Abstract
Background
The advent of oral disease-modifying therapies fundamentally changed the treatment of multiple sclerosis. Nevertheless, impressions of their relative efficacy and tolerability are primarily founded on expert opinion.
Objective
The purpose of this study was to determine whether oral disease-modifying therapies were better tolerated and/or more effective for controlling multiple sclerosis compared to injectable therapies in clinical practice.
Methods
Single-center, retrospective cohort study. 480 patients initiated oral (fingolimod, teriflunomide, or dimethyl fumarate) or injectable therapy between March 2013–March 2015 and follow-up data was collected for 5–31 months. Outcomes included on-drug multiple sclerosis activity and drug discontinuation. Cox proportional hazards models were used to control for baseline differences and sensitivity analyses using propensity-weighted matching were performed.
Results
A higher proportion of teriflunomide-treated patients experienced multiple sclerosis activity compared to those treated with injectable therapies (p = 0.0053) in the adjusted model. Breakthrough multiple sclerosis was equally prevalent among fingolimod and dimethyl fumarate-treated compared to injectable therapy-treated patients. Of patients initiating a disease-modifying therapy, 32–46% discontinued or switched treatments during the study. After controlling for baseline differences, discontinuation rates were comparable across treatment groups.
Conclusions
In this cohort, oral and injectable disease-modifying therapies were equally well tolerated, but teriflunomide appeared less effective for controlling multiple sclerosis activity than injectable therapies. Further study is needed.
Keywords: Multiple sclerosis, disease modifying therapy, dimethyl fumarate, teriflunomide, fingolimod, tolerability
Introduction
Disease modifying therapies (DMTs) became available to treat multiple sclerosis (MS) in the mid-1990s, but for years the only options were injectable (INJ) beta-interferons or glatiramer acetate. More recently, three oral DMTs have become available to treat relapsing MS: fingolimod (FGD, released in the USA in 2010), teriflunomide (TER, released in 2012), and dimethyl fumarate (DMF, released in 2013). Stable treatment with DMT improves the clinical course of MS and reduces long-term disability,1–3 and patients who adhere to DMT incur lower medical costs and are less likely to require hospitalization.4 Nevertheless, the expansion of treatment options for MS has been paralleled by uncertainties regarding the best prescribing practice for these medications.
Many MS clinicians now strive for a benchmark of “no evidence of disease activity” (NEDA) and recommend switching DMT whenever MS activity occurs during treatment,5–7 which has led to increased switching between medications.8 In most cases, the decision to change medications is based on clinical acumen, as little data is available to guide recommendations. This is especially true of the oral agents. These have never been directly compared to one another in clinical trials, and little empirical evidence exists to differentiate them.
Several groups have utilized Phase 3 clinical trial data to indirectly compare the oral DMTs with varying results. When individual level data for FGD were re-analyzed using the same methodologies used in trials for TER and DMF, patients taking FGD were more likely to achieve NEDA than patients taking TER or DMF.9 Others found DMF to be superior to INJ therapy and TER, equivalent to FGD, and inferior to natalizumab for reducing the annualized relapse rate by performing a systematic review and data synthesis of published randomized controlled trials.10 These data were derived from Phase 3 trials, which have stringent inclusion and exclusion criteria and do not represent the full spectrum of MS. The relative efficacy of oral and INJ DMTs may differ in clinical practice.
Observational data gleaned from clinical registries represent a broader spectrum of MS patients. Researchers using the NeuroTransData network, a cohort of patients who switched DMTs due to INJ therapy failure, concluded that patients switching to FGD were more likely to become relapse and progression free than those who initiated a second INJ therapy.11 Data obtained from MS Base, a multi-national registry, suggested that both treatment-naïve patients starting FGD and patients switching to FGD because of breakthrough MS had improved clinical outcomes and were less likely to discontinue treatment than comparable patients prescribed INJ therapy.12,13 Insurance claims analyses also found that MS patients were less likely to discontinue FGD compared to INJ therapy.14,15 These studies did not assess the performance of the other oral agents.
Comparative data to guide DMT selection is lacking. It is therefore important to simultaneously study all three oral DMTs in conjunction with first-line INJ treatments. We evaluated a cohort of 480 patients who initiated treatment with an oral or INJ DMT in order to compare drug effectiveness and persistence with therapy in a real world clinical setting.
