Abstract
Females in a variety of taxa adjust offspring sex ratios to prevailing ecological conditions. However, little is known about whether conditions experienced during a female’s early ontogeny influence the sex ratio of her offspring. We tested for past and present ecological predictors of offspring sex ratios among known-age females that were produced as offspring and bred as adults in a population of house wrens. The body condition of offspring that a female produced and the proportion of her offspring that were male were negatively correlated with the size of the brood in which she herself was reared. The proportion of sons within broods was negatively correlated with maternal hatching date, and varied positively with the quality of a female’s current breeding territory as predicted. However, females producing relatively more sons than daughters were less likely to return to breed in the population the following year. Although correlative, our results suggest that the rearing environment can have enduring effects on later maternal investment and sex allocation. Moreover, the overproduction of sons relative to daughters may increase costs to a female’s residual reproductive value, constraining the extent to which sons might be produced in high-quality breeding conditions. Sex allocation in birds remains a contentious subject, largely because effects on offspring sex ratios are small. Our results suggest that offspring sex ratios are shaped by various processes and trade-offs that act throughout the female life history and ultimately reduce the extent of sex-ratio adjustment relative to classic theoretical predictions.
Keywords: house wren, life history, maternal effect, sex allocation, Trivers-Willard, Troglodytes aedon
Introduction
Although sons and daughters have, on average, equal reproductive value at birth, the production of either sex can affect parental fitness differently depending on a number of ecological conditions (Fisher 1930; West 2009). In a variety of taxa, for example, the quality of the rearing environment can have sex-specific effects on the reproductive success of adult offspring (Clutton-Brock et al. 1984; Bowers et al. 2015), generate differential costs of reproduction for parents if one sex is more costly to rear (Cockburn et al. 2002; Rutkowska et al. 2011), or, if the sexes differ in philopatry, generate local resource competition among kin (Komdeur 2012). Thus, when an interaction between ecological conditions and the offspring sex ratio affect parental fitness, selection should favor the parental ability to adjust the sex of offspring (Trivers and Willard 1973).
An important assumption underlying a major component of sex-allocation theory is that, for selection to favor facultative sex allocation, variation in rearing conditions should affect the reproductive success of sons and daughters differently (Trivers and Willard 1973; see also Hewison and Gaillard 1999; West 2009). In many species, the reproductive success of males is more variable and more strongly affected by variation in body condition than that of females. Thus, when the rearing environment affects body condition and adult reproductive success sex-specifically, females investing heavily in offspring are predicted to overproduce sons because these sons will enjoy increased reproductive success as adults; however, mothers unable to invest as heavily in offspring should overproduce daughters because daughters will have higher reproductive success than sons that are reared under poor-quality conditions (Trivers and Willard 1973; Badyaev et al. 2002, 2006; Krist 2006). Alternatively, mothers on resource-poor territories may overproduce daughters, not to enhance the future reproductive success of their offspring, but simply to maximize the number of offspring that survive or to ameliorate the costs of reproduction if sons require more resources than daughters (Myers 1978; see also Cockburn et al. 2002).
In a population of house wrens, we recently found that the quality of the rearing environment and sibling rivalry have sex-specific effects on offspring reproductive value, with the growth and body condition of male and female nestlings affected differently by the within-brood competitive hierarchy established by the asynchronous hatching of eggs (Bowers et al. 2011). Although male and female offspring hatch from similarly sized eggs and are similar in size shortly after hatching (Bowers et al. 2011, 2014a), males that are days younger than their older siblings (caused by asynchronous hatching) grow more slowly and obtain lower asymptotic body mass than their sisters of a similar age (Bowers et al. 2011). We also found a similar result using two cross-fostering experiments, one in which we manipulated a nestling’s age-related competitive ability relative to nestmates (as occurs when hatching of eggs is asynchronous), and another in which nestling age was held constant while brood size and per-capita food availability for nestlings was manipulated (Bowers et al. 2015). In each of these studies, phenotypic traits did not differ between male and female nestlings, on average. However, males reared under high-quality conditions and increased levels of parental care were heavier, and those reared under low-quality conditions lighter, than females reared under similar conditions. Heavier males reared under high-quality conditions also had higher reproductive success as adults than lighter males reared under adverse conditions, and their reproductive success was positively associated with their body condition prior to independence; however, daughters were less strongly affected by the quality of the rearing environment, and their reproductive success was not associated with their condition prior to independence (Bowers et al. 2015, 2016). As predicted by the generalized Trivers-Willard Model, we also found that females investing more into offspring overproduced sons relative to those investing less (Bowers et al. 2015; see also Whittingham et al. 2002 for a similar result in another house wren population).
