Abstract
Purpose of review
Studies investigating postnatal brain growth disorders inform the biology underlying the development of human brain circuitry. This research is becoming increasingly important for the diagnosis and treatment of childhood neurodevelopmental disorders, including autism and related disorders. Here we review recent research on typical and abnormal postnatal brain growth and examine potential biological mechanisms.
Recent findings
Clinically, brain growth disorders are heralded by diverging head size for a given age and sex, but are more precisely characterized by brain imaging, postmortem analysis, and animal model studies. Recent neuroimaging and molecular biological studies on postnatal brain growth disorders have broadened our view of both typical and pathological postnatal neurodevelopment. Correlating gene and protein function with brain growth trajectories uncovers postnatal biological mechanisms, including neuronal arborization, synaptogenesis and pruning, and gliogenesis and myelination. Recent investigations of childhood neurodevelopmental and neurodegenerative disorders highlight the underlying genetic programming and experience-dependent remodeling of neural circuitry.
Summary
In order to understand typical and abnormal postnatal brain development, clinicians and researchers should characterize brain growth trajectories in the context of neurogenetic syndromes. Understanding mechanisms and trajectories of postnatal brain growth will aid in differentiating, diagnosing, and potentially treating neurodevelopmental disorders.
Keywords: postnatal brain development, brain growth disorders, microcephaly, macrocephaly, connectivity, children, adolescents
Introduction
Prenatal attenuations in brain growth arise largely from disruptions in neurogenesis. Conversely, the postnatal period of human brain development involves a critical period of growth largely driven by experience-dependent formation of neuronal connections. Therefore, disorders of brain growth appearing postnatally offer an opportunity to understand genetic control and environmental input involved in neural circuitry development. Neurodevelopmental processes occurring during postnatal phases of human brain development appear to involve neuronal arborization, synaptogenesis and pruning, and gliogenesis and myelination. The following review describes recent progress regarding the biology underlying development of human brain circuitry through specific investigation of postnatal brain growth. We give examples of postnatal neurodevelopmental disorders that illustrate these aspects of human neurobiology.
Part 1: Clinical observations regarding postnatal brain development
The human brain undergoes dramatic changes in size and connectivity after birth. From birth to age 6, the brain increases in size by four-fold, reaching 90% of adult volume [1]. During normal development, the growth of head circumference (HC) is largely driven by growth of underlying brain tissue. Based on growth charts proposed by Rollins et al. [2] and data from Centers for Disease Control and Prevention [3], occipitofrontal circumference (OFC) at the 50th percentile increases from 34.71 cm (females) and 35.81 cm (males) at birth to 45.20 cm (females) and 46.50 cm (males) at 1 year of age. After the first year, head growth continues more gradually into early adulthood.
OFC is a reliable predictor of brain volume in children younger than 6-years-old [4,5], therefore, clinicians can indirectly detect brain growth abnormalities through HC measurements. Abnormal brain size signaled by microcephaly and macrocephaly in early postnatal years is often indicative of disorders of neurodevelopment [6].
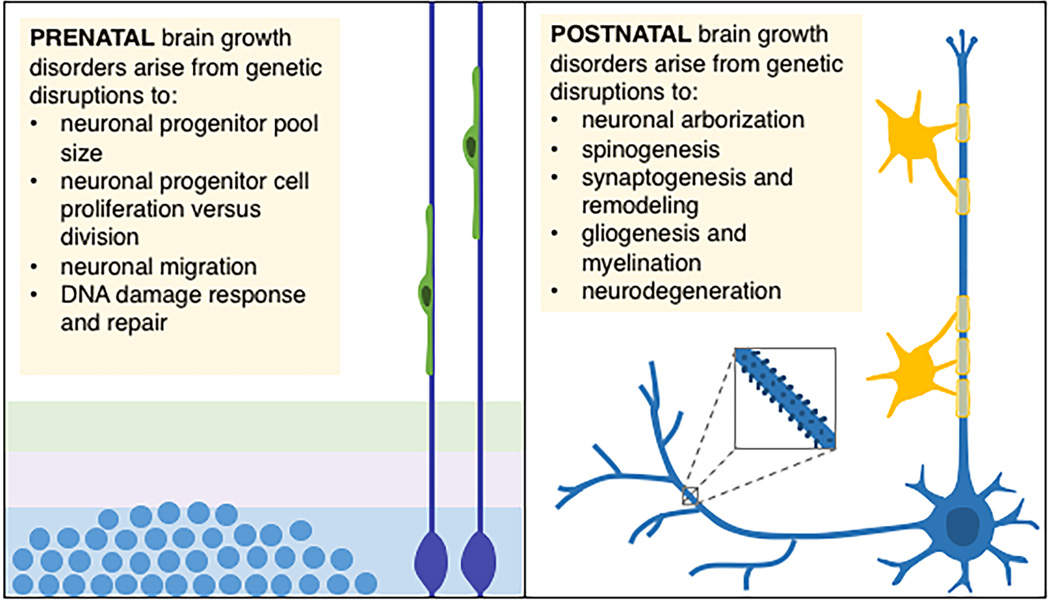
Infants with primary microcephaly exhibit small HC at birth. By contrast, infants with postnatal microcephaly present with normal HC at birth and display subsequent attenuations in HC growth. Microcephaly is defined as OFC <3rd percentile for sex, age, and ethnicity [2]. While both primary and postnatal microcephalies are generally caused by reduced brain growth, the distinction in trajectories of growth abnormalities may reflect different developmental mechanisms. Genetic insults disrupting neurogenesis often lead to primary microcephaly whereas defects in later-stage developmental mechanisms (connectivity or gliogenesis) often result in postnatal microcephaly (Figure 1). Postnatal microcephaly also may herald concern about an early-onset neurodegenerative process. Of course, many conditions may involve components of both prenatal and postnatal processes.
Figure 1.
Biological mechanisms underlying prenatal versus postnatal brain growth disorders. (Left panel) Prenatal brain growth disorders commonly arise from genetic causes associated with centrosomal abnormalities. Disruptions to centrosomal proteins may impact neuronal progenitor cell proliferation and differentiation, neuronal migration, and DNA repair responses (reviewed in [7]). (Right panel) Conversely, postnatal brain growth disorders mainly arise from genetic causes associated with disrupted connectivity (i.e., elaboration of axons and dendrites, spinogenesis and maturation, synaptogenesis and remodeling, and gliogenesis and myelination). Postnatal brain growth disorders may also result from childhood neurodegeneration.
