of ESRD treatment | ||||
| 1 (CT, ME, MA, NH, RI, VT) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| 2 (NY) | 0.63 (0.40–1.02) | 1.33 (0.78–2.28) | 1.56 (1.05–2.32) | 1.27 (0.79–2.06) | 1.07 (0.70–1.63) |
| 3 (NJ) | 0.47 (0.29–0.78) | 1.73 (0.93–3.21) | 1.10 (0.71–1.70) | 0.76 (0.43–1.33) | 1.12 (0.70–1.78) |
| 4 (DE, PA) | 0.64 (0.37–1.08) | 1.09 (0.60–1.98) | 1.78 (1.16–2.73) | 0.56 (0.29–1.09) | 0.79 (0.49–1.28) |
| 5 (DC, MD, VA, WV) | 0.59 (0.36–0.96) | 1.20 (0.69–2.09) | 0.85 (0.55–1.32) | 0.83 (0.50–1.38) | 0.72 (0.46–1.14) |
| 6 (GA, NC, SC) | 0.68 (0.43–1.08) | 0.93 (0.57–1.53) | 0.60 (0.40–0.90) | 0.95 (0.60–1.51) | 0.74 (0.49–1.12) |
| 7 (FL) | 0.55 (0.34–0.88) | 1.15 (0.68–1.95) | 0.38 (0.24–0.61) | 0.89 (0.55–1.44) | 0.58 (0.37–0.91) |
| 8 (AL, MS, TN) | 0.58 (0.36–0.93) | 1.48 (0.85–2.58) | 0.99 (0.65–1.50) | 0.85 (0.52–1.41) | 1.09 (0.71–1.68) |
| 9 (IN, KY, OH) | 0.60 (0.36–0.98) | 1.17 (0.67–2.02) | 0.61 (0.39–0.97) | 0.82 (0.49–1.37) | 0.57 (0.36–0.91) |
| 10 (IL) | 0.45 (0.27–0.73) | 1.29 (0.73–2.29) | 1.02 (0.66–1.57) | 0.82 (0.49–1.38) | 0.47 (0.29–0.77) |
| 11 (MI, MN, ND, SD, WI) | 0.65 (0.40–1.06) | 0.90 (0.53–1.53) | 0.85 (0.56–1.31) | 0.91 (0.55–1.49) | 0.57 (0.36–0.90) |
| 12 (IA, KS, MO, NE) | 0.61 (0.36–1.06) | 0.88 (0.48–1.62) | 0.67 (0.39–1.13) | 0.69 (0.38–1.26) | 0.53 (0.31–0.91) |
| 13 (AR, LA, OK) | 0.48 (0.29–0.79) | 0.88 (0.51–1.53) | 0.61 (0.38–0.99) | 0.68 (0.39–1.16) | 0.65 (0.40–1.05) |
| 14 (TX) | 0.51 (0.32–0.80) | 1.48 (0.89–2.46) | 0.74 (0.50–1.10) | 0.98 (0.62–1.55) | 0.89 (0.59–1.35) |
| 15 (AZ,CO, NM, NV, UT, WY) | 0.54 (0.33–0.88) | 1.01 (0.59–1.75) | 0.76 (0.49–1.18) | 0.69 (0.41–1.15) | 0.68 (0.43–1.08) |
| 16 (AK, ID, MT, OR, WA) | 0.99 (0.54–1.84) | 0.86 (0.46–1.63) | 0.60 (0.34–1.07) | 0.66 (0.35–1.24) | 1.14 (0.66–1.96) |
| 17 (HI, Northern CA) | 0.71 (0.42–1.18) | 0.88 (0.51–1.53) | 1.96 (1.31–2.94) | 0.86 (0.49–1.48) | 0.96 (0.61–1.53) |
| 18 (Southern CA) | 0.46 (0.28–0.74) | 1.16 (0.69–1.95) | 0.70 (0.46–1.07) | 0.84 (0.51–1.36) | 0.51 (0.33–0.80) |
ESRD = end-stage renal disease; 95% CI = 95% confidence interval; ref. = referent.
Figure 2.