Nearly all current sex-allocation models describe sex-ratio variation in relation to either the ecological conditions in which parents find themselves or the environment their offspring are likely to experience in the future (Schwanz and Robert 2014). However, little attention has been paid to the question of whether conditions experienced during a parent’s own ontogeny influence the sex ratio of its progeny (but see Helle et al. 2012; Warner et al. 2013), despite the fact that such carry-over effects can often generate substantial variation in parental investment and fitness, and are likely to be far more widespread than previously thought (Gustafsson and Sutherland 1988; Auer et al. 2010; Bouwhuis et al. 2010; Uller and Olsson 2010; Harrison et al. 2011; Wong and Kölliker 2014). In the context of the Trivers-Willard Model, if variation in natal environmental conditions experienced by females, including resource availability and sibling rivalry, affects their ability to invest in offspring as adults, then the early rearing environment may represent an important source of variation in offspring sex ratios.
In this study, we tested whether past and current ecological conditions experienced by locally recruited females influence the condition and sex ratio of their offspring in a wild population of house wrens. We followed cohorts of known-age females that were produced as offspring in the study population to determine whether carry-over effects from a female’s early ontogeny are associated with the condition of the offspring she produces as an adult (e.g., Naguib and Gil 2005; Naguib et al. 2006). We predicted that, if this is the case, then these conditions would also predict the offspring sex ratio. We predicted that females that were heavier-than-average and those reared in smaller broods as nestlings (nestlings in smaller broods benefit from increased per-capita food availability; Bowers et al. 2014b) would produce heavier offspring and a male-biased sex ratio as adults. In addition, we tested whether a female’s current breeding conditions also influence offspring condition and sex ratios by obtaining an objective proxy of territory quality (the number of offspring successfully fledged from a nestbox over the ten years prior to this study; see Methods). If this measure of territory quality is indicative of local resource availability or male attractiveness, or a combination of both, then females breeding on high-quality territories should rear offspring in better condition than those on poorer-quality territories. We also predicted that the relative production of sons within broods would be positively associated with the quality of a female’s current territory. Finally, because producing sons and daughters may result in differential costs of reproduction to females, we tested whether the proportion of males within broods predicted a female’s probability of returning to breed in the population in subsequent years.
Materials and Methods
Study Site and Species
We studied a population of house wrens breeding in north-central Illinois, USA (40.665°N, 88.89°W) where there are a total of 820 nestboxes available on two forested study areas surrounded by agricultural fields. The subset of available nestboxes used in the present study (N = 258) has been in place since the 1982 breeding season in a nature preserve that has been subject to minimal human disturbance. Nestboxes are spaced 30 m apart along north-south-oriented transects separated by 60 m (5.4 nestboxes/ha; see Figure 1 in DeMory et al. 2010); details of nestbox design can be found in Lambrechts et al. (2010).
House wrens are small (10–12 g), insectivorous songbirds distributed widely across North and South America (Johnson 2014). Males arrive on the study area from spring migration in late April and select and defend nestboxes in which they begin nest construction. Females then select a mate, choosing among possible mates based partly on the quality of the nesting site and the number of available nesting sites they are able to secure and defend from rival males (Johnson and Searcy 1993; Eckerle and Thompson 2006; DeMory et al. 2010). Upon pairing, females complete nest construction and lay a clutch of 4–8 eggs. A nesting cycle spans approximately five weeks, and 50–70% of the females that complete a successful nesting attempt early in the breeding season attempt a second brood on the study area (Finke et al. 1987; Dobbs et al. 2006), with egg-laying for the second brood beginning in late June and early July. Only females incubate eggs and brood young, but both parents provision the young from hatching, and the length of the nestling period is typically 15–17 days (Bowers et al. 2013a, 2014b). Male and female offspring returning to the study area do not differ statistically in natal dispersal distances (median distances: males = 608 m, females = 647 m; Drilling and Thompson 1988), as measured by the distance between the nestbox in which they hatched and the nestbox in which they first bred.
General Procedures
In all years, we checked nestboxes at least twice weekly from May–August to check for female settlement and clutch initiation. Once females finished laying eggs and commenced incubation, we captured the females and males during incubation or early in the nestling-rearing period by either capturing them inside nestboxes or using mist nets near the box. Upon their capture, we measured the body mass (± 0.1 g) and tarsus length (± 0.1 mm) of adults and banded them with a unique U. S. Geological Survey aluminum leg band; males received three additional colored leg bands so they could be identified with binoculars (males are more difficult to capture than females). We visited nests daily when hatching was expected and, 11 days after hatching began, we banded the nestlings, weighed them, and measured their tarsus. Nestling body mass and tarsus length generally reach asymptotic levels by this age, and mass at this age positively predicts recruitment into the breeding population (Bowers et al. 2014c). We also drew a blood sample at this time for molecular sexing (details in Bowers et al. 2011). Although waiting to sample blood until this age introduces the possibility of pre-sampling nestling mortality, we have found no evidence of sex-biased mortality in our study population (Bowers et al. 2015). Nonetheless, we cannot rule out the possibility that sex-biased mortality contributed to the observed sex-ratio variation in the current study. Over the course of this study, we also conducted a number of experimental manipulations to broods (e.g., brood-size manipulations and experimental increases in clutch size), but none of the females in our sample were subjected to such manipulations. Recruitment is low (ca. 2–5%) in house wren populations (e.g., Kendeigh 1941; Poirier et al. 2004; Bowers et al. 2015). Over the course of five breeding seasons (2009–2013), we obtained sex-ratio data for 57 broods (318 nestlings) produced by 37 different females that had been reared on the study area and recruited to breed as adults in subsequent years (average age: 1.6 yr; maximum age: 5 yr).