Methods
Design
This was a retrospective, observational cohort study at an MS center comprised of five board-certified neurologists subspecializing in MS. All patients who initiated therapy with oral (DMF, TER, FGD) or INJ DMT between March 2013–March 2015 were included. All patients had a relapsing form of MS (relapsing–remitting or secondary progressive with relapses) and could be either treatment-naïve or switching between DMTs. For this study, interferon β (IFN-β) medications and glatiramer acetate were pooled as INJ medications. No significant differences in efficacy were observed when subcutaneous IFN-β1a and glatiramer acetate were compared in a randomized trial,16 and pooling INJ medications is a common technique for large observational studies.8,11 Data were collected for an additional six months to ensure a minimum follow-up duration. This study was approved by the Human Research Protection Office at Washington University in St Louis, USA.
Chart reviews
Medical records were reviewed by a board-certified MS neurologist (EEL). Demographic and clinical data were extracted. Demographic data included age, sex, and race. Clinical data included time since diagnosis, number of previous DMTs, expanded disability status scale (EDSS), relapses within the last 12 months (0 vs ≥1) and prescribing neurologist. EDSSs were estimated based on physical examination at the time of DMT initiation, then categorized as mild (EDSS 0–3), moderate (EDSS 3.5–5.5), or severe disability (EDSS ≥ 6). All patients taking TER were prescribed 14 mg daily. Outcomes included on-treatment MS activity, treatment discontinuation, and reasons for discontinuation. MS activity was defined as a clinical relapse (based on the treating physician’s documented impression and/or decision to treat with corticosteroids) or new radiological evidence of disease activity (new T2-weighted or gadolinium-enhancing lesion on follow-up magnetic resonance imaging (MRI) compared to baseline) after treatment for three months or longer. Clinical relapse or MRI activity within the first two months of starting a medication was not considered as MS activity, since most DMTs require several months to reach efficacy. Persistence was defined as the length of time a patient remained on a DMT. When assessing drug discontinuation, relapses, MRI progression, and slow disability accumulation were judged to be MS activity-related reasons for discontinuing. Skin/hair side effects included rash, flushing, injection site reactions, and hair loss. Cardiovascular side effects included chest pain and bradycardia. Neurologic side effects included paresthesia, neuropathy, and headache. “Other” side effects included flu-like symptoms and generalized complaints. Laboratory abnormalities included lymphopenia and transaminitis.
Statistical analysis
Demographic and clinical characteristics were summarized using descriptive statistics. One-way analysis of variance (ANOVA) was used to compare age, disease duration, and follow-up duration. Pearson chi-square tests were used to compare sex, race, number of relapses in the past 12 months, EDSS category, and number of prior medications. Only the first observation for each patient was used for baseline comparisons. A Cox proportional hazards model accounting for recurrent events was used to evaluate the time to discontinuation and MS disease activity to control for differences in baseline covariates.
Sensitivity analysis
Propensity score matching was used as a sensitivity analysis to evaluate the robustness of the adjusted model results in a sample which is more homogeneous at baseline. Patients initiating FGD, TER, or DMF were matched to patients initiating INJ therapy using propensity scores. These were estimated using three separate logistic regression models with FGD/INJ, TER/INJ and DMF/INJ as the dependent variable and age, sex, race, prescribing physician, disease duration, categorized EDSS, presence of relapses in last 12 months, and number of prior DMTs as potential confounders. The matching process used the nearest neighbor method within specified caliper widths (caliper = 0.20×standard deviation (logit of the propensity score)) without replacement.17 Patients who initiated multiple types of medication during the study used only the first observed treatment for matching.
After matching, the balance of covariates was evaluated. The absolute standardized differences of the covariates for the unmatched and matched cohorts were compared between the INJ and matched FGD, TER, and DMF samples. Standardized differences greater than 0.1 have been shown to indicate some covariate imbalance between matched groups.18 After propensity matching, time to event outcomes between matched groups were tested using Cox proportional hazards model with robust standard errors to account for the within-pair homogeneity in matched sample models.19
Results
We identified 480 unique patients who initiated treatment with 579 oral or INJ DMTs during the predefined study period. Eighty-seven patients discontinued and restarted another DMT at least once during the study. There were significant differences between the treatment groups at baseline, reflecting clinical prescribing practice. Patients who initiated INJ therapy had more recent MS diagnoses, milder disability, increased relapse frequency, and fewer prior DMT exposures when compared to patients initiating oral DMTs (Table 1). Patients taking TER tended to be older than those in the other treatment groups. Differences in prescriber practice were also observed. Within the INJ group, interferons (n = 72, 48%) and glatiramer acetate (n = 78, 52%) were equally prescribed. Notably, a third or more (33–46%) of patients taking any given DMT opted to discontinue or switch treatments during the 2.5 years of study observation (Table 1).