Age-, sex-, race/ethnicity-, and insurance-adjusted probability of receipt of nephrology care prior to end-stage renal disease (ESRD) (A), probability of being informed of transplant options at the start of dialysis (B), rate of placement on the kidney transplant waitlist (C), and probability of a permanent vascular access used or in place at first dialysis (D), by US region defined according to Centers for Medicare & Medicaid Services ESRD Networks, among US patients in whom treatment was being initiated for ESRD attributed to lupus nephritis, July 1, 2005–September 30, 2011.
Access to transplant
The likelihood of being informed of transplant options did not differ by ESRD Network (Table 4), and the range of adjusted probabilities was small (Figure 2B), from 0.81 (95% CI 0.74–0.880 (Network 16, Northwest) to 0.90 (95% CI 0.85–0.93) (Network 3, New Jersey). However, there was some evidence that waitlisting for transplant in LN-ESRD patients, particularly in the first year of ESRD, does differ by ESRD Network (Table 4). Age-, sex-, race/ethnicity-, and insurance-adjusted incidence of kidney transplant waitlisting per 1,000 patient-years over the entire period varied by >2.5-fold, from 148 (95% CI 121–175) (Network 7, Florida) to 373 (95% CI 307–440) (Network 17, Northern California and Hawaii) (Figure 2C).
Permanent vascular access
There was substantial, statistically significant ESRD Network–level variation in likelihood of permanent vascular access (Table 4). Age-, sex-, race/ethnicity-, and insurance-adjusted probabilities of permanent vascular access used or in place at first dialysis ranged from 0.17 (95% CI 0.12–0.21) (Network 10) to 0.33 (95% CI 0.24–0.41) (Network 16) (Figure 2D).
DISCUSSION
Despite multiple national and regional quality-of-care initiatives and incentives aimed at improving care in the overall ESRD population (1,2,32), we found that, among patients with LN-ESRD, care remains suboptimal, particularly with respect to permanent vascular access placement. Nearly one-third of patients with LN-ESRD had received no pre-ESRD nephrology care at the start of ESRD treatment, similar to the overall ESRD population, in whom 34–35% had not received this care in 2007–2010 (17). While in some of these patients ESRD may have been the earliest manifestation of SLE, precluding pre-ESRD care, it is likely that most were not referred to a nephrologist in a timely manner, putting them at risk for poor outcomes (39). Most potentially eligible LN-ESRD patients (85%) were reported to be informed of transplant options at the start of ESRD, higher than the percentage in the overall ESRD population (70% in 2005–2007) (29), but the incidence of subsequent waitlisting was only ~20% per year. However, both the proportion of LN-ESRD patients who were informed of transplant options and the proportion who were on the transplant waitlist increased over the study period, and the proportion on the waitlist was much higher than in the general ESRD population, in whom only 11–12% were waitlisted during the first year in 2007–2010 (17). Notably, fewer than one-quarter of LN-ESRD patients treated with hemodialysis had a permanent vascular access in place at the start of treatment, versus 35–36% in 2007–2010 in the overall ESRD population (17). This percentage was higher among those who underwent transplantation early, suggesting that provider decisions to forego vascular access surgery in patients expected to receive a transplant imminently do not explain the low likelihood of permanent vascular access placement among LN-ESRD patients.
Our findings also indicate that there are substantial sociodemographic and regional disparities in the translation of quality-of-care measures related to pre-ESRD care, access to transplant, and placement of permanent vascular access among LN-ESRD patients. Black and Hispanic patients were less likely to have had pre-ESRD nephrology care and permanent vascular accesses than their white counterparts, as in the overall ESRD population (28,32), although differences in permanent vascular access by race/ethnicity were not statistically significant after adjustment for other sociodemographic and clinical factors. Black race and Hispanic ethnicity were also associated with lower likelihood of kidney transplant waitlisting in the first year of ESRD treatment relative to white race, similar to patterns in the overall ESRD population (30,31,40). Faster progression of lupus nephritis (41,42) and reduced engagement with the health care system among minority LN-ESRD patients may contribute to racial and ethnic disparities in pre-ESRD nephrology care and early transplant waitlisting in this population.
