Abstract
OBJECTIVES
To assess whether patient factors, such as age and preoperative kidney function, were associated with receipt of partial in a national integrated health care system.
METHODS
We identified patients treated with a radical or partial nephrectomy from 2002-2014 in the Veterans Health Administration. We examined associations among patient age, sex, race/ethnicity, multi-morbidity, baseline kidney function, tumor characteristics and receipt of partial nephrectomy. We estimated the odds of receiving a partial nephrectomy and assessed interactions between covariates and the year of surgery to explore whether patient factors associated with partial nephrectomy changed over time.
RESULTS
In our cohort of 14,186 patients, 4,508 (31.2%) received a partial nephrectomy. Use of partial nephrectomy increased from 17% in 2002, to 32% in 2008, and to 38% in 2014. Patient race/ethnicity, age, tumor stage, and year of surgery were independently associated with receipt of partial nephrectomy. Black veterans had significantly increased odds of receipt of partial nephrectomy, while older patients had significantly reduced odds. Partial nephrectomy utilization increased for all groups over time, but older patients, and patients with worse baseline kidney function showed the least increase in odds of partial nephrectomy.
CONCLUSIONS
While the utilization of partial nephrectomy increased for all groups, the greatest increase occurred in the youngest patients and those with the highest baseline kidney function. These trends warrant further investigation to ensure that patients at the highest risk of impaired kidney function are considered for partial nephrectomy whenever possible.
MeSH Headings: Nephrectomy, Kidney Neoplasms, Carcinoma, Renal Cell, Renal Insufficiency, Chronic, Healthcare Disparities
INTRODUCTION AND OBJECTIVES
Surgical resection is the primary treatment of localized kidney cancer (renal cell carcinoma, RCC).1 Historically, radical nephrectomy was considered the gold standard oncologically, and partial nephrectomy was reserved for patients with a solitary kidney or bilateral renal masses.2 More recently, partial nephrectomy has been used with increasing frequency, likely motivated by several factors. Increasing use of abdominal imaging has been associated with a shift toward detection of small renal masses where partial nephrectomy is feasible. Studies demonstrating significant risks associated with even small losses of renal function have provided further rationale for partial nephrectomy.3 Adoption of new technologies, such as robot-assisted partial nephrectomy, has aided technically challenging minimally invasive partial nephrectomy procedures.4 Finally, oncologic outcomes have been shown to be equivalent between radical and partial nephrectomy for many patients 5 such that partial nephrectomy is now considered a standard surgical approach for small renal masses.6,7
As a result, the utilization of partial nephrectomy has steadily increased over the past 15 years. With this growing experience, reports have suggested that partial nephrectomy is being applied to larger and more complex tumors,8-10 and is being offered to older and multi-morbid patients.11 While administrative data suggests that partial nephrectomy may improve survival when compared with radical nephrectomy,12 partial nephrectomy has remained concentrated in high-volume academic centers, and reserved for patients with better insurance and higher socioeconomic status.13 It is unknown if the increased overall use of partial nephrectomy has benefited patients with impaired pre-operative kidney function, who could benefit most from the procedure.
The Veterans Health Administration (VHA) operates the largest national integrated health care system in the US. The VHA uses a standardized electronic health record that allows detailed assessment of pre-operative comorbidity and direct measures of kidney function for all patients seen within the VHA. We sought to examine the interaction among patient and tumor characteristics, baseline kidney function, and surgery year with the utilization of partial nephrectomy in the VHA.
MATERIALS AND METHODS
The study was approved by the Stanford University Institutional Review Board. Appropriate approvals were also obtained from Veterans Affairs Palo Alto Health Care System and individual data sources, where required.
Analytic Cohort
An overview of our analysis is provided in Supplemental Figure 1. We identified a national cohort of patients who underwent kidney cancer surgery in the VHA using diagnostic and procedure claims codes from 2002 – 2014 (Radical nephrectomy: ICD9 - 5551, 5552, 5553, 5554 & CPT - 50220, 50225, 50230, 50545, 50546; Partial nephrectomy: ICD9 - 554, 5531, 5539, 5561 & CPT - 50240, 50280, 50290, 50542, 50543). We excluded patients with more than one kidney cancer surgical code over the study period (N = 216). Patient age, race/ethnicity, sex, and treating facility were abstracted from the VHA Corporate Data Warehouse (CDW). The CDW is a comprehensive data repository for all patient encounters at all VHA facilities during the study period. Pre-operative comorbidity was assessed using the Romano adaptation of the Charlson-Deyo comorbidity score.14 The Charlson comorbidity score was examined as a categorical variable, except in models testing for effect modification, where it was included as a continuous variable and in interaction terms.