Table 1.
Uncorrected cohort characteristics.
| INJ (n = 150) | DMF (n = 254) | TER (n = 83) | FGD (n = 92) | p-Value | |
|---|---|---|---|---|---|
| Females, n (%) | 118 (78.7) | 183 (72.0) | 68 (81.9) | 67 (72.8) | 0.113 |
| Age, mean (SD) | 41.6 (13.1) | 44.7 (12.2) | 49.4 (10.4) | 39.8 (9.3) | <0.001 |
| Caucasian, n (%) | 120 (80.0) | 216 (85.0) | 70 (84.3) | 71 (77.2) | 0.020 |
| MS duration, years, mean (SD) | 5.4 (8.2) | 10.6 (9.4) | 12.2 (9.8) | 7.1 (6.9) | <0.001 |
| Follow-up duration, months, mean (SD) | 18.2 (7.7) | 20.4 (6.5) | 20.3 (7.2) | 17.2 (7.2) | 0.015 |
| Number of previous meds, n (%) | |||||
| 0 | 77 (51.3) | 33 (13.0) | 4 (4.8) | 11 (12.0) | <0.001 |
| 1 | 33 (22.0) | 75 (30.0) | 40 (48.2) | 34 (37.0) | |
| 2 | 25 (16.7) | 73 (28.7) | 23 (27.7) | 25 (27.2) | |
| 3+ | 15 (10.0) | 73 (28.7) | 16 (19.3) | 22 (23.9) | |
| Physician, n (%) | |||||
| 1 | 27 (18.0) | 24 (9.5) | 6 (7.2) | 14 (15.2) | <0.001 |
| 2 | 38 (25.3) | 58 (22.8) | 17 (20.5) | 7 (7.6) | |
| 3 | 19 (12.7) | 33 (13.0) | 38 (45.8) | 33 (26.8) | |
| 4 | 19 (12.7) | 23 (9.1) | 3 (3.6) | 7 (7.6) | |
| 5 | 47 (31.3) | 116 (45.7) | 19 (22.9) | 31 (33.7) | |
| Disability | |||||
| Mild (EDSS 0-3) | 110 (73.3) | 143 (56.3) | 43 (51.8) | 52 (56.5) | <0.001 |
| Moderate (EDSS 3.5-5.5) | 29 (19.3) | 46 (18.11) | 19 (22.9) | 30 (32.6) | |
| Severe (EDSS ≥ 6.0) | 11 (7.3) | 65 (25.6) | 21 (25.3) | 10 (10.9) | |
| Relapses in last 12 months, n (%) | 92 (61.3) | 112 (44.1) | 27 (32.5) | 46 (50.0) | <0.001 |
| On-drug MS activity, n (%) | 28 (18.7) | 54 (21.3) | 25 (30.1) | 24 (26.1) | 0.144 |
| Discontinued DMT, n (%) | 58 (38.7) | 90 (35.4) | 38 (45.8) | 30 (32.6) | 0.167 |
| Switched to a different DMT | 46 (79.3) | 74 (82.2) | 31 (81.6) | 24 (80.0) | |
| Permanently discontinued | 12 (20.7) | 15 (16.7) | 6 (15.8) | 6 (20.0) | |
| Death | 0 | 1 (1.1) | 1 (2.6) | 0 |
DMF: dimethyl fumarate; DMT: disease-modifying therapy; EDSS: estimated disability status score; FGD: fingolimod; INJ: injectable; MS: multiple sclerosis; SD: standard deviation; TER: teriflunomide.
A total of 480 unique patients contributed 579 observations to this cohort. Repeated observations were not considered for the statistics reported. One-way analysis of variance (ANOVA) was used to compare age, disease duration, and follow-up duration between groups. Pearson chi-square tests were used to compare sex, race, MS activity, discontinued DMT, and number of prior medications between groups. One patient in the DMF group and one in the TER group died due to comorbid medical conditions; deaths were unrelated to MS treatment.