Microcephaly during the first year is associated with intellectual disability at age 7 years [8]. Postnatal microcephaly is often accompanied by abnormal or absent language, social impairment, and epilepsy [9]. Many childhood brain and neuropsychiatric disorders exhibit postnatal microcephaly, for example, Angelman syndrome (AS), Rett syndrome (RTT), and Christianson syndrome (CS).
Postnatal macrocephaly results from exaggerated head growth after birth, whereby infants are born with normal HC and then exhibit abnormal head enlargement, often due to increased brain growth, or megalencephaly. Macrocephaly is diagnosed when OFC is >97th percentile for sex, age, and ethnicity [2,10]. Accelerated postnatal brain growth is associated with developmental delays of motor, language, and cognitive functions [10–12]. Examples of childhood brain and neuropsychiatric disorders that present with postnatal macrocephaly include monogenic PTEN Hamartoma Tumor Syndrome (PHTS), Tuberous Sclerosis Complex (TSC), and autism spectrum disorder (ASD).
Part 2: Potential biological mechanisms governing postnatal brain growth
Prior to birth, almost all neurons of the brain are generated. The first half of gestation observes birth and migration of neurons. By the second half of gestation, neuronal connectivity begins to develop into immature circuits. Formation and differentiation of glial progenitors have commenced. Yet, the brain will continue to undergo extraordinary developmental changes in connectivity and glial development over the first two decades of life.
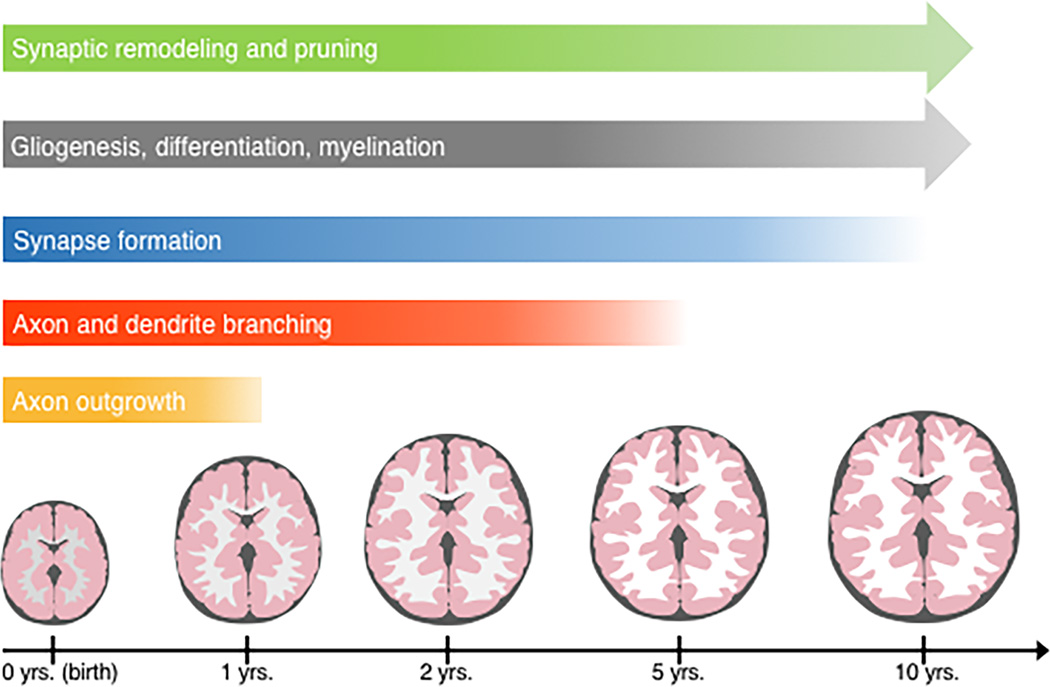
The newborn brain is 36% the size of the adult brain. Within the first postnatal year, the brain grows to approximately 70% of its adult size [13,14]. This growth is due to a rapid increase in neuropil (axons, dendrites, and synapses) and glial cells. Remodeling and myelination of neural circuits extend throughout adolescence. During postnatal development, experience shapes brain connectivity and circuitry. Here, we will address biological mechanisms underlying postnatal brain development (Figure 2).
Figure 2.
Postnatal brain and head growth and underlying neurodevelopmental stages. The human brain and head size increase rapidly during the first and second postnatal years, followed by a more gradual increase into early adulthood. The increase in brain and head size largely results from elaboration of connectivity. Axon and dendrite growth and arborization continue after birth into early postnatal years. Synaptogenesis continues after birth, peaking at various points during childhood (depending on brain region). Synapses mature and remodel to form appropriate connections, and then undergo gradual pruning into early adulthood. Gliogenesis continues after birth, and glial cell differentiation and myelination continue into early adulthood.
Axon and dendrite elaboration
Axonal and dendritic outgrowth increase during the second half of gestation [15,16], forming connections, or synapses, with other cells to give rise to early neural circuits. Elaboration of dendrites accelerates during early childhood [6,17]. Similarly, dendritic spine numbers increase in early childhood, followed by a gradual decline during late childhood and adolescence [18]. Regulation of axonal and dendritic branching is critical for functional organization of circuits. Increasing evidence implicates altered axonal and dendritic formation in a subset of intellectual and developmental disabilities [19–23].
Synaptogenesis and experience-dependent synapse remodeling
During early childhood, synaptic connectivity far exceeds that of an adult. While formation of synapses begins prenatally, the majority of synaptogenesis occurs in early childhood [24–26]. Postmortem studies show that synaptic density increases from birth until late childhood [6,27], followed by a gradual period of pruning (likely largely experience-dependent) that continues until early adulthood. Histological and neuroimaging studies in humans suggest that the time course of synaptogenesis follows a posterior-to-anterior pattern, peaking first in sensory and motor areas, followed by association cortices [26,28]. Subsequent synaptic pruning follows a similar pattern, with primary cortices undergoing synaptic remodeling first, followed by association cortices [6,29]. Synaptogenesis and synaptic pruning may be responsible for cortical thickening and thinning, respectively [28].