Tumor Specific Information
We extracted tumor specific data for patients with kidney cancer entries in the CDW Oncology data using the 2010 TNM staging system. Tumor specific data were available in 9,176 (65%) patients. We excluded patients (N = 767) with evidence of regional or distant metastasis (N or M stage > 0).
Pre-operative Kidney Function Measurement
We identified 120,344 pre-operative serum creatinine measurements in the 12-month period prior to the date of surgery using VHA laboratory data. When more than one serum creatinine value was available, we defined baseline pre-operative kidney function using the average of these results. We estimated the glomerular filtration rate (eGFR) using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation.15 We also performed a companion sensitivity analysis using the serum creatinine value immediately prior to surgery. We excluded patients with stage 4 or 5 chronic kidney disease (eGFR values <30 mL/min/1.73 m2) or with two or more dialysis procedure codes (ICD9 – 3995, 5498, V56, V56.8, V45.1 & CPT – 90935, 90937, 90945, 90947; N = 405, 1.2%).
Statistical Analysis
We examined the use of partial nephrectomy over time using raw case counts as well as the percentage of all kidney cancer surgeries by year. We evaluated the proportion of patients treated with partial nephrectomy, stratified by clinical T1 (<7cm tumors) cutoffs. We examined associations among patient and tumor variables and receipt of partial nephrectomy using Student’s t-test for continuous and the χ2 test for categorical variables. We fit unadjusted and multivariable logistic regression models to estimate the odds of receipt of partial nephrectomy associated with patient and tumor characteristics. To explore changes over time, we tested for effect modification by treatment year for all variables associated with receipt of partial nephrectomy. In addition to main effects, and effect modification over time, we also tested the interaction between comorbidity and preoperative kidney function. We included baseline serum creatinine concentrations in the logistic models, instead of eGFR, to avoid the inclusion of patient age and race in multiple model parameters. We evaluated model discrimination using the concordance (“c”) statistic, corresponding to the area under the receiver operating characteristic (ROC) curve. We used to the Hosmer-Lemeshow χ2 goodness-of-fit test to assess model calibration. All analyses were performed within the VA Informatics and Computing Infrastructure (VINCI) platform using SAS v9.4 (Cary, NC) and figures were generated using JMP Pro v12 (Cary, NC).
RESULTS
Table 1 describes the characteristics of the 14,024 patients that received a radical or partial nephrectomy in the VHA over the study period. Of these, 4,499 (32%) underwent partial nephrectomy. The cohort was predominantly male (96.4%), with an average age of 64.4 years (median age 64 years, interquartile range [IQR] 58 to 71). Patients typically presented with pre-operative chronic kidney disease, with a mean preoperative eGFR of 71.6 mL/min/1.73 m2 (median 70.5 mL/min/1.73 m2, IQR 56.7 to 84.9 mL/min/1.73 m2).
Table 1.
Patient demographics.
