Abstract
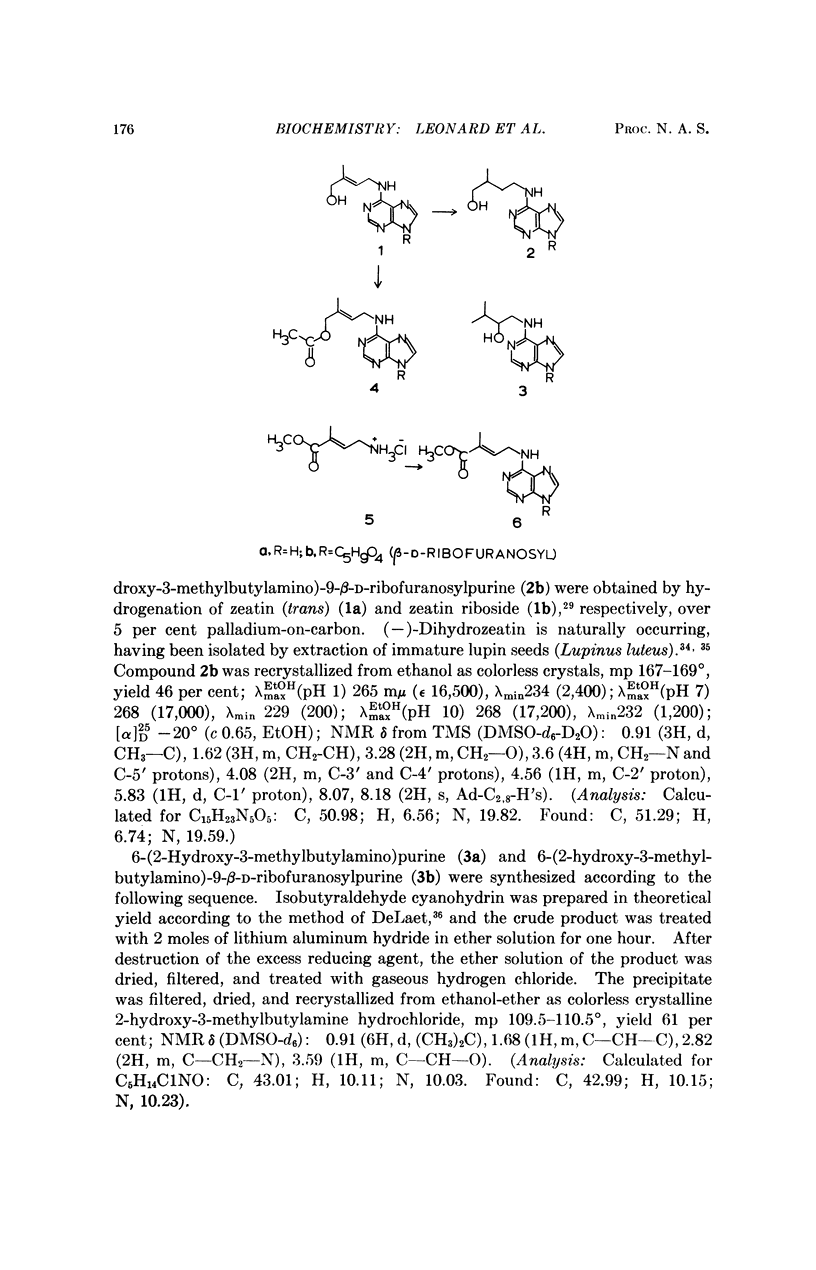
Compounds related to dihydrozeatin that define the influence of the location of the hydroxyl group along the side chain have been synthesized and tested for cytokinin activity. The compounds compared are in the series: 6-(X-hydroxy-3-methylbutylamino)purines and their ribosides, where X = 2, 3, and 4.