MS activity
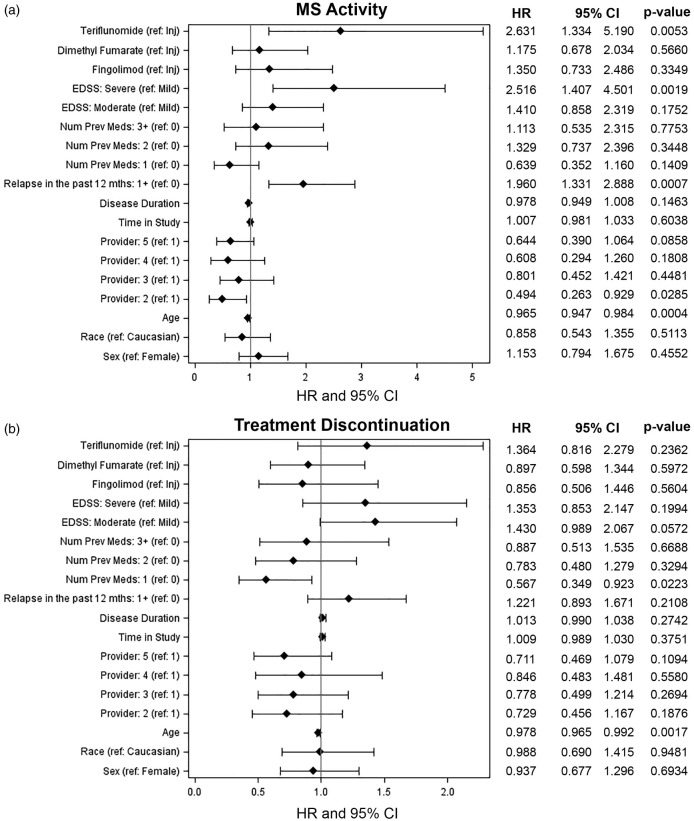
Younger age (hazard ratio (HR) 0.70, 95% confidence interval (CI) 0.58–0.85 per 10-year age increase), severe disability (HR 2.52, 95% CI 1.41–4.50 versus mild disability), and relapses within the last year (HR 1.96, 95% CI 1.33–2.89) were statistically significant predictors of on-treatment MS activity (Figure 1(a)). After controlling for all covariates, treatment with TER (HR 2.63, 95% CI 1.33–5.19 compared to INJ) was associated with an independent risk of MS activity. There were no differences in breakthrough MS activity for patients treated with DMF and FGD compared to those treated with INJ therapy. Given the heterogeneity of the patient population, we then excluded treatment-naïve patients and evaluated only patients switching between DMTs. TER treatment remained a significant risk factor for on-treatment MS activity (HR 2.49, 95% CI 1.14–5.4) (Supplementary Material, Figure 1).
Figure 1.
Forest plots of hazard ratios (HRs) for multiple sclerosis (MS) activity (a) and treatment discontinuation (b) after controlling for measured baseline variables. CI: confidence interval; EDSS: estimated disability status score.
Discontinuation
Since oral therapies are considered more convenient than INJ therapies, we hypothesized that discontinuation rates would be lower for oral compared to INJ DMTs. After controlling for baseline differences, younger age (HR 0.80, 95% CI 0.70–0.92 per 10-year age increase) was associated with an increased risk of discontinuing medication. Patients previously treated with one DMT were less likely to discontinue medication than those who were DMT-naïve (HR 0.57, 95% CI 0.35–0.92). However, there were no differences in discontinuation rate between oral and INJ treatments. (Figure 1(b)).
Sensitivity analysis with propensity matched patients
To validate our findings, we employed propensity matching as an alternative statistical tool. After matching, most variables were balanced although some residual imbalance remained (Table 2, Supplementary Material, Figure 2). Remaining imbalances varied depending on the drug being studied (Supplementary Material, Figure 2), and may be attributable to small numbers within some of these subcategories. Unmatched patients differed from matched patients primarily in number of prior DMTs, disease severity/duration, and age, with unmatched patients being older and having longer disease durations with more DMT exposure compared to matched patients (Supplementary Material, Figure 2).
Table 2.