Gliogenesis and myelination
Beginning prenatally, the proliferation, migration, and differentiation of glial progenitor cells continues for an extended period after birth. Abundant progenitors migrate into neuron-populated areas and differentiate into various glial cells, such as astrocytes and oligodendrocytes. Together, these cells make up over half the cells of the human brain and regulate numerous functions of the developing and adult brain [30*]. The increase in number and size of glial cells are greatly responsible for early rapid brain and head growth.
Mature oligodendrocytes form myelin sheaths to increase axonal conduction [31], beginning at the end of the second trimester [15]. Myelination appears to be regulated by electrical activity of neurons, occurring in a posterior-to-anterior direction [6,32]. White matter fiber tract development rapidly increases in the 6-month to 24-month interval and gradually continues until late adolescence [33].
Part 3: Genetic syndromes exemplifying abnormalities in postnatal brain growth
A wide range of disorders are associated with abnormal postnatal brain growth. These disorders may arise from an attenuation or an exaggeration of growth, and from either monogenic or complex etiologies. In the following section, we will discuss a subset of postnatal brain growth disorders (Table 1).
Table 1.
Genetic neurodevelopmental disorders associated with abnormal postnatal brain growth.
| Disorder | Clinical Description | Genetic Description | Postnatal Disruptions | References |
|---|---|---|---|---|
| Monogenic Postnatal Microcephalies | ||||
| Angelman Syndrome (AS) | Characterized by severe intellectual disability, absent speech, seizures, postnatal microcephaly, movement disorder, developmental delay, and a behavioral profile that includes a happy demeanor and hyperactivity |
Loss of expression of the maternally inherited allele of UBE3A |
Decreased dendritic spine density and abnormal spine morphology, white matter pathology, and altered connectivity |
[34] |
| Rett Syndrome (RTT) | Characterized by typical early development followed by regression, which involves loss of acquired skills and language, intellectual disability, gait abnormalities, stereotypic hand movements, postnatal microcephaly, and seizures (mainly affects females) |
Loss-of-function mutations in the X-linked gene MECP2 (however, can be associated with mutations in CDKL5 and FOXG1) |
Decreased dendritic growth and spine density, fewer spines, white matter pathology, and compromised glial function |
[35,36,37*] |
| Christianson Syndrome (CS) |
Characterized by severe intellectual disability, absent speech, ataxia, and epilepsy Frequently presents with postnatal microcephaly, craniofacial dysmorphology, eye movement abnormalities, progressive neurologic dysfunction, and loss of early acquired motor skills (mainly affects males) |
Loss-of-function mutations in the X-linked gene SLC9A6 |
Decreased axonal and dendritic arborization, decreased spine density, and gray matter atrophy (most notable in cerebellum and brainstem) |
[38–40] |
| Monogenic Postnatal Macrocephalies | ||||
| PTEN Hamartoma Tumor Syndrome (PHTS) |
Characterized by a predisposition of tumors Often associated with Lhermitte-Duclos disease, developmental disabilities, macrocephaly, and autism spectrum disorder (ASD) |
Germline heterozygous loss-of-function mutations in PTEN |
Excess glial population, hypertrophy of dendritic arborization, increased dendritic spine density, and white matter abnormalities |
[41–42] |
| Tuberous Sclerosis Complex (TSC) |
Multi-system disease commonly presenting with dermatological, renal, and neurological manifestations Neurological manifestations include epilepsy, cognitive disabilities, behavioral problems, autism, and macrocephaly |
Heterozygous loss-of-function mutations in either TSC1 or TSC2 |
Astrogliosis, white matter abnormalities, structurally compromised axons, and altered structural connectivity Projection neurons within cortical tubers exhibit shortened dendrites, abnormal spine morphology, and decreased dendritic spine density |
[43–46] |
| Neurodegenerative Disorders | ||||
| Cockayne Syndrome | Neurodegenerative disorder characterized by severe motor and cognitive developmental delays, intellectual disability, microcephaly, multi- organ degeneration, progessive hearing loss, retinopathy, and sun sensitivity |
Autosomal recessive mutations in ERCC6 (also known as CSB) or ERCC8 (also known as CSA) |
Neurological manifestations include neuronal loss, gliosis, demyelination, and axonal degeneration |
[47–49] |
| KIF1A-associated Neurodegenerative Syndrome |
Neurodegenerative disorder characterized by severe developmental delay, hypotonia, microcephaly, cortical visual impairment, ataxia, epilepsy, and movement disorders |
De novo (likely dominant-negative) mutations in KIF1A |
Progressive brain atrophy and cerebral white matter reduction, likely caused by impaired axonal synaptic vesicle transport |
[50*] |
| Complex Disorders | ||||
| Autism Spectrum Disorder (ASD) |
Genetically and clinically heterogeneous neurodevelopmental disorder characterized by impaired communication and social interactions, and stereotyped behaviors |
Ongoing area of research, but examples include: PTEN mutations (associated with macrocephaly) DYRK1A mutations (associated with microcephaly) 16p11.2 (duplications associated with microcephaly and deletions with macrocephaly) 1q21.1 (deletions associated with microcephaly and duplications with macrocephaly) |
Increased cortical thickness and spine density, decreased diffusion and resting state connectivity, and widespread reductions in white matter tract integrity (however, may be limited to right inferior longitudinal fasiculus after matching for head motion) |
[51–53,54**,55– 59,60**,61*] |
| Schizophrenia | Criteria include positive symptoms (i.e., hallucinations, delusions, disorganized behavior) and negative symptoms (i.e., blunted affect, lack of motivation) |
Ongoing area of research, but examples include: Altered expression of C4 genes (i.e., C4A and C4B) |
Exaggerated parieto-frontal-temporal gray matter loss (predominantly in prefrontal and temporal cortices), reduced spine density and neuropil in deep layer III and layer V of prefrontal cortex, abnormal functional and structural connectivity, and altered white matter integrity |
[62–64,65**] |
Monogenic postnatal microcephalies
ANGELMAN SYNDROME (AS)
First described in 1965, AS is a classic postnatal microcephaly disorder. There is evidence of developmental delay by age 6–12 months, and in the majority of cases, decelerated growth in HC resulting in microcephaly by age 2 years [34]. AS arises from loss of expression of the maternally inherited allele of the imprinted ubiquitin protein ligase E3A (UBE3A) gene, most frequently due to a genomic deletion on chromosome 15q11q13 [66–68]. In mature neurons, UBE3A is expressed from the maternally inherited copy, whereas in most other tissues and cell types UBE3A is expressed from both alleles [66,69,70]. The biological role of UBE3A in AS remains poorly understood. UBE3A encodes E6-associated protein (E6-AP), an E3 ubiquitin ligase. E6-AP is believed to participate primarily in protein degradation in proteasomes via the ubiquitin pathway [34], functioning as a cellular quality control. E6-AP targets proteins involved in cell-cycle regulation and synaptic function and plasticity [71,72].