House wren males are highly territorial and compete vigorously over breeding territories (Johnson and Kermott 1990). Heavier, larger, and more attractive males typically out-compete other males for breeding territories and mates and have increased reproductive success (DeMory et al. 2010; Bowers et al. 2015); competition among males over highly limiting breeding sites is intense, and can occasionally result in one male killing another (Belles-Isles and Picman 1987). Indeed, female mate decisions are often based more strongly on aspects of a male’s territory than they are on a male’s morphology (Eckerle and Thompson 2006). Therefore, we tested for an effect of territory quality on offspring condition and sex ratios, using a proxy of territory quality quantified as the number of offspring successfully fledged from a nestbox over the ten years preceding this study (Fig 1). Although approximately 20–30% of females and 25%–45% of males breeding in one year return to breed the next year (Johnson 2014), in no case did a bird in the current study contribute to an estimate of the quality of its breeding territory. Therefore, these estimates of territory quality are independent of the reproductive success of focal birds in the current study, as there is a high rate of turnover among the birds that use these territories. For example, the focal females and their mates in the current study (37 different females and 29 different males that we identified) produced a total of 168 nesting attempts on the study area in their lifetimes. Of these, six different birds each reused the same territory across different years; five birds (three females and two males) each reused the same territory once across different years, and one bird, a male, reused each of three different territories across different years. Therefore, this is a reliable index of territory quality in our study species that reflects the attractiveness of nest sites and the productivity of birds that use them (Fig 1; see also Janiszewski et al. 2013).
Fig 1.
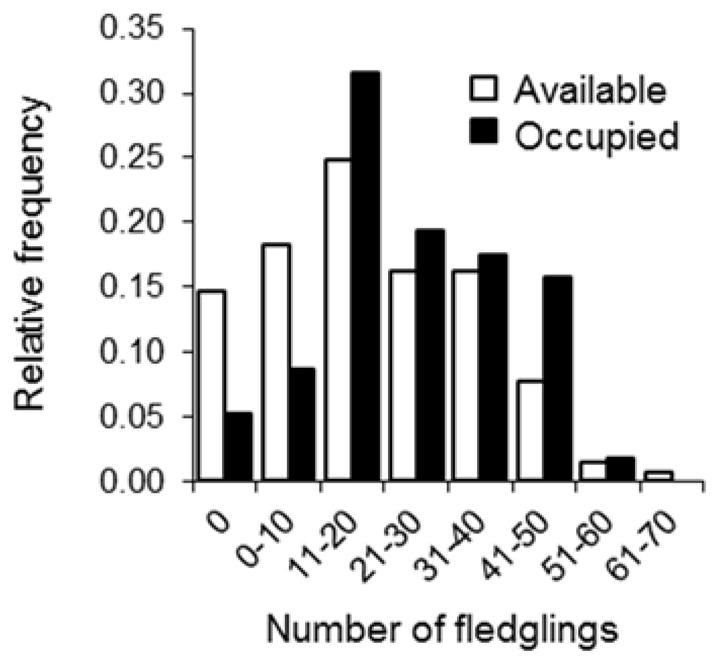
Variation in territory quality, quantified as the total number of fledglings produced at a given nesting site over the ten years prior to this study (open bars). Open bars depict variation in the relative success of birds breeding at available territories (N = 258 nesting sites) in previous years, and filled bars represent the frequency with which these nesting sites were occupied by birds in the present study (N = 57). The average number of total fledglings produced by a territory over the ten years prior to this study was 19.2 ± 0.9 (mean ± SE); thus, low-quality territories were under-occupied and high-quality territories were over-occupied ( , P = 0.015)
Data and Analyses
We used SAS (version 9.3) for all analyses, all tests are two-tailed, and we converted data to z-scores prior to analysis to obtain standardized parameter estimates (Schielzeth 2010). We also used Satterthwaite’s degrees-of-freedom approximation, which can result in non-integer denominator degrees of freedom. We accounted statistically for the non-independence of multiple broods produced by individual females by including female identity and year as random effects in analyses of offspring body condition, sex ratios, and maternal return rates (see below).