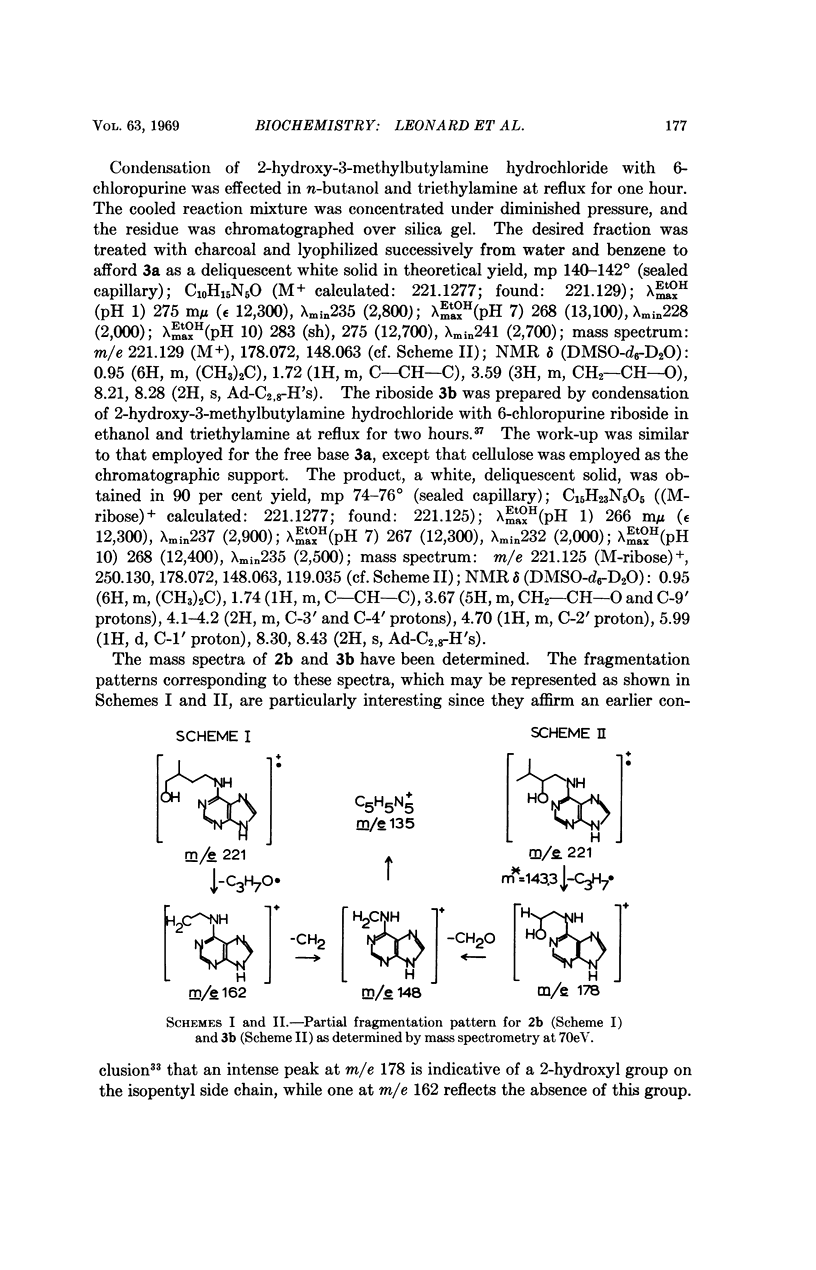
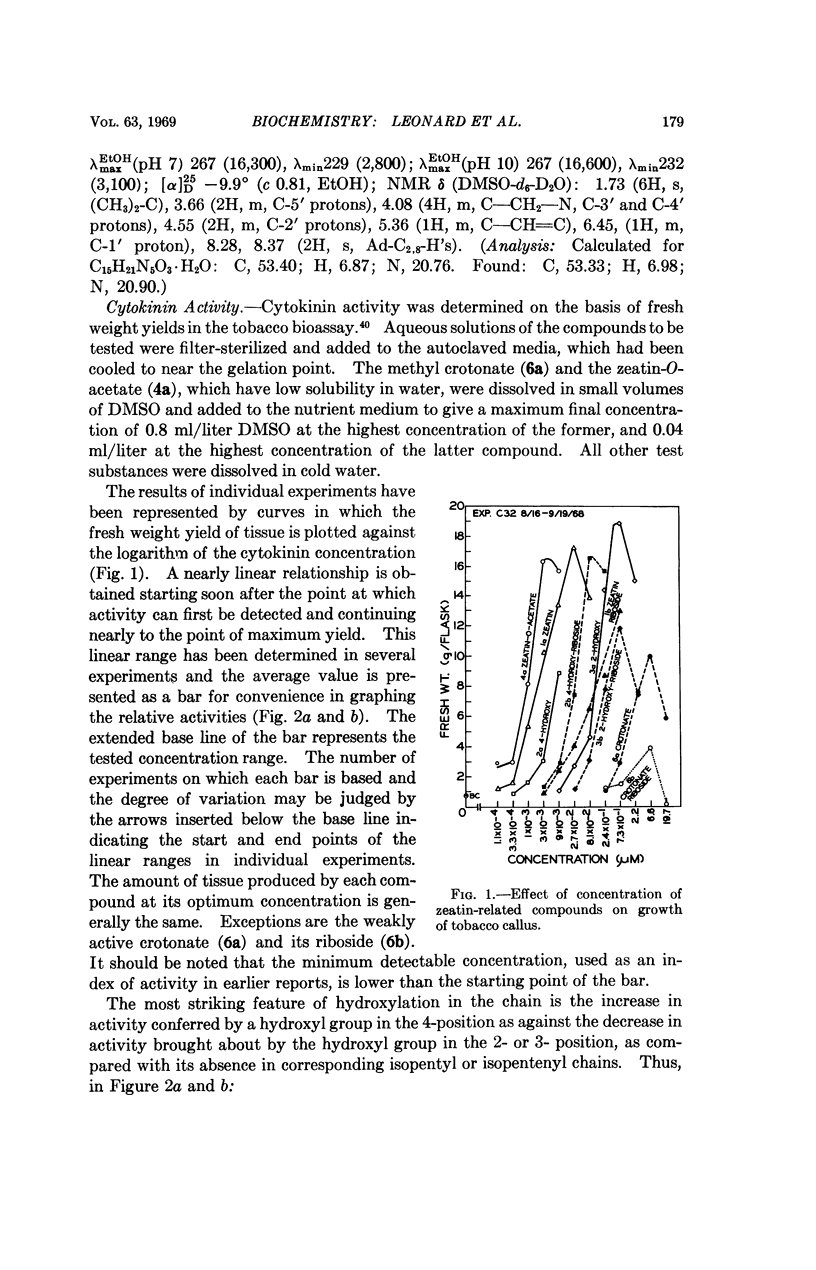
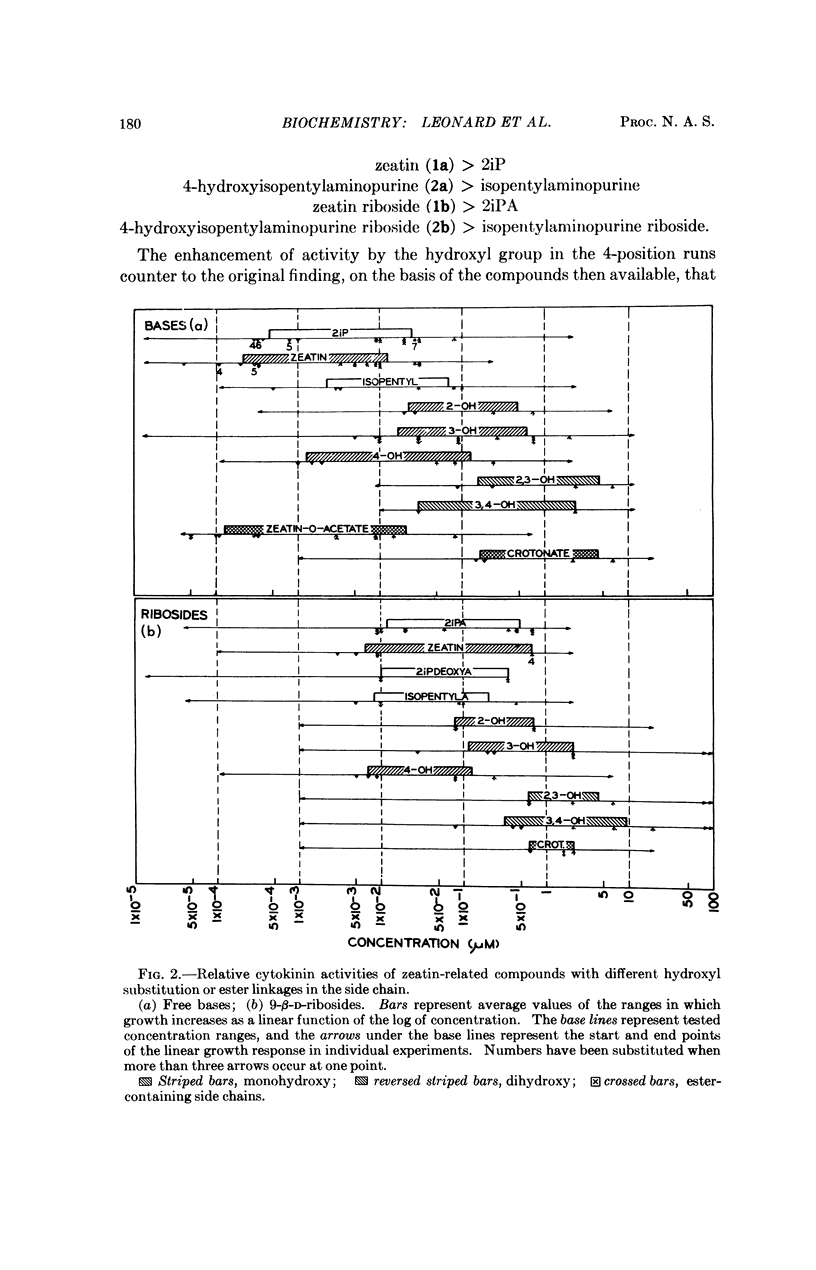
Hydroxy substitution on the 4-position of the side chain enhances, but in the 2-, 3-, or 2- and 3- positions, decreases cytokinin activity as compared with the unsubstituted isopentyl (or isopentenyl) chains. This differential influence of the position of the hydroxyl group in the N6-chain holds also for the similarly related 9-β-D-ribofuranosides. The relatively higher activity of 3,4-dihydroxy as compared with 2,3-dihydroxy derivatives is consistent with this position effect.