While patient race/ethnicity was not associated with being informed of transplant options or, after the first year, with waitlisting, these apparent equivalencies may not be sufficient to close racial and ethnic gaps in kidney transplantation created by the early lag in wait-listing, relative to white patients. Further, how well patients are actually informed of transplant options—and whether this translates to usable knowledge of the options—may differ by race/ethnicity. Among patients who are not informed, it is likely that reasons for failure to provide information differ by race/ethnicity, and reasons for failure to provide information to minority and female patients (the majority of patients with LN-ESRD) are more likely to represent subjective assessments (e.g., “psychologically unfit”) (29). Thus, being appropriately and thoroughly informed of options may not be equivalent by race/ethnicity.
Similar to reported findings in the overall ESRD population (43,44), lack of insurance at the start of ESRD was strongly associated with less successful translation of all examined quality-of-care measures after adjustment for other sociodemographic and clinical factors. This disparity is likely at least partially related to the actual or perceived inability of uninsured patients to cover expenses associated with specialty care, including nephrology, transplant evaluation, and vascular access surgery. However, not having private insurance at the time of ESRD development was also associated with a lower likelihood of pre-ESRD nephrology care, being informed of transplant options, and waitlisting. After the first year of ESRD, when all treated patients have CMS coverage for ESRD services, the association of having no or public insurance with lower likelihood of waitlisting persisted. For example, LN-ESRD patients with Medicaid remained nearly 50% less likely to be wait-listed than LN-ESRD patients with private insurance at the start of ESRD, suggesting that these patients are less likely to be perceived as suitable candidates for transplant even after they gain equivalent access to CMS ESRD coverage. The 3-year limit on coverage for immunosuppressive treatment among transplant patients who qualify for Medicare based solely on ESRD may act as a provider deterrent to waitlisting young, uninsured, or publicly insured LN-ESRD patients. However, the pattern is likely not fully explained by this policy (45).
Area-based socioeconomic measures of lower educational attainment and greater poverty were associated with inadequate pre-ESRD nephrology care and access to transplantation, although after adjustment for individual factors the impact was generally more modest and less statistically significant than that of individual race/ethnicity or insurance status. Whether the area-level associations represent proxy effects for individual poverty and education level not captured by individual race/ethnicity and insurance status or contextual effects is unknown without information on individual education and poverty status. After adjustment, the racial composition of patients’ residential area was not associated with most of these quality-of-care measures except transplant waitlisting over the entire followup period, suggesting that individual race/ethnicity, along with age, sex, insurance status, and comorbid conditions, may explain most differences in quality of care by residential-area racial composition.
Translation of most of the quality-of-care measures examined, with the exception of being informed of transplant options, also differed among LN-ESRD patients by US region as defined by ESRD Network. Even with adjustment for ESRD Network differences in age, sex, race/ethnicity, and insurance, LN-ESRD patients in the Northeast, and especially New England, had a relatively high likelihood of pre-ESRD nephrology care, transplant waitlisting, and permanent vascular access placement, mirroring patterns seen in the overall ESRD population (26,33,46–48). Patients in the Northwest similarly had a high likelihood of pre-ESRD care and permanent vascular access placement, but these same patients had a relatively low likelihood of transplant waitlisting. LN-ESRD patients in Southern California were generally less likely than patients in other ESRD Networks to have pre-ESRD nephrology care, transplant waitlisting, and permanent vascular access placement.
The inconsistency of these geographic disparities across quality measures may be the result of differences between ESRD Networks in resources and priorities—despite national programs designed to eliminate these regional differences, such as the Fistula First Breakthrough Initiative (32,33,49). Alternatively, the varying prevalence of LN-ESRD across ESRD Networks could lead to differences in provider experience and comfort with the care of lupus nephritis and associated ESRD, leading to differences in the translation of these quality-of-care measures. Of course, for ESRD Networks with small proportions of the overall LN-ESRD population, statistical differences may also be due to chance.