We first tested for effects on the body condition (i.e., size-adjusted body mass) of offspring using a linear mixed model that included clutch (i.e., a female’s first or second clutch of the season) as a within-female effect. Analyzing the condition of individual nestlings, with nest as a random effect, resulted in some independent variables (offspring tarsus length and brood size) having denominator degrees of freedom that were greater than the number of broods from which we collected data. Thus, we analyzed nestling condition as brood means, although results are qualitatively similar if the condition of individual nestlings is analyzed with nest as a random effect (data not shown). We included main effects of a female’s natal brood size, her pre-fledging body mass, and the day of the year on which hatching began (hatching date) in her natal nest; we also included a female’s current age, body mass, brood size, and territory quality, and we included nestling tarsus length as a covariate to obtain results for body mass that control for skeletal size. Analyzing nestling body condition using the scaled mass index (Peig and Green 2009) or using residuals from a log(mass) × log(tarsus) linear regression yields similar results (data not shown). For some of the focal females in this study (15), we lacked data on their tarsus length as nestlings prior to fledging; for this reason, we used raw values of body mass for maternal females when they were nestlings. However, we were not missing any data on tarsus length for the nestlings produced by the focal females. There was a correlation between a female’s natal brood size and hatching date in her natal nest (r35 = −0.39, P = 0.016), which is a common pattern, but a correlation of this magnitude should not result in variance inflation attributable to collinearity among independent variables. There were no other correlations among independent variables (all P > 0.05). Following this, we analyzed variation in brood sex ratios using the number of male offspring in a nest as the dependent variable and the number of sexed offspring as the binomial denominator using a generalized linear mixed model with a binomial distribution (PROC GLIMMIX in SAS). We included the same main effects as for the analysis of nestling condition, with the exception of tarsus length. Finally, we used a GLMM with a binary response to test whether the proportion of males within broods predicted a female’s probability of returning to the population to breed in subsequent years while controlling for variation in brood size, maternal body condition and age, and territory quality.
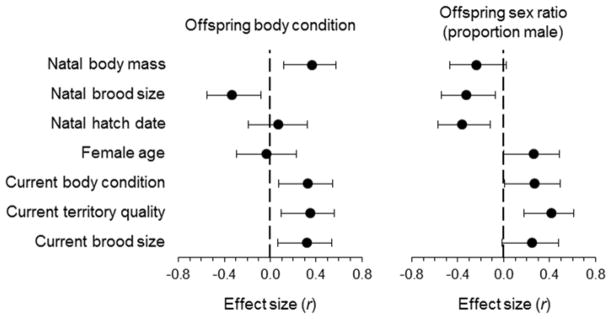
We calculated effect sizes ± 95 % confidence intervals for the independent variables in our analyses (Nakagawa and Cuthill 2007). To do this, we used F-statistics from the analyses above to calculate correlation coefficients as , where DDF represents the error degrees of freedom from an F-test (Rosenthal 1994), and we calculated confidence limits using z-transformation (Sokal and Rohlf 1995). Typically, values of 0.1, 0.3, and 0.5 are considered to represent small, medium, and large effects, respectively.
Results
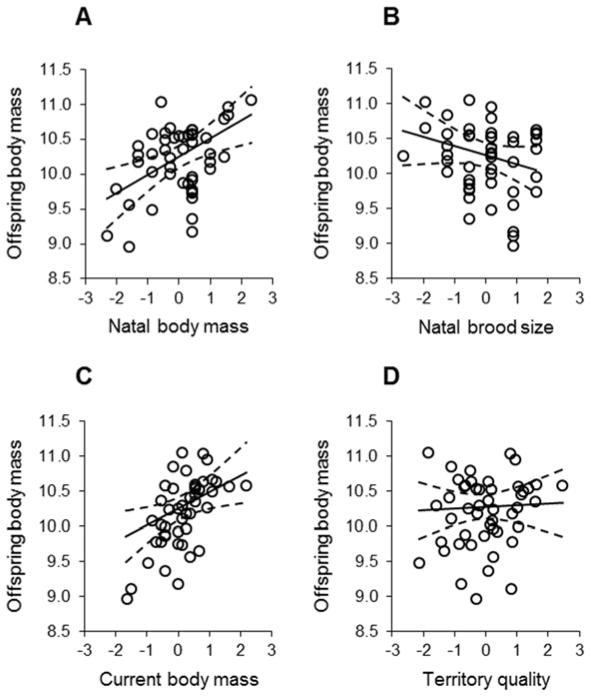
While controlling for variation in territory quality and seasonal variation (day of the year), there was a significant correlation between female body mass as a nestling and her condition as an adult (estimate ± SE = 0.260 ± 0.118, F1,32 = 4.84, P = 0.035; effect of territory quality: estimate ± SE = 0.164 ± 0.104, F1,39.6 = 2.50, P = 0.122; day of the year: estimate ± SE = −0.231 ± 0.089, F1,33.6 = 6.71, P = 0.014). The natal conditions that a female experienced were associated with variation in the body condition of offspring she later produced as an adult (Table 1A; Fig 2). The condition of offspring that a female produced was positively correlated with her body mass as a nestling, and negatively correlated with the size of the brood in which she was reared (Table 1A; Fig 3A,B). The body mass of offspring that a female produced as an adult was also positively associated with her current, adult body mass (Fig 3C) and with the quality of her current breeding territory (Table 1A, Fig 3D).