| Characteristic | Radical Nephrectomy N = 9525 | Partial Nephrectomy N = 4499 | P Value |
|---|---|---|---|
| Age | |||
| Mean (SD) | 65.2 (9.3) | 62.5 | |
| Median (IQR) | 65 (59, 72) | 63 (57, 68) | |
| Age Group (%): | |||
| <45 | 268 (2.8) | 165 (3.7) | < 0.0001 |
| 45 - 60 | 2668 (28.0) | 1535 (34.1) | |
| 60 - 75 | 4911 (51.6) | 2425 (53.9) | |
| ≥75 | 1678 (17.6) | 374 (8.3) | |
| Sex (%): | |||
| Male | 9176 (96.3) | 4341 (96.5) | 0.65 |
| Female | 349 (3.7) | 158 (3.5) | |
| Race / Ethnicity (%): | |||
| White | 6903 (72.5) | 2974 (66.1) | < 0.0001 |
| Black | 1230 (12.9) | 884 (19.6) | |
| Other or unknown | 1392 (14.6) | 641 (14.2) | |
| Charlson Score | |||
| Mean (SD) | 1.93 (1.66) | 1.91 (1.55) | |
| Median (IQR) | 2 (1, 3) | 2 (1,3) | |
| Charlson Score (%): | |||
| 0 | 2437 (25.6) | 1048 (23.3) | 0.0003 |
| 1 | 1406 (14.9) | 727 (16.2) | |
| 2 | 2581 (27.0) | 1310 (29.1) | |
| 3 | 1743 (18.3) | 835 (18.5) | |
| ≥4 | 1358 (14.2) | 579 (12.9) | |
| Pre-operative eGFR: | |||
| Mean (SD) | 70.1 (19.8) | 74.7 (20.3) | |
| Median (IQR) | 68.7 (55.4, 83.0) | 74.5 (60.2, 88.4) | |
| Pre-operative eGFR (%) | |||
| ≥ 90 | 1564 (16.4) | 1016 (22.6) | < 0.0001 |
| 60 - 89 | 4821 (50.6) | 2370 (52.7) | |
| 45 - 59 | 2188 (23.0) | 762 (16.9) | |
| 30 - 44 | 952 (10.0) | 351 (7.8) | |
| Geographic Region (%): | 2615 (27.5) | 1187 (26.4) | 0.0011 |
| Midwest | 2261 (23.7) | 1092 (24.3) | |
| West | 1302 (13.7) | 720 (16.0) | |
| Northeast | 3347 (35.1) | 1500 (33.3) | |
| South | |||
| Tumor stage (%): | |||
| T1 | 3099 (32.5) | 2664 (59.2) | < 0.0001 |
| T2 | 976 (10.2) | 87 (1.9) | |
| T3 | 605 (6.4) | 66 (1.5) | |
| unknown | 4845 (50.9) | 1682 (37.4) |
Patients treated with partial nephrectomy were younger (mean age 62.5 versus 65.2 years; P < 0.0001), and healthier in general (mean Charlson score 1.92 versus 1.93, P = 0.0003). Patients treated with partial nephrectomy also had better preoperative kidney function (mean eGFR 74.8 versus 70.1 mL/min/1.73 m2; P < 0.0001), and less likely to have grade 3 chronic kidney disease (24.7% versus 33.0%). Black patients were more commonly treated with a partial nephrectomy than white patients (19.6% versus 12.9%; P < 0.0001). Geographic region was also associated with receipt of partial nephrectomy with most partial nephrectomies performed in the South region (P = 0.001).
Use of partial nephrectomy increased for all stages of RCC from 17% in 2002, to 32% in 2008, and to 38% in 2014 (Figure 1A). Use of partial nephrectomy was highest for patients with smaller renal masses (T stage 1, <7cm), and increased from 22% in 2002, to 36% in 2008, and to 44% in 2014. While the use of partial nephrectomy was similar across ages in 2002, by 2014 two-thirds of patients under age 45 underwent partial nephrectomy, compared with approximately one-fourth of patients over age 75 (Figure 1B). Furthermore, the percentage of patients with the normal pre-operative kidney function receiving partial nephrectomy increased but changed little for those with impaired kidney function (Figure 1C). There were no significant trends in receipt of partial nephrectomy by Charlson comorbidity score (Figure 1D). The percentage of patients treated with partial nephrectomy stratified by patient age, comorbidity, and pre-operative kidney function are shown in Supplemental Figure 2.
Figure 1.