This study has several limitations. The USRDS does not capture non–Medicare-eligible individuals, including undocumented residents who are likely to be socioeconomically deprived and geographically concentrated. Also, attribution of ESRD cause on the CMS-2728 has unknown validity; one small validation study (50) conducted using biopsy samples obtained prior to 2001 suggests that sensitivity is potentially low although attributed causes were mostly missing, contributing to low agreement. If these validation results apply in the more modern era, with nearly complete data on attributed cause, our study population may not have captured all individuals with LN-ESRD and, if differential by sociodemographics, region, and/or quality of care, this could potentially bias our results. Provider accuracy in recording other patient variables, including race/ethnicity and insurance status as well as quality measures, may also be imperfect. Death may factor as a competing risk to analyses of time to waitlisting, although sensitivity analyses using only patients who did not die during the first year suggest that the effect of this bias is likely minimal. ZCTAs and measures at the ZCTA level may serve as insufficient proxies for neighborhoods and for characteristics of the individuals within these areas, respectively. Further, more granular information on ethnicity and language barriers, which may be important for the comprehensive assessment of disparities, was not available. There also is the potential for selection bias due to excluded data in analyses of pre-ESRD care, since included and excluded patients differed on several characteristics. However, pre-ESRD care status was unknown in <10%, and sensitivity analyses showed that any bias was likely minimal. Misclassification of quality of care on the CMS-2728 is also possible, although pre-ESRD care appears to be accurately captured with respect to patients with preemptive transplants and waitlisting. Many confounding factors may instead or also serve as mediating factors, leading to potential overadjustment, and, as with any observational study, there is possible residual confounding, particularly due to provider factors such as availability of nephrologists and rheumatologists.
However, the study also has several powerful strengths. These include the capture of all US patients treated for ESRD, limited loss to followup due to universal coverage of ESRD services by CMS, and the provision of the Medicare eligibility form (CMS-2728)—which includes ESRD quality-of-care information of interest to CMS—for all treated patients.
Despite its limitations, this investigation provides a comprehensive, national snapshot of ESRD quality of care for US patients with LN-ESRD, overall and by patient characteristics and US region. The results encourage hypothesis generation and further analysis regarding potential barriers to improving quality of ESRD care in this population at the levels of the health system, ESRD Networks, providers (including rheumatologists, nephrologists, and transplant and vascular access surgeons), and patients. Our findings also identify potential specific targets with respect to inadequate translation of quality-of-care measures in this population (particularly, permanent vascular access placement) and the LN-ESRD patient subpopulations that are least likely to receive high-quality care, as assessed by these measures. For example, an ESRD Network–level intervention to enhance rheumatology–nephrology partnerships aimed at improving ESRD care could be targeted to a region with a large population of uninsured, black LN-ESRD patients, such as the Southeast. Such efforts have the potential to ensure better and more equitable quality of ESRD care among patients with SLE.
Acknowledgments
The data reported herein have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government or the official views of the National Institutes of Health.
Supported in part by the NIH (grant R01-AR-065493 to Drs. Drenkard and Lim, National Center for Advancing Translational Sciences grants ULl-TR-000454 and KL2-TR-000455 to Dr. Patzer, National Institute on Minority Health and Health Disparities grant 1R24-MD-008077-01 to Drs. Patzer and Pastan, and Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K01-HD-074726 to Dr. Kramer) and by the CDC (grant U01-DP-005119 to Drs. Drenkard and Lin). Dr. Plantinga’s work was supported by the Laney Graduate School, Emory University.
Footnotes
Dr. Drenkard has received speaking fees from Questcor Pharmaceuticals (less than $10,000).
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Plantinga had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Plantinga, Drenkard, Patzer, Lim, McClellan.
Acquisition of data. Plantinga, Lim.
Analysis and interpretation of data. Plantinga, Drenkard, Patzer, Klein, Kramer, Pastan, Lim, McClellan.
References
- 1.Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program: end-stage renal disease prospective payment system for calendar year 2014. Federal Register. 2013 Dec 2;38(231) CMS-1526-F http://www.gpo.gov/fdsys/pkg/FR-2013-12-02/pdf/2013-28451.pdf. [Google Scholar]
- 2.Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. Healthy people 2020. www.healthypeople.gov.
