Abstract
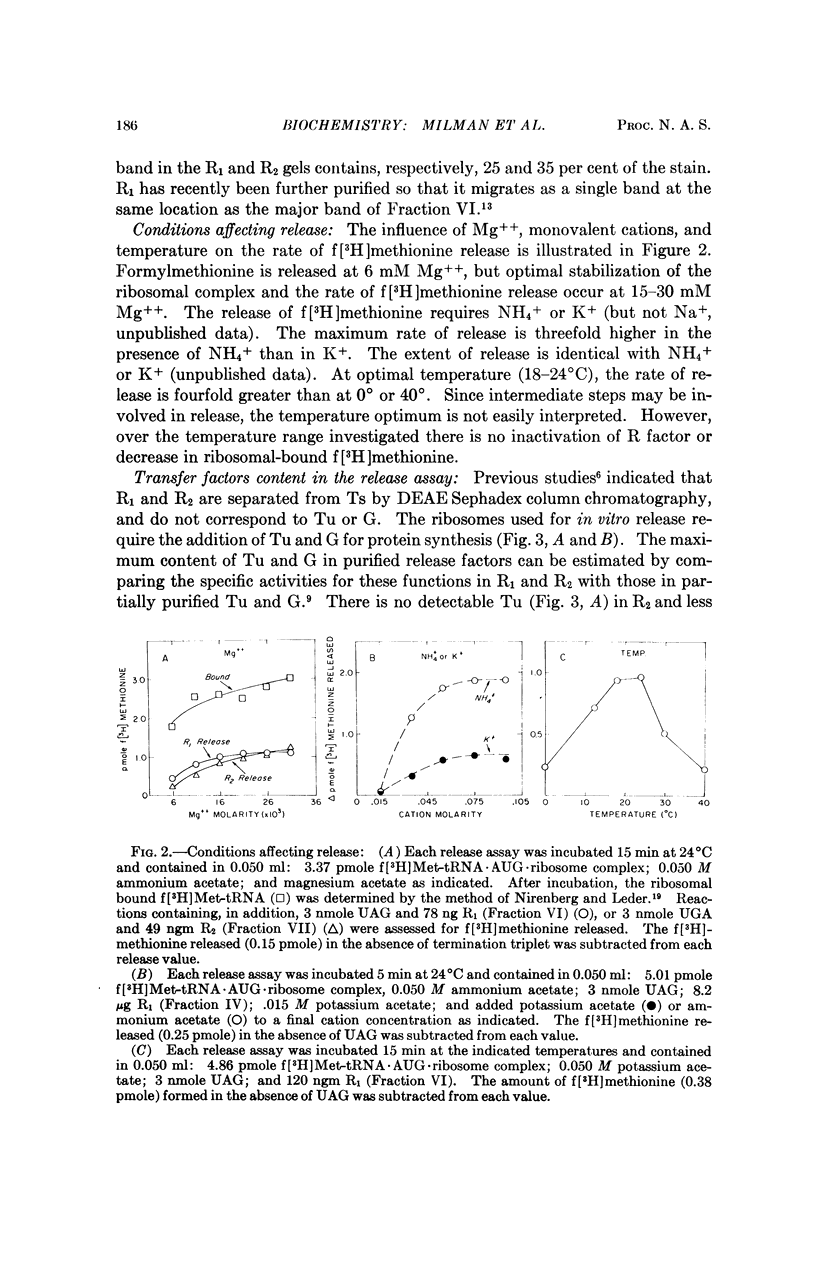
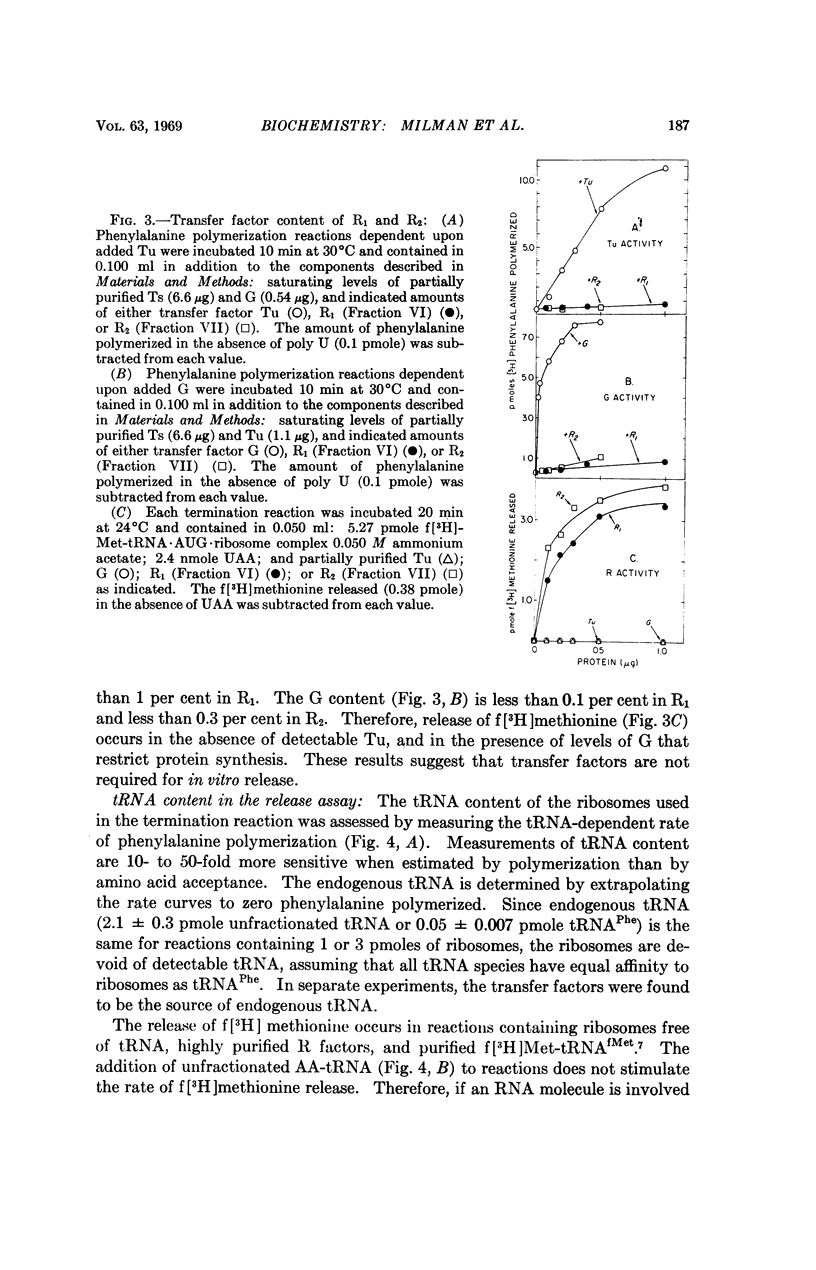
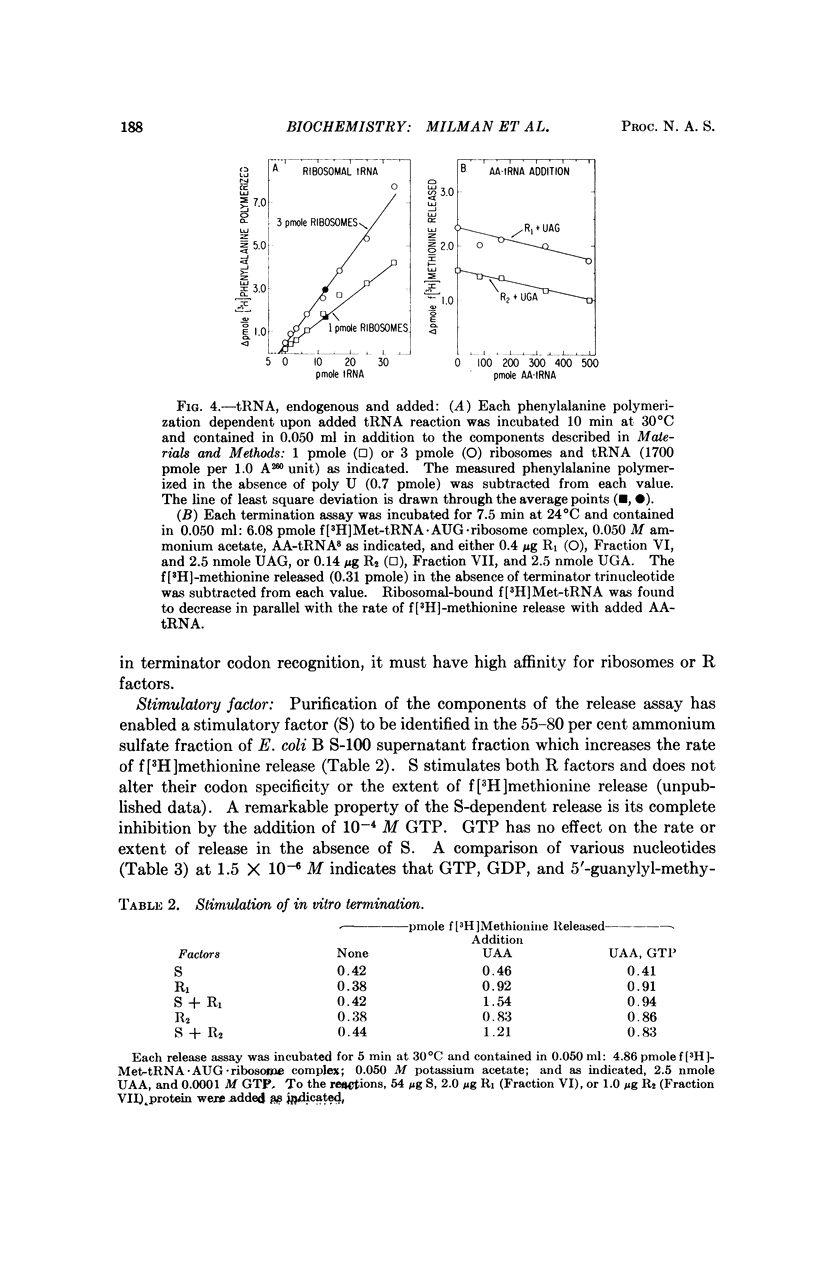
Throughout extensive purification, the release factors R1 and R2 each behave as a single molecular species with alternate codon recognition (R1, UAA or UAG; R2, UAA or UGA). The release of f[3H]methionine from f[3H]-Met-tRNA·AUG·ribosome complex requires R factor and terminator codon and does not appear to require tRNA or transfer factors T and G. Purification of the components of the release assay has enabled identification of a protein factor S in the 55-80 per cent ammonium sulfate fraction of E. coli B supernatant fraction which stimulates the rate but not the extent of release dependent upon R factor and appropriate termination codon. The S factor has properties similar to T, but further purification is required to determine the nature and function of S in peptide chain termination.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende J. E., Seeds N. W., Conway T. W., Weissbach H. Guanosine triphosphate interaction with an amino acid polymerization factor from E. coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1566–1573. doi: 10.1073/pnas.58.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Barnett L., Katz E. R., Crick F. H. UGA: a third nonsense triplet in the genetic code. Nature. 1967 Feb 4;213(5075):449–450. doi: 10.1038/213449a0. [DOI] [PubMed] [Google Scholar]