Patient characteristics for propensity-matched groups for each oral DMT.
|
Matched for dimethyl fumarate |
Matched for teriflunomide |
Matched for fingolimod |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| INJ (n = 82) | DMF (n = 82) | SD | INJ (n = 48) | TER (n = 48) | SD | INJ (n = 57) | FGD (n = 57) | SD | |
| Females, n (%) | 61 (74.4) | 60 (73.2) | 0.028 | 40 (83.3) | 41 (85.4) | 0.057 | 45 (79.0) | 46 (80.7) | 0.044 |
| Age, mean (SD) | 43.7 (12.6) | 44.1 (12.8) | 0.032 | 47.9 (11.8) | 49.4 (11.0) | 0.128 | 41.1 (11.7) | 39.4 (9.5) | 0.160 |
| Caucasian, n (%) | 68 (82.9) | 70 (85.4) | 0.067 | 42 (87.5) | 41 (85.4) | 0.061 | 42 (73.7) | 44 (77.2) | 0.081 |
| MS duration, years,mean (SD) | 7.8 (8.8) | 8.3 (9.3) | 0.053 | 11.0 (9.5) | 11.5 (11.0) | 0.053 | 6.8 (6.6) | 7.7 (7.7) | 0.118 |
| Number of previous meds, n (%) | |||||||||
| 0 | 26 (31.7) | 30 (36.6) | 0.103 | 3 (6.3) | 4 (8.3) | 0.080 | 10 (17.5) | 11 (19.3) | 0.045 |
| 1 | 31 (37.8) | 25 (30.5) | 0.154 | 21 (43.8) | 19 (39.6) | 0.085 | 22 (38.6) | 17 (29.8) | 0.186 |
| 2 | 17 (20.7) | 15 (18.3) | 0.062 | 14 (29.2) | 15 (31.3) | 0.045 | 16 (28.1) | 16 (28.1) | 0.000 |
| 3+ | 8 (9.8) | 12 (14.6) | 0.149 | 10 (20.8) | 10 (20.8) | 0.000 | 9 (15.8) | 13 (22.8) | 0.179 |
| Physician, n (%) | |||||||||
| 1 | 7 (8.5) | 10 (12.2) | 0.12 | 6 (12.5) | 6 (12.5) | 0.000 | 9 (15.8) | 8 (14.0) | 0.049 |
| 2 | 21 (25.6) | 19 (23.2) | 0.057 | 16 (33.3) | 14 (29.2) | 0.089 | 6 (10.5) | 7 (12.3) | 0.055 |
| 3 | 9 (11.0) | 8 (9.8) | 0.04 | 6 (12.5) | 6 (12.5) | 0.000 | 13 (22.8) | 11 (19.3) | 0.086 |
| 4 | 9 (11.0) | 7 (8.5) | 0.082 | 3 (6.3) | 3 (6.3) | 0.000 | 4 (7.0) | 4 (7.0) | 0.000 |
| 5 | 36 (43.9) | 38 (46.3) | 0.049 | 17 (35.4) | 19 (39.6) | 0.086 | 25 (43.9) | 27 (47.4) | 0.070 |
| Disability | |||||||||
| Mild (EDSS 0–3) | 57 (69.5) | 58 (70.7) | 0.027 | 29 (60.4) | 26 (54.2) | 0.127 | 40 (70.2) | 40 (70.2) | 0.000 |
| Moderate (EDSS 3.5–5.5) | 16 (19.5) | 12 (14.6) | 0.130 | 11 (22.9) | 14 (29.2) | 0.143 | 13 (22.8) | 11 (19.3) | 0.086 |
| Severe (EDSS ≥ 6.0) | 9 (11.0) | 12 (14.6) | 0.110 | 8 (16.7) | 8 (16.7) | 0.000 | 4 (7.0) | 6 (10.5) | 0.124 |
| Relapses in last 12 months, n (%) | 43 (52.4) | 43(52.4) | 0.000 | 17 (35.4) | 19 (39.6) | 0.087 | 26 (45.6) | 27 (47.4) | 0.035 |
DMF: dimethyl fumarate; EDSS: estimated disability status score;INJ: injectable; MS: multiple sclerosis; SD: standard difference; TER: teriflunomide; FGD: fingolimod.
SD ≤ 0.1 indicates covariate balance between matched groups.