The majority of individuals with AS exhibit decelerated HC growth resulting in microcephaly [73]. Microcephaly is more frequent among patients with a deletion in chromosome 15q11q13 [74]. Microcephaly in AS may be caused by decreased brain volume arising from impaired connectivity. Mouse models exhibit reduced brain size, learning and memory impairment [75,76], and impaired synaptic transmission [77]. In addition, they show abnormal dendritic spine morphology and decreased dendritic spine density [21]. Finally, diffusion tensor imaging (DTI) studies reveal altered white matter pathways and connectivity in participants with AS [78]. Unlike neurons, mature oligodendrocytes express UBE3A biallelically. Nonetheless, disruption to the maternal copy may compromise myelination in AS [69].
RETT SYNDROME (RTT)
RTT is an X-linked progressive neurodevelopmental disorder mainly affecting females. Classic RTT is characterized by typical early development until age 6–18 months, followed by gradual developmental regression, and in most cases, decelerated postnatal head growth [79]. The majority of RTT cases result from loss-of-function mutations in the X-linked gene MECP2 [80]. MECP2 encodes for methyl-CpG-binding protein 2 (MeCP2), a protein highly expressed in the brain that regulates transcription [37*]. MeCP2 can act as both a repressor and an activator of transcription through interacting with histone deacetylase-containing complexes and with promoter regions, respectively [81,82]. It is believed that RTT arises mainly from loss of transcriptional activation, rather than loss of repression [81]. For example, loss of MECP2 leads to repression of several genes involved in brain development, including brain-derived neurotrophic factor (BDNF) [81,82]. MeCP2 is likely involved in neuronal maturation and maintenance, rather than in neuronal proliferation, demonstrated by increasing postnatal MECP2 expression levels as neurons mature [83–85].
Postnatal deceleration of head growth resulting in microcephaly is found in the majority of RTT cases [35,36,86]. Decelerated head growth may result from a deficit in both development and maintenance of neuronal connectivity. Postmortem studies reveal a profound decrease in dendritic growth in cortex [87], corroborated by studies in RTT mouse models [19,88]. In addition, postmortem brain and mouse model studies demonstrate decreased dendritic spine density [19,89], particularly in later stages of development, suggesting an error in maintenance [88,90]. Decreased dendritic growth is at least in part due to MECP2-deficient glia, which are unable to support dendritic morphology of both wild-type and MECP2-null neurons [91–93]. In addition, RTT patients likely present with mild progressive white matter pathology [94,95].
CHRISTIANSON SYNDROME (CS)
CS is an X-linked neurodevelopmental disorder primarily affecting males [38]. Boys with CS exhibit severe intellectual disability, absent speech, ataxia, and epilepsy. The majority of patients display postnatal microcephaly [38–40]. CS arises from loss-of-function mutations in SLC9A6 [40], which encodes for the Na+/H+ exchanger 6 (NHE6) protein. NHE6 localizes to early, recycling, and late endosomal membranes and transiently associates with the plasma membrane [96–98]. NHE6 regulates endosomal lumen pH by allowing for electroneutral exchange of proton ions out of the endosome for monovalent cations into the endosome [97,99]. Over-acidification of endosomal pH in absence of functional NHE6 may disrupt endosomal trafficking normally needed for growth of axonal and dendritic arbors, as well as for dendritic spines during long-term potentiation (LTP) [100] and neuronal development [101]. In the mouse model, loss of NHE6 results in over-acidification of endosomal compartments and reduced endosomal signaling by neurotrophins and their receptors, such as BDNF and TrkB, respectively [98].
The majority of CS patients exhibit decelerated postnatal head growth. In a cohort of twelve independent pedigrees, 92% of CS participants displayed postnatal microcephaly with decelerated head growth [39]. Postnatal microcephaly is likely due to a deficit in development and maintenance of connectivity, as supported by the CS mouse model. Slc9a6-null mouse neurons exhibit diminished axonal and dendritic branching, decreased synapse number and spine density, and a greater number of immature spines [98]. In addition, a subset of CS patients shows brainstem and cerebellar atrophy, as evidenced by magnetic resonance imaging (MRI), presenting after 12 months of age [38,102] and particularly after the first decade [39]. Clinical findings are corroborated by findings in the CS mouse model demonstrating Purkinje cell loss with age [103].
Monogenic postnatal macrocephalies
PTEN-RELATED DISORDERS
Mutations in phosphatase and tensin homolog (PTEN) are implicated in various distinct disorders with concomitant macrocephaly, including Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome (BRRS), Proteus syndrome, and Proteus-like syndrome [41]. These disorders, and other conditions with germline heterozygous loss-of-function mutations in PTEN, share a predisposition of tumors, and therefore are collectively referred to as PHTS (PTEN Hamartoma Tumor Syndrome). PTEN mutations are also described in patients with ASD with pronounced macrocephaly [56,57].
PTEN encodes a widely expressed tumor suppressor phosphatase. PTEN’s lipid-phosphatase activity inhibits the phosphoinositide 3-kinase (PI3K)-AKT pathway, which activates mammalian target of rapamycin (mTOR) signaling. The PI3K-AKT-mTOR pathway is involved in cell functions such as survival, proliferation, and cellular architecture (reviewed in [42]). In the brain, the PI3K-AKT pathway is implicated in functions including neuronal survival, outgrowth, synaptic plasticity, learning, and memory. In addition, PTEN acts as a protein phosphatase involved in regulation of various cell-survival pathways, for example, in mediation of growth suppression via the mitogen-activated protein kinase (MAPK) pathway [104]. Therefore, in the context of loss of PTEN protein or function, cell signaling and growth lack regulation.