- Brenner S., Stretton A. O., Kaplan S. Genetic code: the 'nonsense' triplets for chain termination and their suppression. Nature. 1965 Jun 5;206(988):994–998. doi: 10.1038/206994a0. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Polypeptide chain termination in vitro: isolation of a release factor. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1144–1151. doi: 10.1073/pnas.58.3.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A., Nirenberg M. RNA codons and protein synthesis. 15. Dissimilar responses of mammalian and bacterial transfer RNA fractions to messenger RNA codons. J Mol Biol. 1968 Oct 14;37(1):99–118. doi: 10.1016/0022-2836(68)90076-4. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Tompkins R., Scolnick E., Caryk T., Nirenberg M. Sequential translation of trinucleotide codons for the initiation and termination of protein synthesis. Science. 1968 Oct 4;162(3849):135–138. doi: 10.1126/science.162.3849.135. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Separation of three microbial amino acid polymerization factors. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1562–1566. doi: 10.1073/pnas.55.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHAEI J. H., NIRENBERG M. W. Characteristics and stabilization of DNAase-sensitive protein synthesis in E. coli extracts. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1580–1588. doi: 10.1073/pnas.47.10.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Ravel J. M. Demonstration of a guanosine triphosphate-dependent enzymatic binding of aminoacyl-ribonucleic acid to Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1811–1816. doi: 10.1073/pnas.57.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M. G., Garen A. Base composition of nonsense codons in E. coli. Evidence from amino-acid substitutions at a tryptophan site in alkaline phosphatase. Nature. 1965 Jun 5;206(988):992–994. doi: 10.1038/206992a0. [DOI] [PubMed] [Google Scholar]
- Weiss J. F., Pearson R. L., Kelmers A. D. Two additional reversed-phase chromatographic systems for the separation of transfer ribonucleic acids and their application to the preparation of two formylmethionine and a valine transfer ribonucleic acid from Escherichia coli B. Biochemistry. 1968 Oct;7(10):3479–3487. doi: 10.1021/bi00850a024. [DOI] [PubMed] [Google Scholar]