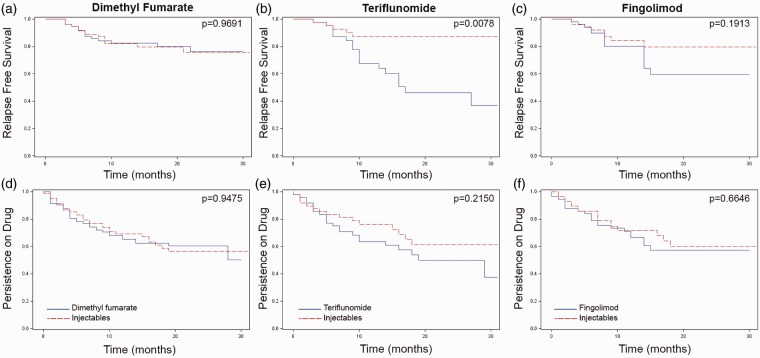
We compared breakthrough MS activity between propensity-matched patients taking oral and INJ DMTs using Cox proportional hazard analyses and controlling for time in study. As with the prior models, there were no differences in MS activity between DMF-treated (HR 0.985, 95% CI 0.47–2.08) or FGD-treated (HR 1.79, 95% CI 0.75–4.26) when compared to INJ-treated patients (Figure 2(a) and (c)). However, patients taking TER were more likely to experience breakthrough MS activity than matched INJ patients (HR 3.9, 95% CI 1.43–10.64) (Figure 2(b)). Once again, patients taking oral and INJ DMTs were equally likely to discontinue or switch treatments (Figure 2(d)–(f)).
Figure 2.
Multiple sclerosis (MS) activity and persistence on therapy for oral (blue lines) versus injectable (red lines) disease modifying therapies (DMTs). After propensity weighted matching, 82 dimethyl fumarate (DMF)-treated patients, 48 teriflunomide (TER)-treated patients and 57 fingolimod (FGD)-treated patients were matched with comparable injectable (INJ)-treated patients. On-drug MS activity (a)–(c) and persistence on drug (d)–(f) were evaluated. Kaplan-Meier time to event analyses are shown.
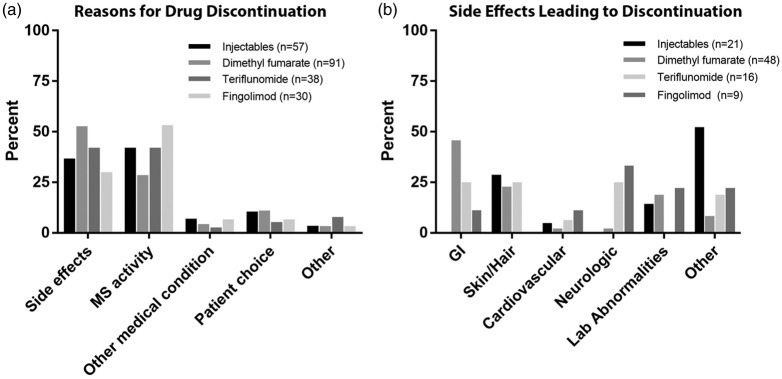
Reasons for stopping DMT
The most common reasons for stopping any given DMT were side effects (30–52%) and breakthrough MS activity (17–42%) (Figure 3). As expected, the type of side effects leading to drug discontinuation differed between medications. Injection reactions and flu-like symptoms commonly led to discontinuation among patients taking INJ therapies, and GI side effects were notable among patients taking both DMF and TER (Figure 3). Side effects were less common among patients taking FGD, but included headache and macular edema.
Figure 3.
Factors contributing to multiple sclerosis (MS) disease-modifying therapy discontinuation. Primary reasons for drug discontinuation (a). Side effects precipitating discontinuation (b). Number (n) of discontinuation events (a) or discontinuations due to drug side effects (b).
Discussion
Randomized, placebo-controlled trials have demonstrated that both oral and INJ DMTs are effective in relapsing MS, yet many questions remain about their relative efficacy. We retrospectively assessed the use and performance of these medications in a real world cohort. As expected, there was variability among the patients chosen to receive each drug. INJ therapies were commonly prescribed for patients who were early in their disease course and had not previously been exposed to immunomodulators. In contrast, oral medications were prescribed for individuals later in their disease course who had failed or could not tolerate other forms of DMT. There was also variability among patients initiating oral DMTs, with FGD-treated patients tending to be younger and having a more recent diagnosis than those starting the other oral medications. Physician prescribing practice varied widely, illustrating the current lack of evidence available to guide treatment decisions.