In a cohort of 161 patients with pathogenic germline PTEN mutations, 94% of PHTS individuals presented with macrocephaly [105], supported by MRI studies [106]. In addition, mouse models show a gene dose-dependent increase in brain weight and macrocephaly, which increases from birth to adulthood [22,105,107**]. Brain overgrowth is likely due to abnormalities in proliferation and connectivity. PTEN haploinsufficiency in a mouse model leads to hyperplasia, specifically an excess neuronal population at birth and an excess glial population in adulthood, suggesting a role of PTEN in controlling cell number [107**]. Furthermore, neurons exhibit hypertrophy of neuronal soma and dendritic arborization and increased dendritic spine density [22]. In addition, MRI revealed white matter abnormalities and dilated perivascular spaces in patients with PTEN mutations [106]. This suggests that loss of PTEN protein or function disrupts neurodevelopmental events occurring prenatally (i.e., neurogenesis) and postnatally (i.e., gliogenesis, dendritic growth, myelination).
TUBEROUS SCLEROSIS COMPLEX (TSC)
TSC is an autosomal dominant disorder characterized by hamartomas in multiple organ systems and variable symptom presentation. Common neurological manifestations include epilepsy, cognitive disability, and an ASD phenotype [43,44]. TSC results from heterozygous germline mutations in either TSC1 or TSC2 [43]. Tumors likely develop from a “second hit,” in which disruption to the functional allele or other TSC protein leads to uncontrolled cell growth. Indeed, several downstream protein cascades are disrupted in TSC, particularly the mTOR signaling pathway [46]. TSC1 and TSC2 encode for hamartin and tuberin, respectively, which form a heterodimer complex [108]. Loss of a functional hamartin-tuberin complex leads to increased activation of mTOR signaling and subsequent unregulated cell growth and proliferation. The hamartin-tuberin complex is implicated in neurodevelopment, specifically in the regulation of somatic size, dendritic arborization, dendritic spine formation and morphogenesis, axon specification and guidance, astrocyte proliferation, and cortical lamination [46].
Neurological pathology associated with TSC includes macrocephaly [109], hamartomatous brain lesions, cellular cytomegaly, lamination defects, astrogliosis, and white matter abnormalities [45,110]. While several TSC neurological abnormalities arise prenatally, many occur postnatally. For example, there is increasing evidence of abnormal neuronal connectivity [111], which experiences large changes after birth. Postmortem studies of TSC individuals show multipolar neurons, shortened dendrites, abnormal spine morphology, and decreased dendritic spine density of projection neurons in tubers [112,113], corroborated by rodent models [114]. Furthermore, recent brain imaging studies show diffuse white matter abnormalities, structurally compromised axons, and altered structural connectivity in TSC [78,110,115**,116,117].
NEURODEGENERATIVE DISORDERS
While postnatal microcephaly usually results from abnormal neurodevelopment, this clinical finding can also herald an early-onset neurodegenerative process. A heterogeneous group of neurodegenerative disorders cause regression and progressive loss of neurological function in children. Pathologic hallmarks of neurodegeneration include neuronal loss and gliosis in the nervous system. Many neurodegenerative disorders are due to neurometabolic disease, related to synthesis, metabolism, transport, or storage of biochemical compounds [118]. For example, Cockayne syndrome is a childhood autosomal recessive disorder arising from abnormal nucleotide excision repair [49]; it is characterized by both postnatal growth failure and degeneration [48]. Cockayne syndrome is generally caused by mutations in ERCC6 or ERCC8, genes involved in DNA damage repair mechanisms. HC is typically normal at birth, followed by postnatal decelerated brain growth resulting in microcephaly. In addition, de novo mutations in KIF1A, which encodes for a microtubule-based motor protein involved in axonal transport of synaptic vesicle precursors, have been described in patients with a severe and progressive neurodegenerative syndrome presenting within the first months of life [50*]. The majority of patients exhibit microcephaly, potentially as a result of brain atrophy and cerebral white matter reduction.
Complex disorders
AUTISM SPECTRUM DISORDER (ASD)
ASD comprises genetically and clinically heterogeneous disorders of atypical neurodevelopment characterized by impaired communication and social interactions and stereotyped behaviors [51,52]. A subset of ASD appears to be associated with larger HC and brain volume [60**], perhaps with most rapid increase in head and brain size during the earliest stages of postnatal development [60**,119–121]. A variety of subphenotypes exist within ASD depending on genetic etiology. For example, high rates of macrocephaly are found in PTEN-associated ASD [56,57]. Also, postmortem ASD brains show increased spine density in frontal, parietal, and temporal lobes [101,122], likely due to a defect in postnatal pruning and associated with hyperactivated mTOR and impaired autophagy [122]. However, some cohorts describe ASD populations with microcephaly [123]. For example, ASD patients carrying DYRK1A mutations present with microcephaly [61*]. In addition, there are examples of genetic reciprocity in ASD. Duplications within chromosome 16p11.2 are associated with decreased brain volume and HC, whereas deletions are associated with the mirror pattern of increased brain volume and macrocephaly [59]. Additionally, reciprocal deletions and duplications within 1q21.1 are associated with microcephaly and macrocephaly, respectively [58]. Interestingly, extreme HC (small or large) is associated with lower IQ and higher autism symptom severity in ASD patients from the Simons Simplex Collection (SSC) [124].
SCHIZOPHRENIA
According to the widely accepted neurodevelopmental model, schizophrenia may arise in part from abnormal brain growth beginning years before symptom onset [62]. A number of MRI studies describe reduced brain and gray matter volume and increased extracerebral cerebral spinal fluid (CSF) in schizophrenia (reviewed in [64]). Also, typical back-to-front gray matter loss is exaggerated, predominantly in prefrontal and temporal cortices [125–127]. Greatest reduction has been noted 3 months following onset of psychosis, advancing into most of the frontal cortex by one year after onset [128]. Postmortem studies indicate that loss of cortical gray matter is due to reduced dendritic complexity and synaptic density, rather than from decreased neuron number [63]. Given its complexity, identification of potential genetic etiologies for observed brain matter changes in schizophrenia is an ongoing area of research. As an example, results from a recent study revealed an association between schizophrenia and altered expression of complement component 4 (C4) genes, the encoded proteins of which are involved in synapse elimination during postnatal development [65**]. Finally, abnormal functional and structural connectivity and altered white matter integrity are observed in schizophrenia [129–132].
Conclusions
In conclusion, the study of brain growth disorders illustrates that postnatal neurodevelopment is supported by regulation of neuronal arborization, synaptogenesis and pruning, and gliogenesis and myelination. However, our understanding of biological events underlying typical and pathological brain development in the postnatal period is still incomplete. Additional longitudinal studies will be important in correlating gene and protein function with brain growth trajectories. Understanding the pathogenesis of childhood neurodevelopmental disorders will help in differentiating and diagnosing neurogenetic syndromes, as well as in developing interventions to normalize divergent postnatal brain growth trajectories.