- 3.Astor BC, Eustace JA, Powe NR, Klag MJ, Sadler JH, Fink NE, et al. Timing of nephrologist referral and arteriovenous access use: the CHOICE Study. Am J Kidney Dis. 2001;38:494–501. doi: 10.1053/ajkd.2001.26833. [DOI] [PubMed] [Google Scholar]
- 4.Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, et al. Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol. 2002;55:711–6. doi: 10.1016/s0895-4356(02)00415-8. [DOI] [PubMed] [Google Scholar]
- 5.Winkelmayer WC, Glynn RJ, Levin R, Owen W, Jr, Avorn J. Late referral and modality choice in end-stage renal disease. Kidney Int. 2001;60:1547–54. doi: 10.1046/j.1523-1755.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 6.Winkelmayer WC, Glynn RJ, Levin R, Mittleman MA, Pliskin JS, Avorn J. Late nephrologist referral and access to renal transplantation. Transplantation. 2002;73:1918–23. doi: 10.1097/00007890-200206270-00012. [DOI] [PubMed] [Google Scholar]
- 7.Winkelmayer WC, Owen WF, Jr, Levin R, Avorn J. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol. 2003;14:486–92. doi: 10.1097/01.asn.0000046047.66958.c3. [DOI] [PubMed] [Google Scholar]
- 8.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137:479–86. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Stack AG. Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis. 2003;41:310–8. doi: 10.1053/ajkd.2003.50038. [DOI] [PubMed] [Google Scholar]
- 10.Kazmi WH, Obrador GT, Khan SS, Pereira BJ, Kausz AT. Late nephrology referral and mortality among patients with end-stage renal disease: a propensity score analysis. Nephrol Dial Transplant. 2004;19:1808–14. doi: 10.1093/ndt/gfg573. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa T, Bragg-Gresham JL, Yamazaki S, Fukuhara S, Akizawa T, Kleophas W, et al. Greater first-year survival on hemodialysis in facilities in which patients are provided earlier and more frequent pre-nephrology visits. Clin J Am Soc Nephrol. 2009;4:595–602. doi: 10.2215/CJN.03540708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270:1339–43. [PubMed] [Google Scholar]
- 13.Ojo AO, Port FK, Wolfe RA, Mauger EA, Williams L, Berling DP. Comparative mortality risks of chronic dialysis and cadaveric transplantation in black end-stage renal disease patients. Am J Kidney Dis. 1994;24:59–64. doi: 10.1016/s0272-6386(12)80160-0. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 15.Kutner NG. Improving compliance in dialysis patients: does anything work? Semin Dial. 2001;14:324–7. doi: 10.1046/j.1525-139x.2001.00080.x. [DOI] [PubMed] [Google Scholar]
- 16.Evans RW, Manninen DL, Garrison LP, Jr, Hart LG, Blagg CR, Gutman RA, et al. The quality of life of patients with end-stage renal disease. N Engl J Med. 1985;312:553–9. doi: 10.1056/NEJM198502283120905. [DOI] [PubMed] [Google Scholar]
- 17.National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. United States Renal Data System. USRDS 2013 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. www.usrds.org.