Compounds related to zeatin possessing side-chain ester moieties have also been synthesized and tested comparatively. Among these, 6-(4-acetoxy-3-methyl-trans-2-butenylamino)purine is at least as active as zeatin, the most active presently known cytokinin in the tobacco bioassay, whereas the analog, methyl 2-methyl-4-(purin-6-ylamino)-trans-crotonate, with the ester function effectively reversed, has vastly lower activity, and its riboside is practically inactive.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hall R. H., Csonka L., David H., McLennan B. Cytokinins in the soluble RNA of plant tissues. Science. 1967 Apr 7;156(3771):69–71. doi: 10.1126/science.156.3771.69. [DOI] [PubMed] [Google Scholar]
- Helgeson J. P. The cytokinins. Synthetic and naturally occurring N6-substituted adenine derivatives profoundly affect plant growth. Science. 1968 Sep 6;161(3845):974–981. doi: 10.1126/science.161.3845.974. [DOI] [PubMed] [Google Scholar]
- Klämbt D., Thies G., Skoog F. Isolation of cytokinins from Corynebacterium fascians. Proc Natl Acad Sci U S A. 1966 Jul;56(1):52–59. doi: 10.1073/pnas.56.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LETHAM D. S. ZEATIN, A FACTOR INDUCING CELL DIVISION ISOLATED FROM ZEA MAYS. Life Sci. 1963 Aug;8:569–573. doi: 10.1016/0024-3205(63)90108-5. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Hecht S. M., Skoog F., Schmitz R. Y. CYTOKININS: SYNTHESIS OF 6-(3-METHYL-3-BUTENYLAMINO)-9-beta-D-RIBOFURANOSYLPURINE (3IPA), AND THE EFFECT OF SIDE-CHAIN UNSATURATION ON THE BIOLOGICAL ACTIVITY OF ISOPENTYLAMINOPURINES AND THEIR RIBOSIDES. Proc Natl Acad Sci U S A. 1968 Jan;59(1):15–21. doi: 10.1073/pnas.59.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison J. T., Everett G. A., Kung H. K. Oligonucleoides from yeast tyrosine transfer ribonucleic acid. J Biol Chem. 1967 Mar 25;242(6):1318–1323. [PubMed] [Google Scholar]
- Miller C. O. A KINETIN-LIKE COMPOUND IN MAIZE. Proc Natl Acad Sci U S A. 1961 Feb;47(2):170–174. doi: 10.1073/pnas.47.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Evidence for the natural occurrence of zeatin and derivatives: compounds from maize which promote cell division. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1052–1058. doi: 10.1073/pnas.54.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Zeatin and zeatin riboside from a mycorrhizal fungus. Science. 1967 Sep 1;157(3792):1055–1057. doi: 10.1126/science.157.3792.1055. [DOI] [PubMed] [Google Scholar]
- Skoog F., Armstrong D. J., Cherayil J. D., Hampel A. E., Bock R. M. Cytokinin activity: localization in transfer RNA preparations. Science. 1966 Dec 9;154(3754):1354–1356. doi: 10.1126/science.154.3754.1354. [DOI] [PubMed] [Google Scholar]
- Skoog F., Strong F. M., Miller C. O. Cytokinins. Science. 1965 Apr 23;148(3669):532–533. doi: 10.1126/science.148.3669.532-a. [DOI] [PubMed] [Google Scholar]
- Staehelin M., Rogg H., Baguley B. C., Ginsberg T., Wehrli W. Structure of a mammalian serine tRNA. Nature. 1968 Sep 28;219(5161):1363–1365. doi: 10.1038/2191363a0. [DOI] [PubMed] [Google Scholar]
- Zachau H. G., Dütting D., Feldmann H. The structures of two serine transfer ribonucleic acids. Hoppe Seylers Z Physiol Chem. 1966;347(4):212–235. doi: 10.1515/bchm2.1966.347.1.212. [DOI] [PubMed] [Google Scholar]