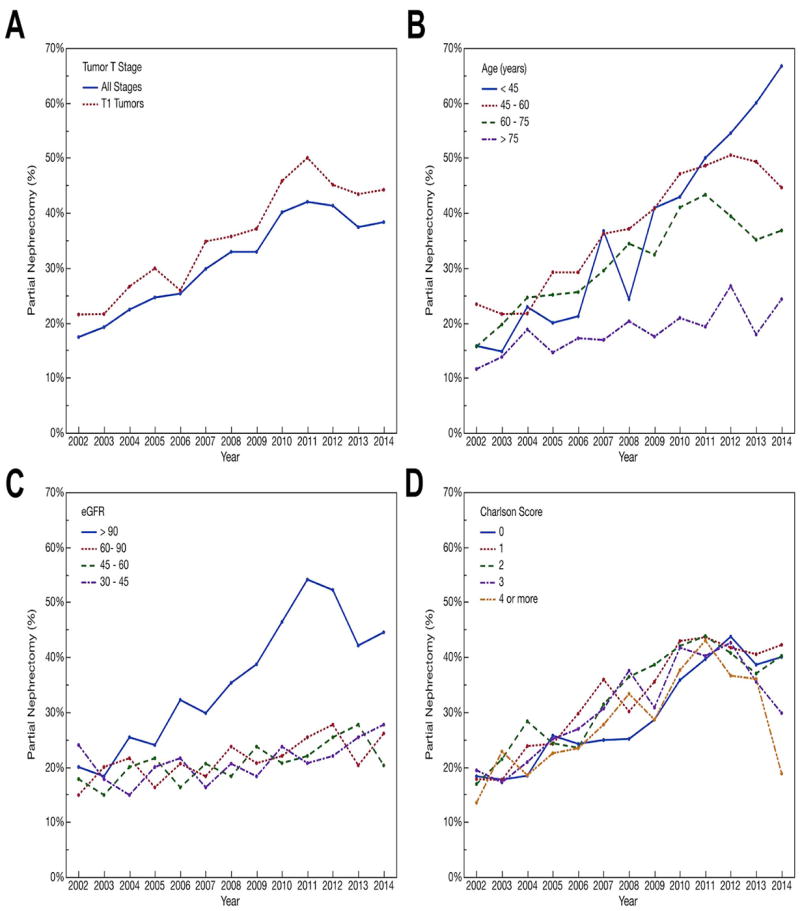
A) The percentage of all patients (blue), and patients with T1 tumors (red), receiving partial nephrectomy each year. There was a steady increase in the utilization of partial nephrectomy over the study period. B) The percentage of patients receiving partial nephrectomy by year and age. Younger patients had the greatest increase in the proportion receiving partial nephrectomy. C) The percentage of patients receiving partial nephrectomy by year and pre-operative eGFR. Patients with the best pre-operative kidney function showed the greatest increase in proportion receiving partial nephrectomy. D) The percentage of patients receiving partial nephrectomy by year and Charlson score.
In unadjusted logistic regression models, patients that were younger, Black, with better pre-operative kidney function, with more comorbid conditions, with lower stage tumors, and who were treated more recently had increased odds of receipt of partial nephrectomy (Table 2). In multivariable models, younger age, female sex, non-white race/ethnicity, year of surgery, and tumor stage remained significantly associated with the odds of receipt of partial nephrectomy. The odds of receiving partial nephrectomy increased 10% per year for all patients. Pre-operative kidney function (using serum creatinine measurements) did not meet statistical significance in the multivariable model (P = 0.083). Using the creatinine measurement immediately prior to surgery, instead of the average of pre-operative creatinine measurements, did not materially change the results.
Table 2.
Uni- and multivariable logistic regression models estimating the odds of receipt of partial nephrectomy.
| Characteristic | Univariable OR (95%CI) | Multivariable OR (95%CI) |
|---|---|---|
| Age (per 5 year increase) | 0.88 (0.86, 0.89) | 0.88 (0.86, 0.89) |
| Sex (Female vs. Male) | 0.96 (0.79, 1.16) | 0.76 (0.61, 0.93) |
| Year of surgery | 1.10 (1.09, 1.16) | 1.10 (1.09, 1.11) |
| Race / Ethnicity: | ||
| Black vs. white | 1.67 (1.51, 1.84) | 1.52 (1.37, 1.69) |
| Other/Unknown vs. white | 1.07 (0.96, 1.18) | 1.14 (1.02, 1.27) |
| Charlson Score | ||
| 1 vs. 0 | 1.20 (1.07, 1.35) | 1.30 (1.15, 1.48) |
| 2 vs. 0 | 1.18 (1.07, 1.30) | 1.25 (1.12, 1.39) |
| 3 vs. 0 | 1.11 (1.00, 1.24) | 1.20 (1.06, 1.35) |
| ≥4 vs. 0 | 0.99 (0.88, 1.12) | 1.07 (0.94, 1.22) |
| Serum Creatinine (per 0.1 mg/dL increase) | 0.96 (0.94, 0.97) | 0.99 (0.98, 1.00) |
| Clinical T Stage: | ||
| T2 vs. T1 | 0.10 (0.08, 0.13) | 0.10 (0.08, 0.13) |
| T3 vs. T1 | 0.13 (0.10, 0.16) | 0.13 (0.10, 0.18) |
| Unknown vs. T1 | 0.40 (0.37, 0.44) | 0.45 (0.41, 0.48) |
| VA Region: | ||
| North East vs. Mid West | 1.22 (1.09, 1.37) | 0.89 (0.79, 1.01) |
| South vs. Mid West | 0.99 (0.90, 1.08) | 1.30 (1.15, 1.47) |
| West vs. Mid West | 1.06 (0.96, 1.18) | 0.98 (0.88, 1.08) |
Odds Ratios in bold indicate a statistical significance threshold of P < 0.05.