After controlling for baseline differences, we found that DMF and FGD were comparable to INJ therapies for controlling breakthrough MS activity. This is in line with Phase 3 trials, where DMF performed similarly to glatiramer acetate.20 FGD out-performed low dose IFN-β in its Phase 3 trial,21 but that interferon formulation was previously found to be less effective than a higher dose formulation.22 We considered all INJ therapies together, which may contribute to the lack of an observed difference in efficacy between FGD and INJ therapies. The sample size in this study may also have been too small to detect differences between these groups. In contrast, while TER performed similarly to high dose IFN-β in a Phase 3 clinical trial,23 cohort patients treated with TER experienced significantly more breakthrough MS activity than patients treated with INJ therapies. This finding was reproduced when data were re-analyzed using propensity score matching. The observation is likely driven by patients switching to TER from a different DMT, as few treatment-naïve patients initiated TER. Subgroup analyses of data from patients who were not treatment-naive support this hypothesis. Other significant predictors of MS activity included neurologic disability (based on EDSS) and relapse frequency, both of which are consistent with the published literature.
The baseline patient heterogeneity makes evaluating the comparative effectiveness of DMTs challenging, but statistical methods can account for measured differences in factors known to affect disease course.24 The consistency of the adjusted models with the propensity matched models support our results. Nevertheless, Cox models and propensity score matching are not perfect substitutes for randomization and only control for measured variables. Unmeasured variables may account for some of the observed difference in efficacy between TER and INJ therapies. For example, prior MRI activity often plays a role in treatment decisions, but because these data were not consistently captured in the clinical record, they were not statistically accounted for. Moreover, although propensity score matching markedly reduced the measured differences between the treatment groups, residual bias remained for several variables (Table 2, Supplementary Material, Figure 2).
It is worth noting that the patients prescribed TER in this clinical cohort differed markedly from those studied in Phase 3 trials. For example, the average age for TER-treated patients in this real-world sample was 49 years, with patients as old as 76 years initiating the drug. In contrast, the upper age limit for patients entering the Phase 3 trials was 55 years, and the average age was 38 years.25,26 Real-world patients also included a larger proportion of African-Americans, increased prior DMT exposure, and a longer duration of MS compared to clinical trial patients.25,26 Overall, these data suggest that as it is being used in clinical practice, TER may be less effective than INJ DMTs for controlling inflammatory MS activity.
A surprisingly high proportion of patients discontinued DMT during the study period; within a 2.5 year window, 33–46% of patients newly prescribed any given DMT subsequently discontinued or switched therapies. These proportions are higher than those previously reported for observational studies. One systematic review reported that 22–43% of patients taking INJ therapy long-term (>24 months) ultimately discontinued or switched medications, but only 10–15% discontinued within the first year.27 Another large observational study found annual discontinuation rates of 20–25% 24. These numbers largely reflect the pre-oral DMT epoch. Since the advent of oral options, patients are more likely to discontinue other DMTs in favor of an oral medication.8,12 Previous work suggested that MS patients were more adherent with oral DMTs and were less likely to discontinue treatment when compared with INJ or infused medications.14,15 Despite this, we were unable to confirm improved efficacy or tolerability for oral therapies in this cohort. This may be due in part to our limited sample size; a larger cohort would increase the power for detecting differences between the treatment groups. Nevertheless, when combined with a well-established safety record, our data suggest that INJ therapies will continue to play an important role in MS treatment for the foreseeable future.
Drug persistence may depend partly on the geo-political situation. The published literature is primarily based on data from countries with nationalized healthcare, and the unique healthcare situation in the USA may contribute to high rates of switching between medications. Increased ease of switching likely has both positive and negative effects. DMT adherence improves MS outcomes and helps manage healthcare costs.4 When physicians have access to many DMTs, this increases the likelihood that an effective and tolerable treatment can be found. On the other hand, since DMTs are expensive and come with a substantial administrative burden, rapidly switching between therapies can strain the resources of clinical practices.
The expanding armamentarium of therapies against relapsing MS has encouraged physicians to strive for NEDA. This may result in multiple medication changes. Notably, the cumulative health effects of multiple immunomodulatory therapies are not known. Past exposure to immunosuppressants increases the likelihood of developing progressive multifocal leukoencephalopahy during natalizumab therapy.28 Serial exposure to multiple DMTs may change the immune systems of patients and affect risk of future complications. The immunologic effects of sequential DMT exposures deserves more study.
These data should be generalized with caution, given the limited size of the patient cohort and potential for bias among the patients selected to initiate each therapy, which may remain despite statistical corrections. This study was relatively short, with an average follow-up period of around 18 months. Additional differences between groups may emerge with longer follow up. Additionally, our cohort consisted of patients treated at a single Midwestern US center and thus may not be representative of larger regions. Further evaluation of the comparative effectiveness of the oral DMTs is needed.
Acknowledgements
EEL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors would like to thank Gregory Wu for discussion of the data and critique of the manuscript – he did not receive compensation for this activity.