Table 1.
Effects on offspring condition and sex ratio. Significant effects in bold type
| Estimate ± SE | F | d.f. | P | |
|---|---|---|---|---|
|
|
||||
| (a) Effects of maternal experiences on offspring body mass | ||||
| Natal body massa | 0.327 ± 0.140 | 5.48 | 1, 35.6 | 0.025 |
| Natal brood sizea | −0.321 ± 0.151 | 4.52 | 1, 35.4 | 0.041 |
| Natal hatch datea | 0.063 ± 0.145 | 0.19 | 1, 36.6 | 0.665 |
| Female age | −0.020 ± 0.098 | 0.04 | 1, 36.1 | 0.840 |
| Current body mass | 0.363 ± 0.171 | 4.49 | 1, 36.7 | 0.041 |
| Current tarsus length | 0.079 ± 0.136 | 0.34 | 1, 32.7 | 0.563 |
| Current territory qualityb | 0.246 ± 0.114 | 4.66 | 1, 34.1 | 0.038 |
| Current brood size | 0.272 ± 0.133 | 4.18 | 1, 36.2 | 0.048 |
| Nestling tarsus length | 0.426 ± 0.137 | 9.71 | 1, 37.0 | 0.004 |
| Intercept | 0.058 ± 0.156 | |||
| (b) Effects of maternal experiences on the brood sex ratio (proportion of offspring that were male) | ||||
| Natal body massa | −0.243 ± 0.166 | 2.15 | 1, 35.8 | 0.152 |
| Natal brood sizea | −0.431 ± 0.203 | 4.49 | 1, 36.8 | 0.041 |
| Natal hatch datea | −0.487 ± 0.202 | 5.81 | 1, 38.0 | 0.021 |
| Female age | 0.190 ± 0.117 | 2.65 | 1, 36.9 | 0.112 |
| Current body mass | 0.311 ± 0.189 | 2.72 | 1, 34.7 | 0.108 |
| Current tarsus length | −0.091 ± 0.185 | 0.24 | 1, 37.5 | 0.627 |
| Current territory qualityb | 0.365 ± 0.136 | 7.16 | 1, 34.0 | 0.011 |
| Current brood size | 0.284 ± 0.182 | 2.44 | 1, 37.5 | 0.126 |
| Intercept | −0.155 ± 0.269 | |||
| (c) Effects on maternal probability of returning in subsequent years | ||||
| Brood sex ratio (prop. male) | −1.472 ± 0.674 | 4.76 | 1, 39.6 | 0.035 |
| Current brood size | 1.461 ± 0.585 | 6.23 | 1, 40.0 | 0.017 |
| Current body mass | 0.889 ± 0.712 | 1.56 | 1, 40.0 | 0.219 |
| Current tarsus length | 0.332 ± 0.547 | 0.37 | 1, 9.25 | 0.558 |
| Female age | −0.126 ± 0.349 | 0.13 | 1, 15.5 | 0.723 |
| Current territory qualityb | 0.323 ± 0.458 | 0.50 | 1, 34.3 | 0.485 |
| Intercept | −1.207 ± 0.559 | |||
prior to fledgling from a female’s natal nest;
the number of fledglings produced at a given nest site during the ten years leading up to this study
Fig 2.
Effect sizes (correlation coefficients, r) ± 95 % confidence limits for independent variables in our analyses of nestling body condition and sex ratio. Effects are plotted to depict the relationship between the independent and dependent variables (e.g., an increase in current territory quality is associated with an increase in the condition of offspring and the proportion of offspring that are male, whereas an increase in natal brood size is associated with a reduction in the condition of offspring and proportion of males produced in the future). The effect of a female’s current body condition represents an effect of her current body mass as an adult with her tarsus length as a covariate
Fig 3.