We observed significant interactions with surgery year for both patient age (P = 0.017) and pre-operative serum creatinine measurements (P = 0.0002). (Supplemental Table 1) While the odds of receipt of partial nephrectomy increased for all groups, older patients and patients with the lowest pre-operative kidney function showed the slowest rate of increase in odds of receipt of partial nephrectomy. The interaction of surgery year and Charlson comorbidity score, and surgery year and race/ethnicity were not significant. The interaction between Charlson comorbidity score and pre-operative kidney function was significant (P= 0.036). After adjusting for patient comorbidity and tumor characteristics, the odds ratios and probabilities of receipt of partial nephrectomy declined for older patients throughout the study period, as shown in Figure 2. Patients with lower pre-operative kidney function initially had increased odds of receipt of partial nephrectomy, but this declined such that these patients had the lowest odds of receipt of partial nephrectomy by 2014.
Figure 2.
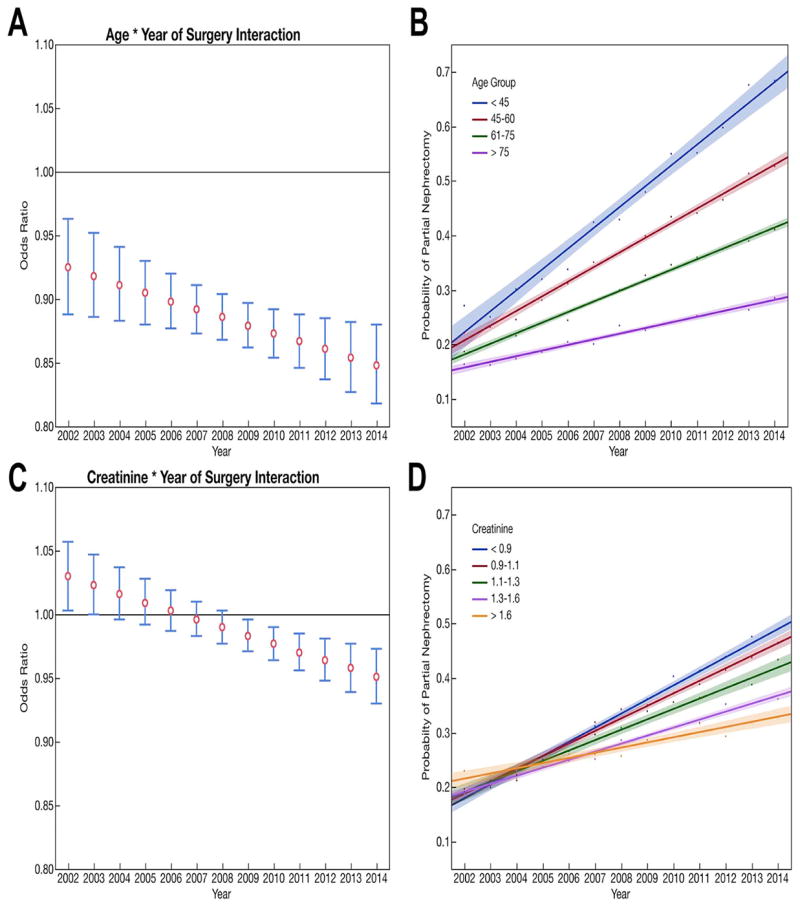
Graphical representation of the interactions among patient age, pre-operative kidney function, and the year of surgery. A) The odds ratio of receipt of partial nephrectomy by increasing age (per 5 year increase) shows a steady decline. B) The probability a reference patient would receive of partial nephrectomy by age group and year is shown with the oldest patients having the slowest rise. Reference patients were defined as white, male, with T1 tumors with continuous variables set at their average value (serum creatinine = 1.15 mg/dL, Charlson score = 1.92). C) Similarly, patients with impaired kidney function initially had increased odds of receipt of partial nephrectomy that decreased over the study period. D) The probability of reference patient receiving partial nephrectomy by quintile of pre-operative kidney function and year initially showed higher probabilities for patients with the lowest baseline function that reversed over the study period. Reference patients were defined as white, male, with T1 tumors with continuous variables set at their average value (age = 64.3 years, Charlson score = 1.92).