Conflicts of interest.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EEL is funded by a Sylvia Lawry Fellowship from the National MS Society. She has received honoraria for speaking/consulting from Genzyme, Teva, and Biogen. AHC was supported by the Manny & Rosalyn Rosenthal–John L. Trotter MS Center Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation. She has received honoraria for consulting from AbbVie, Biogen, Genzyme/Sanofi Aventis, Genentech/Roche, Novartis, and Teva Neuroscience. She has research support from Biogen and Genentech/Roche. ARS has no disclosures.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by National Institute of Health (NIH) training grant UL1 TR000448. No corporate funding was received for this study.
References
- 1.Trojano M, Pellegrini F, Paolicelli D, et al. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol 2009; 66: 513–520. [DOI] [PubMed] [Google Scholar]
- 2.Freedman MS. Long-term follow-up of clinical trials of multiple sclerosis therapies. Neurology 2011; 76: S26–S34. [DOI] [PubMed] [Google Scholar]
- 3.Kappos L, Edan G, Freedman MS, et al. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology 2016; 87: 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011; 28: 51–61. [DOI] [PubMed] [Google Scholar]
- 5.Havrdova E, Galetta S, Stefoski D, et al. Freedom from disease activity in multiple sclerosis. Neurology 2010; 74(Suppl. 3): S3–S7. [DOI] [PubMed] [Google Scholar]
- 6.Bevan CJ, Cree BA. Disease activity free status: A new end point for a new era in multiple sclerosis clinical research? JAMA Neurol 2014; 71: 269–270. [DOI] [PubMed] [Google Scholar]
- 7.Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
- 8.Zhornitsky S, Greenfield J, Koch MW, et al. Long-term persistence with injectable therapy in relapsing–remitting multiple sclerosis: An 18-year observational cohort study. PLoS One 2015; 10: e0123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nixon R, Bergvall N, Tomic D, et al. No evidence of disease activity: Indirect comparisons of oral therapies for the treatment of relapsing–remitting multiple sclerosis. Adv Ther 2014; 31: 1134–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchinson M, Fox RJ, Havrdova E, et al. Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing–remitting multiple sclerosis: A systematic review and mixed treatment comparison. Curr Med Res Opin 2014; 30: 613–627. [DOI] [PubMed] [Google Scholar]
- 11.Braune S, Lang M, Bergmann A, NeuroTransData Study Group. Efficacy of fingolimod is superior to injectable disease modifying therapies in second-line therapy of relapsing remitting multiple sclerosis. J Neurol 2016; 263: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warrender-Sparkes M, Spelman T, Izquierdo G, et al. The effect of oral immunomodulatory therapy on treatment uptake and persistence in multiple sclerosis. Mult Scler 2016; 22: 520–532. [DOI] [PubMed] [Google Scholar]
- 13.He A, Spelman T, Jokubaitis V, et al. Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. JAMA Neurol 2015; 72: 405–413. [DOI] [PubMed] [Google Scholar]
- 14.Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: A retrospective US claims database analysis. J Med Econ 2014; 17: 696–707. [DOI] [PubMed] [Google Scholar]
- 15.Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol 2013; 13: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): A multicentre, randomised, parallel, open-label trial. Lancet Neurol 2008; 7: 903–914. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum P, Rubin D. Constructing a control-group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985; 39: 33–38. [Google Scholar]
- 18.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J Clin Epidemiol 2001; 54: 387–398. [DOI] [PubMed] [Google Scholar]
- 19.Klein JP, Moeschberger ML. Survival analysis: Techniques for censored and truncated data, New York: Springer-Verlag, 1997. [Google Scholar]
- 20.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 22.Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE trial. Neurology 2002; 59: 1496–1506. [DOI] [PubMed] [Google Scholar]
- 23.Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: A randomised, controlled phase 3 trial. Mult Scler 2014; 20: 705–716. [DOI] [PubMed] [Google Scholar]
- 24.Kalincik T, Spelman T, Trojano M, et al. Persistence on therapy and propensity matched outcome comparison of two subcutaneous interferon beta 1a dosages for multiple sclerosis. PLoS One 2013; 8: e63480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Confavreux C, O'Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 247–256. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 27.Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: Tolerability and adherence. Mult Scler 2012; 18: 932–946. [DOI] [PubMed] [Google Scholar]
- 28.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012; 366: 1870–1880. [DOI] [PubMed] [Google Scholar]