Variation in nestling body mass (brood means) in relation to female (A) body mass and (B) brood size as nestlings, and in relation to females’ current (C) body mass and (D) territory quality as breeding adults. In (C), female body mass is depicted for graphing purposes only; female body condition as an adult was included as the independent variable in this analysis, but her body mass as a nestling (B) was used in analyses as our morphological data for females at that age were incomplete. Values on x-axes are z-scores
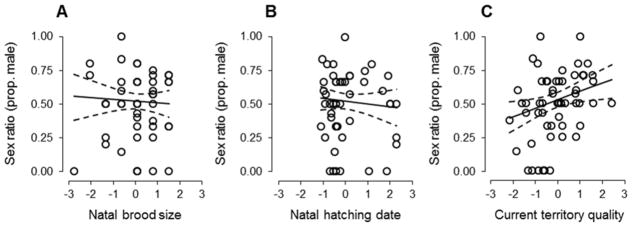
Consistent with the effects on offspring body mass, we also detected a correlation between the natal conditions in which females were reared and the sex ratios that they produced as adults (Table 1B; Fig 2). Females that were reared in larger-than-average broods and produced later within breeding seasons overproduced daughters relative to those that were reared in smaller broods and produced earlier within seasons (Fig 4A,B). The proportion of offspring that were male also increased with the quality of a female’s current breeding territory (Table 1B; Figs 2, 4C). While controlling for variation in brood size, maternal body condition and age, and territory quality, increases in the proportion of males within broods were negatively associated with a female’s probability of returning to breed in the study population the following year (Table 1C; Fig. 5).
Fig 4.
Variation in offspring sex ratio (proportion male) in relation to (A) brood size and (B) hatching date as nestlings, and (C) the quality of a female’s breeding territory. Values on x-axes are z-scores
Fig 5.
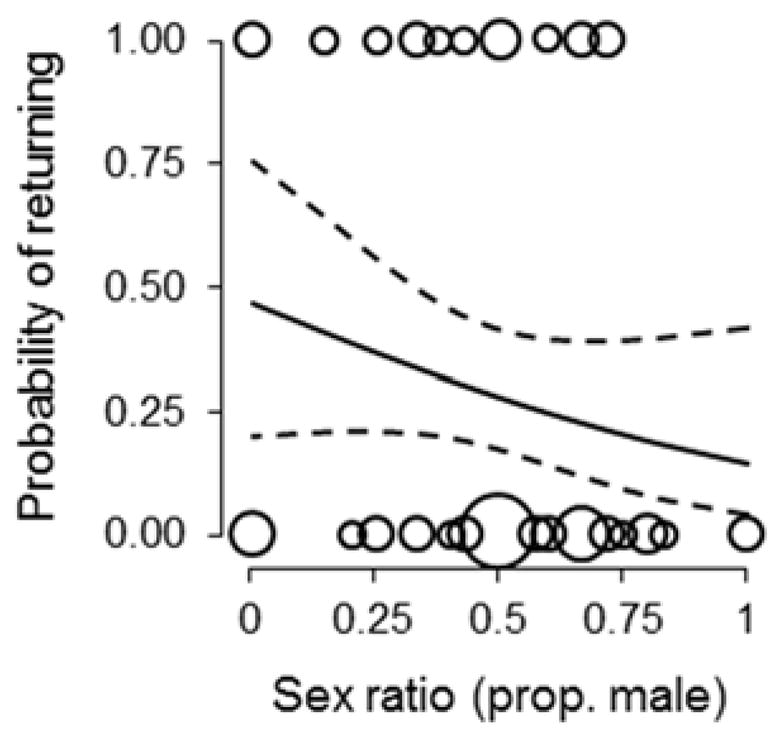
The probability that a given female would return to breed in the local population in subsequent years in relation to the brood sex ratio. Curves represent predictions from a generalized linear mixed model ± 95 % confidence limits. Points represent individual females (point size is proportional to the number of overlapping values)
Discussion
Our results suggest persistent effects of natal conditions on important components of a female’s life history, including the body condition and sex ratio of offspring produced as an adult. The condition and sex ratio of offspring were also associated with the quality of a female’s current breeding territory. Territory quality and resource abundance during development can affect life-history trajectories in a variety of taxa (Haywood and Perrins 1992; Lindström 1999; Millon et al. 2011; Douhard et al. 2013; Zedrosser et al. 2013; but see also Drummond and Ancona 2015). For example, in great tits (Parus major), the long-term survival and reproductive success of neonates are negatively correlated with the altitude and positively correlated with the size of the territories on which they are reared, and to a greater extent for male than for female offspring (Wilkin and Sheldon 2009). Consistent with these sex-biased patterns of selection, offspring sex ratios often vary with local conditions in association with the fitness returns these offspring are expected to provide (Dijkstra et al. 1990; Pen et al. 1999; Cordero et al. 2001; Romano et al. 2012; Bowers et al. 2013b, 2015; Gaukler et al. 2016). We recently found that the quality of the rearing environment and the body mass of offspring prior to independence has sex-specific effects on their reproductive success as adults: large sons produced more fledglings as adults than smaller sons, and the reproductive success of daughters was not affected by their body mass prior to independence (Bowers et al. 2015). We also found that females investing heavily into reproduction overproduce sons relative to those investing less, as measured by the number of broods they produce within seasons (Bowers et al. 2015). Thus, the effect of current territory quality on offspring sex ratios detected in the current study is consistent with the prediction that mothers with a reduced ability to invest in offspring should overproduce daughters, the sex that is more strongly limiting to population growth, and those able to invest highly in offspring, or those breeding on high-quality territories, should overproduce sons.