DISCUSSION
Veterans treated in the VHA have worse physical and mental health compared with the general population, and may be more susceptible to loss of kidney function after kidney cancer surgery.16 Landrum et al. demonstrated lower performance status and higher rates of severe comorbidity among cancer patients treated in the VHA.17 Despite caring for a complex patient population, data suggests that the VHA generally outperforms community care across a broad range of conditions. For example, after adjusting for comorbidity, cancer outcomes in the VHA have been shown to be equal or better than in the Medicare system.17 Several groups have suggested that the underutilization of partial nephrectomy is a quality concern,18,19 and that the proportion of patients treated with partial nephrectomy may be an indicator of high-quality care.13 In this study, we show that the utilization of partial nephrectomy increased rapidly in the VHA between 2002 and 2014. By 2014, nearly 38% of all extirpative kidney surgeries were partial nephrectomies, including 44% of surgeries for clinical t1 renal masses. These data suggest that the VHA is adopting partial nephrectomy at a comparable, or perhaps faster rate, than the community.13,19-21
Despite increasing partial nephrectomy use, this study also identifies concerning disparities in receipt of partial nephrectomy in the VHA. Since we had access to the clinical laboratory data, we were able directly measure pre-operative kidney function, which was not feasible in previous work. Surprisingly, patients treated with partial nephrectomy had better baseline kidney function. Among patients with stage 3b chronic kidney disease (eGFR < 45 mL/min/1.73 m2), the proportion of patients receiving partial nephrectomy remained relatively stable over the study period (24% in 2002, to 21% in 2008, and 28% in 2014). Prior reports using data from the Nationwide Inpatient Sample from 2007 to 2010 also found that pre-operative claims for chronic kidney disease were not associated with higher odds of receipt of partial nephrectomy.20 “Renalism” is a term previously used to describe inappropriately low rates of coronary angiography in patients with impaired kidney function.22 Here we find evidence of renalism – an aversion to perform a potentially more complex partial nephrectomy surgery in patients with impaired kidney function – despite the historical presumption that these patients derive the most benefit from partial nephrectomy.
We also show that older patients had slower increases in their odds of receipt of partial nephrectomy over time. Evidence suggesting “ageism” has consistently been seen in reports of utilization of partial nephrectomy in the community.13,20,21 However, it is unclear why partial nephrectomy would evolve to be used least in older patients. Urologists may believe that older patients may derive fewer long-term benefits from the preservation of kidney function by partial nephrectomy. In 2012, Tan et al. reported an instrumental variable analysis of Medicare patients and demonstrated that receipt of a partial nephrectomy was associated with significantly improved overall survival.12 In sub-group analysis, this survival benefit was not statistically significant among Medicare beneficiaries older than 75 years of age. It is also possible that urologists consider partial nephrectomy too risky in older patients. However, reports demonstrate that partial nephrectomy is safe. Perioperative morbidity and mortality rates have been shown to be equivalent for Veterans undergoing partial nephrectomy and radical nephrectomy in the VHA.23 In centers where robot-assisted surgery is not available, the benefits of a minimally invasive laparoscopic radical nephrectomy may be considered preferable to an open partial nephrectomy.