Key points.
Postnatal brain development is supported by genetic programming with experience-dependent remodeling.
Correlations between gene and protein function and postnatal brain growth trajectories illuminate underlying biological mechanisms of neurodevelopment.
Postnatal brain growth involves neuronal arborization, synaptogenesis and pruning, and gliogenesis and myelination.
Investigation of the pathogenesis of brain growth disorders can teach us about typical postnatal neurodevelopment and help in the development of interventions to correct aberrant trajectories in brain development.
Acknowledgments
None
Financial support and sponsorship
This work was supported in part by the following grants and institutional funds to EMM: National Institute of Mental Health (MH105442, MH102418), Simons Foundation Autism Research Initiative (286756), Simons Foundation Autism Research Initiative and Nancy Lurie Marks Family Foundation for Autism Research (296318), and the Hassenfeld Child Health Innovation Institute at Brown University.
Footnotes
Conflicts of interest
None
References
- 1.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rollins JD, Collins JS, Holden KR. United States head circumference growth reference charts: birth to 21 years. J Pediatr. 2010;156:907–913. doi: 10.1016/j.jpeds.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 4.Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- 5.Lindley AA, Benson JE, Grimes C, et al. The relationship in neonates between clinically measured head circumference and brain volume estimated from head CT-scans. Early Hum Dev. 1999;56:17–29. doi: 10.1016/s0378-3782(99)00033-x. [DOI] [PubMed] [Google Scholar]
- 6.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmore EC, Walsh CA. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol. 2013;2:461–478. doi: 10.1002/wdev.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolk H. The predictive value of microcephaly during the first year of life for mental retardation at seven years. Dev Med Child Neurol. 1991;33:974–983. doi: 10.1111/j.1469-8749.1991.tb14813.x. [DOI] [PubMed] [Google Scholar]
- 9.Seltzer LE, Paciorkowski AR. Genetic disorders associated with postnatal microcephaly. Am J Med Genet C Semin Med Genet. 2014;166C:140–155. doi: 10.1002/ajmg.c.31400. [DOI] [PubMed] [Google Scholar]
- 10.Williams CA, Dagli A, Battaglia A. Genetic disorders associated with macrocephaly. Am J Med Genet A. 2008;146A:2023–2037. doi: 10.1002/ajmg.a.32434. [DOI] [PubMed] [Google Scholar]
- 11.Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 12.Hazlett HC, Poe M, Gerig G, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 13.Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- 14.Knickmeyer RC, Gouttard S, Kang C, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland MA, Miller KE, Kuhl E. Emerging Brain Morphologies from Axonal Elongation. Ann Biomed Eng. 2015;43:1640–1653. doi: 10.1007/s10439-015-1312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raybaud C, Ahmad T, Rastegar N, et al. The premature brain: developmental and lesional anatomy. Neuroradiology. 2013;55 Suppl(2):23–40. doi: 10.1007/s00234-013-1231-0. [DOI] [PubMed] [Google Scholar]
- 17.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 18.Penzes P, Cahill ME, Jones KA, et al. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belichenko PV, Wright EE, Belichenko NP, et al. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J Comp Neurol. 2009;514:240–258. doi: 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- 20.de Anda FC, Rosario AL, Durak O, et al. Autism spectrum disorder susceptibility gene TAOK2 affects basal dendrite formation in the neocortex. Nat Neurosci. 2012;15:1022–1031. doi: 10.1038/nn.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- 22.Kwon CH, Luikart BW, Powell CM, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 25.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Glantz LA, Gilmore JH, Hamer RM, et al. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 29.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 30. Zuchero JB, Barres BA. Glia in mammalian development and disease. Development. 2015;142:3805–3809. doi: 10.1242/dev.129304. This review extensively discusses the latest research on glia in the context of neurodevelopment. This article highlights the numerous and diverse roles of glia.
- 31.Gibson EM, Purger D, Mount CW, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demerens C, Stankoff B, Logak M, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 34.Bird LM. Angelman syndrome: review of clinical and molecular aspects. Appl Clin Genet. 2014;7:93–104. doi: 10.2147/TACG.S57386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagberg G, Stenbom Y, Witt Engerstrom I. Head growth in Rett syndrome. Acta Paediatr. 2000;89:198–202. doi: 10.1080/080352500750028834. [DOI] [PubMed] [Google Scholar]
- 36.Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pohodich AE, Zoghbi HY. Rett syndrome: disruption of epigenetic control of postnatal neurological functions. Hum Mol Genet. 2015;24:R10–R16. doi: 10.1093/hmg/ddv217. This review provides a comprehensive perspective of the molecular basis of Rett syndrome. This article considers potential mechanisms underlying postnatal onset of the disorder.
- 38.Christianson AL, Stevenson RE, van der Meyden CH, et al. X linked severe mental retardation, craniofacial dysmorphology, epilepsy, ophthalmoplegia, and cerebellar atrophy in a large South African kindred is localised to Xq24–q27. J Med Genet. 1999;36:759–766. doi: 10.1136/jmg.36.10.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pescosolido MF, Stein DM, Schmidt M, et al. Genetic and phenotypic diversity of NHE6 mutations in Christianson syndrome. Ann Neurol. 2014;76:581–593. doi: 10.1002/ana.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilfillan GD, Selmer KK, Roxrud I, et al. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet. 2008;82:1003–1010. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med. 2009;11:687–694. doi: 10.1097/GIM.0b013e3181ac9aea. [DOI] [PubMed] [Google Scholar]
- 42.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 43.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 44.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 45.DiMario FJ., Jr Brain abnormalities in tuberous sclerosis complex. J Child Neurol. 2004;19:650–657. doi: 10.1177/08830738040190090401. [DOI] [PubMed] [Google Scholar]
- 46.Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cockayne EA. Dwarfism with Retinal Atrophy and Deafness. Arch Dis Child. 1946;21:52–54. [PubMed] [Google Scholar]
- 48.Rapin I, Lindenbaum Y, Dickson DW, et al. Cockayne syndrome and xeroderma pigmentosum. Neurology. 2000;55:1442–1449. doi: 10.1212/wnl.55.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 50. Esmaeeli Nieh S, Madou MR, Sirajuddin M, et al. De novo mutations in KIF1A cause progressive encephalopathy and brain atrophy. Ann Clin Transl Neurol. 2015;2:623–635. doi: 10.1002/acn3.198. This article is the first to genetically and clinically characterize a novel neurodegenerative syndrome associated with de novo mutations in KIF1A.