We also found increasing brood sizes to be associated with an increased probability that a female would return to breed in the study population in the future, an apparently paradoxical result if increased reproductive effort imposes costs to a female’s long-term survival and future reproduction (Williams 1966; Gustafsson and Sutherland 1988; Hodges et al. 2015). However, a number of hypotheses may explain this result, even if reproduction is generally costly. For example, interannual return rates to local populations in wild birds are a function of both survival and site fidelity, and in a number of species are positively affected by breeding success the previous year (e.g., Drilling and Thompson 1988; Pärt and Gustafsson 1989; Bensch and Hasselquist 1991; Hoover 2003). Alternatively, females producing a greater-than-average number of young may be breeding on high-quality territories that contain abundant resources for reproduction, or these females may simply be of greater individual quality (sensu Wilson and Nussey 2010) than those producing fewer young, thereby reducing the extent to which these females incur reproductive costs associated with rearing large broods. Each of these patterns (increased site fidelity or resource availability mitigating the cost of reproduction) could generate a positive relationship between reproductive success within a season and a female’s probability of returning the following year, but it important to note that neither of them precludes the existence of a survival cost of reproduction that accrues over an individual’s life.
It must be acknowledged that our sample size is small and that the results here are correlative, making the patterns observed subject to potential confounds. For example, in our study population, there is statistically significant, heritable genetic variation contributing to nestling body condition (h2 = 0.135; Sakaluk et al. 2014), which may partially explain the correlations between a female’s mass and that of her offspring; however, the low narrow-sense heritability suggests that additive genetic variation likely does not account for a large component of the effects on offspring condition that we have detected here, particularly considering the magnitude of the effects we detected (Fig 2).
Although we have found offspring sex ratios in our study population to vary according to predictions of the Trivers-Willard Model, this does not preclude other selective forces from acting on maternal sex-allocation strategies. For example, increases in the proportion of sons within broods in the current study was associated with a reduced probability that a female would return to breed in the population in subsequent years, suggesting a cost associated with overproducing sons for females of any given age and number of offspring produced. Particularly if sons require a greater share of parental resources than their sisters to become successful breeders, then mothers may adjust offspring sex ratios to ameliorate the cost of reproduction for themselves or simply to enhance the total number of surviving offspring regardless of their sex. Known as the cost-of-reproduction hypothesis (Cockburn et al. 2002; see also Myers 1978; Merkling et al. 2015), this idea could be tested by cross-fostering offspring among nests, thereby forcing females to rear sex ratios other than those they produced. Additionally, female house wrens are known to choose their mates based, in part, on the quality of the breeding territory, particularly the number of nest cavities that males are able to secure and defend from rivals (Johnson and Searcy 1993; Eckerle and Thompson 2006). Thus, the increased body mass and excess production of sons on high-quality territories may be a product of (i) the current breeding territory having a direct effect on food availability for offspring, or (ii) differential allocation by females in relation to male attractiveness. We suspect that the latter scenario is unlikely, as recent experiments in our study population that have manipulated male attractiveness have found no effect on pre- and post-natal allocation by females (DeMory et al. 2010; Grana et al. 2012), suggesting that females do not differentially allocate offspring on the basis of male attractiveness. Nonetheless, separating territory quality from male attractiveness and intrasexual competitive ability experimentally could shed light on the factors contributing to the effect of territory quality on offspring condition and sex ratios.