We identified additional disparities in the use of partial nephrectomy in the VHA. White patients had lower odds of receipt of a partial nephrectomy compared with Black or non-white non-Black patients. This finding contrasts with reports that Black patients, despite data showing a higher prevalence of chronic kidney disease, have lower odds of receipt of partial nephrectomy at academic and comprehensive community centers.24-26 One possible explanation for these previous observations was due in part to inability to adjust for pre-operative kidney function.27 In our study, a larger percentage of Black patients underwent partial nephrectomy, and had increased odds of receipt of partial nephrectomy after adjusting for pre-operative kidney function. Female patients, while a minority in the VHA, also had increased odds of receipt of a partial nephrectomy. Finally, we observed geographic variation in the use of partial nephrectomy. In the setting of an integrated health care system, where patients have theoretically equal access to services, the reasons for these disparities are unknown and warrant further attention.
The increasing use of partial nephrectomy is encouraging and reflects guideline concordant care. However, the use of elective partial nephrectomy may be associated with the selection of the youngest and most fit patients and dilute the potential benefits of partial nephrectomy. For example, most patients in an EORTC trial comparing partial to radical nephrectomy had no chronic medical conditions and normal or near-normal preoperative kidney function. For these patients, randomization to partial nephrectomy did not confer a significant survival benefit at a median follow-up of nine years.28 While these data suggest that younger and healthier patients may not benefit from partial nephrectomy, partial nephrectomy is increasing most in these patient groups. For patients that are poor surgical candidates or with limited life expectancies due to comorbidity, active surveillance – instead of partial or radical nephrectomy – may offer the best nephron-sparing alternative to nephrectomy.29,30
There are several important limitations of this study. We could not adjust for patient characteristics and preferences that might dictate the use of non-nephron sparing approaches, even for small tumors (e.g., bleeding disorders, refusal of blood transfusion consent). Furthermore, it is possible that some procedures were misclassified as a “radical nephrectomy” instead of a “simple nephrectomy” and were performed for benign conditions that would not normally be suitable for partial nephrectomy. Among patients with a renal mass, the anatomic features of the tumor, including size, location, or complexity of the renal mass (e.g., nephrometry scores), that could impact use of partial nephrectomy were not available. We also were not able to determine the surgical approach (open versus laparoscopic versus robot-assisted) or the availability of local expertise using these approaches. Furthermore, many VHA facilities are associated with academic medical centers and the VHA does centralize complex surgical care to higher volume facilities. These forces are highly likely to influence adoption of partial nephrectomy and might not reflect practice patterns in the community.13 Finally, the VHA population is predominantly male and may not be representative of the general population.
Despite these limitations, this study has notable strengths. The cohort includes granular data for a large group of patients treated across the period of rapid adoption of partial nephrectomy. In addition, we had direct access to pre-operative laboratory values so that we could fully characterize pre-operative kidney function – which was not available in prior population-based studies of partial nephrectomy use. We also had sufficient sample size to test for temporal trends and effect modification by year of surgery in a large fixed integrated health care system.
CONCLUSIONS
While the utilization of partial nephrectomy has increased over time for all groups in the VHA, the greatest increase has occurred in the youngest patients and those with the highest baseline kidney function. Older patients, and patients with impaired kidney function, were less likely to receive partial nephrectomy even though they potentially have the most to gain from the procedure.
Supplementary Material
Overview of cohort creation and analytic methods.
The proportion of patients receiving a partial or radical nephrectomy stratified by age group, pre-operative kidney function, and Charlson comorbidity score.