- 18.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61:305–16. doi: 10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 19.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7:523–35. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40:611–22. doi: 10.1053/ajkd.2002.34924. [DOI] [PubMed] [Google Scholar]
- 21.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60:1443–51. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 22.Woods JD, Port FK. The impact of vascular access for haemodialysis on patient morbidity and mortality. Nephrol Dial Transplant. 1997;12:657–9. doi: 10.1093/ndt/12.4.657. [DOI] [PubMed] [Google Scholar]
- 23.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–9. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol. 2004;15:477–86. doi: 10.1097/01.asn.0000109668.05157.05. [DOI] [PubMed] [Google Scholar]
- 25.Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002;62:620–6. doi: 10.1046/j.1523-1755.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- 26.McClellan WM, Wasse H, McClellan AC, Kipp A, Waller LA, Rocco MV. Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol. 2009;20:1078–85. doi: 10.1681/ASN.2008060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakash S, Rodriguez RA, Austin PC, Saskin R, Fernandez A, Moist LM, et al. Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol. 2010;21:1192–9. doi: 10.1681/ASN.2009101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan G, Cheung AK, Ma JZ, Yu AJ, Greene T, Oliver MN, et al. The associations between race and geographic area and quality-of-care indicators in patients approaching ESRD. Clin J Am Soc Nephrol. 2013;8:610–8. doi: 10.2215/CJN.07780812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucirka LM, Grams ME, Balhara KS, Jaar BG, Segev DL. Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant. 2012;12:351–7. doi: 10.1111/j.1600-6143.2011.03865.x. [DOI] [PubMed] [Google Scholar]
- 30.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20:1333–40. doi: 10.1681/ASN.2008030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patzer RE, Perryman JP, Schrager JD, Pastan S, Amaral S, Gazmararian JA, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. Am J Transplant. 2012;12:358–68. doi: 10.1111/j.1600-6143.2011.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch JR, Mohan S, McClellan WM. Achieving the goal: results from the Fistula First Breakthrough Initiative. Curr Opin Nephrol Hypertens. 2011;20:583–92. doi: 10.1097/MNH.0b013e32834b33c4. [DOI] [PubMed] [Google Scholar]
- 33.Hopson S, Frankenfield D, Rocco M, McClellan W. Variability in reasons for hemodialysis catheter use by race, sex, and geography: findings from the ESRD Clinical Performance Measures Project. Am J Kidney Dis. 2008;52:753–60. doi: 10.1053/j.ajkd.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63:1681–8. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiraki LT, Lu B, Alexander SR, Shaykevich T, Alarcon GS, Solomon DH, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum. 2011;63:1988–97. doi: 10.1002/art.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, FitzGerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res (Hoboken) 2014;66:617–24. doi: 10.1002/acr.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minnesota Population Center. National Historical Geographic Information System: version 2.0. Minneapolis (MN): University of Minnesota; 2011. www.nhgis.org. [Google Scholar]
- 39.Minutolo R, Lapi F, Chiodini P, Simonetti M, Bianchini E, Pecchioli S, et al. Risk of ESRD and death in patients with CKD not referred to a nephrologist: a 7-year prospective study. Clin J Am Soc Nephrol. 2014;9:1586–93. doi: 10.2215/CJN.10481013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall YN, Choi AI, Xu P, O’Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol. 2011;22:743–51. doi: 10.1681/ASN.2010080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alarcon GS, McGwin G, Jr, Petri M, Ramsey-Goldman R, Fessler BJ, Vila LM, et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med. 2006;3:e396. doi: 10.1371/journal.pmed.0030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barr RG, Seliger S, Appel GB, Zuniga R, D’Agati V, Salmon J, et al. Prognosis in proliferative lupus nephritis: the role of socioeconomic status and race/ethnicity. Nephrol Dial Transplant. 2003;18:2039–46. doi: 10.1093/ndt/gfg345. [DOI] [PubMed] [Google Scholar]
- 43.Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol. 2012;7:1490–7. doi: 10.2215/CJN.13151211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: still “catheter first. Hemodialysis Int. 2009;13:533–42. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 45.Grubbs V, Plantinga LC, Vittinghoff E, O’Hare AM, Dudley RA. Medicare immunosuppressant coverage and access to kidney transplantation: a retrospective national cohort study. BMC Health Serv Res. 2012;12:254. doi: 10.1186/1472-6963-12-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClellan WM, Wasse H, McClellan AC, Holt J, Krisher J, Waller LA. Geographic concentration of poverty and arteriovenous fistula use among ESRD patients. J Am Soc Nephrol. 2010;21:1776–82. doi: 10.1681/ASN.2009121235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB. Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant. 2007;7:1412–23. doi: 10.1111/j.1600-6143.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O’Hare AM. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med. 2007;146:493–501. doi: 10.7326/0003-4819-146-7-200704030-00005. [DOI] [PubMed] [Google Scholar]
- 49.Reddan D, Klassen P, Frankenfield DL, Szczech L, Schwab S, Coladonato J, et al. National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol. 2002;13:2117–24. doi: 10.1097/01.asn.0000022422.79790.a8. [DOI] [PubMed] [Google Scholar]
- 50.Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, et al. Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol. 2010;5:2046–52. doi: 10.2215/CJN.03550410. [DOI] [PMC free article] [PubMed] [Google Scholar]