- 51.DiCicco-Bloom E, Lord C, Zwaigenbaum L, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol. 2014;10:74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koldewyn K, Yendiki A, Weigelt S, et al. Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proc Natl Acad Sci U S A. 2014;111:1981–1986. doi: 10.1073/pnas.1324037111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rane P, Cochran D, Hodge SM, et al. Connectivity in Autism: A Review of MRI Connectivity Studies. Harv Rev Psychiatry. 2015;23:223–244. doi: 10.1097/HRP.0000000000000072. This review discusses the latest perspectives of connectivity in ASD. This article highlights convergent MRI findings of abnormal intra- and inter-hemispheric circuitry in ASD.
- 55.Travers BG, Adluru N, Ennis C, et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buxbaum JD, Cai G, Chaste P, et al. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:484–491. doi: 10.1002/ajmg.b.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McBride KL, Varga EA, Pastore MT, et al. Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res. 2010;3:137–141. doi: 10.1002/aur.132. [DOI] [PubMed] [Google Scholar]
- 58.Brunetti-Pierri N, Berg JS, Scaglia F, et al. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet. 2008;40:1466–1471. doi: 10.1038/ng.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qureshi AY, Mueller S, Snyder AZ, et al. Opposing brain differences in 16p11.2 deletion and duplication carriers. J Neurosci. 2014;34:11199–11211. doi: 10.1523/JNEUROSCI.1366-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sacco R, Gabriele S, Persico AM. Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Res. 2015;234:239–251. doi: 10.1016/j.pscychresns.2015.08.016. This article is the first to compile numerous studies in order to provide an overall estimate of head and brain size in ASD.
- 61. van Bon BW, Coe BP, Bernier R, et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Mol Psychiatry. 2016;21:126–132. doi: 10.1038/mp.2015.5. This article describes a microcephaly-associated ASD due to DYRK1A mutations. The described study is the first to genetically and clinically characterize this subgroup of ASD.
- 62.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 64.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 65. Sekar A, Bialas AR, de Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. This article identifies genes strongly associated with schizophrenia and their involvement in synapse elimination. Importantly, this research puts forth a potential biological mechanism, namely, altered expression of different alleles of C4 genes and an associated increase in C4-mediated postnatal synaptic pruning, to explain the reduced number of synapses observed in patient brains.
- 66.Albrecht U, Sutcliffe JS, Cattanach BM, et al. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat Genet. 1997;17:75–78. doi: 10.1038/ng0997-75. [DOI] [PubMed] [Google Scholar]
- 67.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 68.Matsuura T, Sutcliffe JS, Fang P, et al. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet. 1997;15:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 69.Judson MC, Sosa-Pagan JO, Del Cid WA, et al. Allelic specificity of Ube3a expression in the mouse brain during postnatal development. J Comp Neurol. 2014;522:1874–1896. doi: 10.1002/cne.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamasaki K, Joh K, Ohta T, et al. Neurons but not glial cells show reciprocal imprinting of sense and antisense transcripts of Ube3a. Hum Mol Genet. 2003;12:837–847. doi: 10.1093/hmg/ddg106. [DOI] [PubMed] [Google Scholar]
- 71.Sato M, Stryker MP. Genomic imprinting of experience-dependent cortical plasticity by the ubiquitin ligase gene Ube3a. Proc Natl Acad Sci U S A. 2010;107:5611–5616. doi: 10.1073/pnas.1001281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yashiro K, Riday TT, Condon KH, et al. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009;12:777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan WH, Bacino CA, Skinner SA, et al. Angelman syndrome: Mutations influence features in early childhood. Am J Med Genet A. 2011;155A:81–90. doi: 10.1002/ajmg.a.33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fridman C, Varela MC, Kok F, et al. Paternal UPD15: further genetic and clinical studies in four Angelman syndrome patients. Am J Med Genet. 2000;92:322–327. doi: 10.1002/1096-8628(20000619)92:5<322::aid-ajmg6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 75.Jiang YH, Armstrong D, Albrecht U, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 76.Miura K, Kishino T, Li E, et al. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol Dis. 2002;9:149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- 77.Greer PL, Hanayama R, Bloodgood BL, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peters JM, Sahin M, Vogel-Farley VK, et al. Loss of white matter microstructural integrity is associated with adverse neurological outcome in tuberous sclerosis complex. Acad Radiol. 2012;19:17–25. doi: 10.1016/j.acra.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 80.Amir RE, Van den Veyver IB, Wan M, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 81.Chahrour M, Jung SY, Shaw C, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yasui DH, Peddada S, Bieda MC, et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci U S A. 2007;104:19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balmer D, Goldstine J, Rao YM, LaSalle JM. Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J Mol Med (Berl) 2003;81:61–68. doi: 10.1007/s00109-002-0396-5. [DOI] [PubMed] [Google Scholar]
- 84.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet. 2002;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- 86.Schultz RJ, Glaze DG, Motil KJ, et al. The pattern of growth failure in Rett syndrome. Am J Dis Child. 1993;147:633–637. doi: 10.1001/archpedi.1993.02160300039018. [DOI] [PubMed] [Google Scholar]
- 87.Armstrong D, Dunn JK, Antalffy B, Trivedi R. Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol. 1995;54:195–201. doi: 10.1097/00005072-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 88.Baj G, Patrizio A, Montalbano A, et al. Developmental and maintenance defects in Rett syndrome neurons identified by a new mouse staging system in vitro. Front Cell Neurosci. 2014;8:18. doi: 10.3389/fncel.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chapleau CA, Calfa GD, Lane MC, et al. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol Dis. 2009;35:219–233. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marchetto MC, Carromeu C, Acab A, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010;30:5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maezawa I, Swanberg S, Harvey D, et al. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horska A, Farage L, Bibat G, et al. Brain metabolism in Rett syndrome: age, clinical, and genotype correlations. Ann Neurol. 2009;65:90–97. doi: 10.1002/ana.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahmood A, Bibat G, Zhan AL, et al. White matter impairment in Rett syndrome: diffusion tensor imaging study with clinical correlations. AJNR Am J Neuroradiol. 2010;31:295–299. doi: 10.3174/ajnr.A1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brett CL, Wei Y, Donowitz M, Rao R. Human Na(+)/H(+) exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol. 2002;282:C1031–C1041. doi: 10.1152/ajpcell.00420.2001. [DOI] [PubMed] [Google Scholar]