Acknowledgments
Funding
The authors were supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK089086 to JTL and DK085446 to GMC) at the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969;101:297–301. doi: 10.1016/s0022-5347(17)62331-0. [DOI] [PubMed] [Google Scholar]
- 2.Novick AC, Gephardt G, Guz B, et al. Long-term follow-up after partial removal of a solitary kidney. N Engl J Med. 1991;325:1058–1062. doi: 10.1056/NEJM199110103251502. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Sivarajan G, Taksler GB, Walter D, et al. The Effect of the Diffusion of the Surgical Robot on the Hospital-level Utilization of Partial Nephrectomy. Med Care. 2015;53:71–78. doi: 10.1097/MLR.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belldegrun A, Tsui KH, deKernion JB, et al. Efficacy of nephron-sparing surgery for renal cell carcinoma: analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol. 1999;17:2868–2875. doi: 10.1200/JCO.1999.17.9.2868. [DOI] [PubMed] [Google Scholar]
- 6.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Kopp RP, Mehrazin R, Palazzi KL, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int. 2014;114:708–718. doi: 10.1111/bju.12580. [DOI] [PubMed] [Google Scholar]
- 9.Breau RH, Crispen PL, Jimenez RE, et al. Outcome of stage T2 or greater renal cell cancer treated with partial nephrectomy. J Urol. 2010;183:903–908. doi: 10.1016/j.juro.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Long CJ, Canter DJ, Kutikov A, et al. Partial nephrectomy for renal masses >/= 7 cm: technical, oncological and functional outcomes. BJU Int. 2012;109:1450–1456. doi: 10.1111/j.1464-410X.2011.10608.x. [DOI] [PubMed] [Google Scholar]
- 11.Maurice MJ, Zhu H, Kim SP, et al. Increased use of partial nephrectomy to treat high-risk disease. BJU Int. 2015 doi: 10.1111/bju.13262. [DOI] [PubMed] [Google Scholar]
- 12.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307:1629–1635. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SG, Penson DF, Pabla B, et al. National trends in the use of partial nephrectomy: a rising tide that has not lifted all boats. J Urol. 2012;187:816–821. doi: 10.1016/j.juro.2011.10.173. [DOI] [PubMed] [Google Scholar]
- 14.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081-1090. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 17.Landrum MB, Keating NL, Lamont EB, et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1072–1079. doi: 10.1200/JCO.2011.35.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller DC, Hollingsworth JM, Hafez KS, et al. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853–857. doi: 10.1016/S0022-5347(05)00422-2. discussion 858. [DOI] [PubMed] [Google Scholar]
- 19.Hollenbeck BK, Taub DA, Miller DC, et al. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254–259. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 20.Bjurlin MA, Walter D, Taksler GB, et al. National trends in the utilization of partial nephrectomy before and after the establishment of AUA guidelines for the management of renal masses. Urology. 2013;82:1283–1289. doi: 10.1016/j.urology.2013.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SP, Shah ND, Weight CJ, et al. Contemporary trends in nephrectomy for renal cell carcinoma in the United States: results from a population based cohort. J Urol. 2011;186:1779–1785. doi: 10.1016/j.juro.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Chertow GM, Normand SL, McNeil BJ. "Renalism": inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15:2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 23.Corman JM, Penson DF, Hur K, et al. Comparison of complications after radical and partial nephrectomy: results from the National Veterans Administration Surgical Quality Improvement Program. BJU Int. 2000;86:782–789. doi: 10.1046/j.1464-410x.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 24.Kiechle JE, Abouassaly R, Gross CP, et al. Racial Disparities in Partial Nephrectomy Persist Across Hospital Types: Results From a Population-based Cohort. Urology. 2016;90:69–75. doi: 10.1016/j.urology.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 25.Colli J, Sartor O, Grossman L, et al. Underutilization of partial nephrectomy for stage T1 renal cell carcinoma in the United States, trends from 2000 to 2008. A long way to go. Clinical genitourinary cancer. 2012;10:219–224. doi: 10.1016/j.clgc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Small AC, Tsao CK, Moshier EL, et al. Trends and variations in utilization of nephron-sparing procedures for stage I kidney cancer in the United States. World J Urol. 2013;31:1211–1217. doi: 10.1007/s00345-012-0873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strope SA. Editorial Comment. Urology. 2016;90:74. doi: 10.1016/j.urology.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 28.Scosyrev E, Messing EM, Sylvester R, et al. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65:372–377. doi: 10.1016/j.eururo.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Tomaszewski JJ, Kutikov A. Small renal mass management in the elderly and the calibration of risk. Urol Oncol. 2015;33:197–200. doi: 10.1016/j.urolonc.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Daskivich TJ, Tan HJ, Litwin MS, et al. Life Expectancy and Variation in Treatment for Early-Stage Kidney Cancer. J Urol. 2016 doi: 10.1016/j.juro.2016.03.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of cohort creation and analytic methods.
The proportion of patients receiving a partial or radical nephrectomy stratified by age group, pre-operative kidney function, and Charlson comorbidity score.