- 97.Nakamura N, Tanaka S, Teko Y, et al. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 98.Ouyang Q, Lizarraga SB, Schmidt M, et al. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohgaki R, van ISC, Matsushita M, et al. Organellar Na+/H+ exchangers: novel players in organelle pH regulation and their emerging functions. Biochemistry. 2011;50:443–450. doi: 10.1021/bi101082e. [DOI] [PubMed] [Google Scholar]
- 100.Park M, Salgado JM, Ostroff L, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 102.Bosemani T, Zanni G, Hartman AL, et al. Christianson syndrome: spectrum of neuroimaging findings. Neuropediatrics. 2014;45:247–251. doi: 10.1055/s-0033-1363091. [DOI] [PubMed] [Google Scholar]
- 103.Stromme P, Dobrenis K, Sillitoe RV, et al. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain. 2011;134:3369–3383. doi: 10.1093/brain/awr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Waite KA, Eng C. Protean PTEN: form and function. Am J Hum Genet. 2002;70:829–844. doi: 10.1086/340026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mester JL, Tilot AK, Rybicki LA, et al. Analysis of prevalence and degree of macrocephaly in patients with germline PTEN mutations and of brain weight in Pten knock-in murine model. Eur J Hum Genet. 2011;19:763–768. doi: 10.1038/ejhg.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vanderver A, Tonduti D, Kahn I, et al. Characteristic brain magnetic resonance imaging pattern in patients with macrocephaly and PTEN mutations. Am J Med Genet A. 2014;164A:627–633. doi: 10.1002/ajmg.a.36309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chen Y, Huang WC, Sejourne J, et al. Pten Mutations Alter Brain Growth Trajectory and Allocation of Cell Types through Elevated beta-Catenin Signaling. J Neurosci. 2015;35:10252–10267. doi: 10.1523/JNEUROSCI.5272-14.2015. This article describes the first study correlating TSC mouse brain overgrowth with hyperplasia of specific cell types at birth versus adulthood. This article highlights a potential cellular mechanism underlying brain overgrowth during development in TSC.
- 108.van Slegtenhorst M, Nellist M, Nagelkerken B, et al. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet. 1998;7:1053–1057. doi: 10.1093/hmg/7.6.1053. [DOI] [PubMed] [Google Scholar]
- 109.Fidler DJ, Bailey JN, Smalley SL. Macrocephaly in autism and other pervasive developmental disorders. Dev Med Child Neurol. 2000;42:737–740. doi: 10.1017/s0012162200001365. [DOI] [PubMed] [Google Scholar]
- 110.Krishnan ML, Commowick O, Jeste SS, et al. Diffusion features of white matter in tuberous sclerosis with tractography. Pediatr Neurol. 2010;42:101–106. doi: 10.1016/j.pediatrneurol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsai P, Sahin M. Mechanisms of neurocognitive dysfunction and therapeutic considerations in tuberous sclerosis complex. Curr Opin Neurol. 2011;24:106–113. doi: 10.1097/WCO.0b013e32834451c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huttenlocher PR, Heydemann PT. Fine structure of cortical tubers in tuberous sclerosis: a Golgi study. Ann Neurol. 1984;16:595–602. doi: 10.1002/ana.410160511. [DOI] [PubMed] [Google Scholar]
- 113.Machado-Salas JP. Abnormal dendritic patterns and aberrant spine development in Bourneville's disease--a Golgi survey. Clin Neuropathol. 1984;3:52–58. [PubMed] [Google Scholar]
- 114.Tavazoie SF, Alvarez VA, Ridenour DA, et al. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- 115. Im K, Ahtam B, Haehn D, et al. Altered Structural Brain Networks in Tuberous Sclerosis Complex. Cereb Cortex. 2016;26:2046–2058. doi: 10.1093/cercor/bhv026. This article represents the first unbiased study of global and regional white matter connectivity in TSC. This article correlates connectivity with tuber load and developmental delay, relating brain imaging to clinical phenotype.
- 116.Makki MI, Chugani DC, Janisse J, Chugani HT. Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol. 2007;28:1662–1667. doi: 10.3174/ajnr.A0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Widjaja E, Simao G, Mahmoodabadi SZ, et al. Diffusion tensor imaging identifies changes in normal-appearing white matter within the epileptogenic zone in tuberous sclerosis complex. Epilepsy Res. 2010;89:246–253. doi: 10.1016/j.eplepsyres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 118.Wong V. Neurodegenerative diseases in children. Hong Kong Med J. 1997;3:89–95. [PubMed] [Google Scholar]
- 119.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 120.Dementieva YA, Vance DD, Donnelly SL, et al. Accelerated head growth in early development of individuals with autism. Pediatr Neurol. 2005;32:102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 121.Webb SJ, Nalty T, Munson J, et al. Rate of head circumference growth as a function of autism diagnosis and history of autistic regression. J Child Neurol. 2007;22:1182–1190. doi: 10.1177/0883073807306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang G, Gudsnuk K, Kuo SH, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fombonne E, Roge B, Claverie J, et al. Microcephaly and macrocephaly in autism. J Autism Dev Disord. 1999;29:113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- 124.Chaste P, Klei L, Sanders SJ, et al. Adjusting head circumference for covariates in autism: clinical correlates of a highly heritable continuous trait. Biol Psychiatry. 2013;74:576–584. doi: 10.1016/j.biopsych.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 126.Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 127.Wiegand LC, Warfield SK, Levitt JJ, et al. Prefrontal cortical thickness in first-episode psychosis: a magnetic resonance imaging study. Biol Psychiatry. 2004;55:131–140. doi: 10.1016/j.biopsych.2003.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thompson PM, Bartzokis G, Hayashi KM, et al. Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb Cortex. 2009;19:1107–1123. doi: 10.1093/cercor/bhn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fornito A, Yoon J, Zalesky A, et al. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull. 2008;34:72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lawrie SM, Buechel C, Whalley HC, et al. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 132.Lim KO, Hedehus M, Moseley M, et al. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]