The effects of natal conditions on progeny sex ratios that we observed in the current study, in addition to the reduced interannual return rate of mothers producing male-biased broods, may help explain, in part, why sex ratios in birds and mammals often do not conform to classic expectations. Although females in a variety of taxa have been shown to adjust offspring sex ratios according to various environmental conditions (Cockburn et al. 2002; West and Sheldon 2002; West 2009), effect sizes in sex-ratio analyses and sex-ratio variation among nests are often smaller than expected if females have facultative control over sex determination (Williams 1979; Harmsen and Cooke 1983; Postma et al. 2011). Thus, uncertainty and disagreement has arisen regarding whether mothers can possibly exert any kind of influence on sex-ratio variation. For example, Williams (1979) concluded that “sex seems to be just another Mendelian unit character” based solely on a lack of pronounced sex-ratio variation (see also Harmsen and Cooke 1983); however, such a result does not demonstrate that females are unable to adjust offspring sex ratios either within or among broods. Mendelian sex determination is not thought to impose a major constraint on avian sex allocation (West and Sheldon 2002), but other forms of constraint may reduce the extent of sex-ratio variation even under conditions of non-random sex-chromosome segregation (Leimar 1996; Uller 2006; Bowers et al. 2015, 2016). Here, we found that increases in the proportion of sons within broods is associated with a reduced probability that a female will return to breed in the study population in subsequent years. Thus, although selection may favor an increased production of sons on high-quality territories (sensu Trivers and Willard 1973), if these sons are more sensitive than daughters and require an increased share of parental resources to become successful breeders (e.g., Bowers et al. 2015), then producing sons may impose greater costs to a female’s future reproduction than producing daughters (see also Myers 1978; Cockburn et al. 2002; Merkling et al. 2015), ultimately reducing the extent to which sons are produced within broods. A question that arises, then, is under what conditions should sons be over-produced, despite the apparent costs associated with overproducing sons? Although empirical work to date has provided insights into the evolution of sex-allocation strategies, the complex life histories of many organisms, including iteroparous species in which individuals produce different numbers of offspring at any given time and for which multiple processes shape between-individual differences in return rates, means that multiple selective forces are likely to interact in shaping an individual’s optimal offspring sex ratio at any point in time and space (Cockburn et al. 2002). Thus, greater integration of classical ideas (e.g., the Trivers-Willard Model) with the complex life-cycles of our study organisms (e.g., Wild and West 2007), may shed light on the selective forces shaping sex-ratio variation in the wild.
Acknowledgments
We thank the 1999–2013 Wren Crews for field assistance and the ParkLands Foundation (Merwin Preserve) and the Sears and Butler families for the use of their properties for this work. Financial support was provided by the School of Biological Sciences, Illinois State University; National Science Foundation grants IBN-0316580, IOS-0718140 and IOS-1118160; National Institutes of Health grant R15HD076308-01; and student research grants from the Sigma Xi Society, the Animal Behavior Society, the American Ornithologists’ Union, the American Museum of Natural History’s Frank M. Chapman Memorial Fund, the Champaign County Audubon Society, and the Beta Lambda Chapter of the Phi Sigma Biological Sciences Honor Society.
Footnotes
Ethical Standards
All research activities complied with current laws of the United States of America, and were performed in accordance with Illinois State University Institutional Animal Care and Use Committee permits 10-2009, 05-2010, 04-2013; U. S. Geological Survey banding permit 09211; and U. S. Fish and Wildlife Service collecting permit MB692148-0.
References
- Auer SK, Arendt JD, Chandramouli R, Reznick DN. Juvenile compensatory growth has negative consequences for reproduction in Trinidadian guppies (Poecilia reticulata) Ecology Letters. 2010;13:998–1007. doi: 10.1111/j.1461-0248.2010.01491.x. [DOI] [PubMed] [Google Scholar]
- Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002;295:316–318. doi: 10.1126/science.1066651. [DOI] [PubMed] [Google Scholar]
- Badyaev AV, Hamstra TL, Oh KP, Acevedo Seaman DA. Sex-biased maternal effects reduce ectoparasite-induced mortality in a passerine bird. Proceedings of the National Academy of Sciences of the USA. 2006;103:14406–14411. doi: 10.1073/pnas.0602452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belles-Isles JC, Picman J. Suspected adult intraspecific killing by house wrens. Wilson Bulletin. 1987;99:497–498. [Google Scholar]
- Bensch S, Hasselquist D. Territory infidelity in the polygynous great reed warbler Acrocephalus arundinaceus: the effect of variation in territory attractiveness. J Anim Ecol. 1991;60:857–871. [Google Scholar]
- Bouwhuis S, Charmantier A, Verhulst S, Sheldon BC. Individual variation in rates of senescence: natal origin effects and disposable soma in a wild bird population. Journal of Animal Ecology. 2010;79:1251–1261. doi: 10.1111/j.1365-2656.2010.01730.x. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Adaptive sex allocation in relation to hatching synchrony and offspring quality in house wrens. American Naturalist. 2011;177:617–629. doi: 10.1086/659630. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Sibling cooperation influences the age of nest-leaving in an altricial bird. American Naturalist. 2013a;181:775–786. doi: 10.1086/670244. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Munclinger P, Bureš S, Nádvorník P, Uvírová L, Krist M. Cross-fostering eggs reveals that female collared flycatchers adjust clutch sex ratios according to parental ability to invest in offspring. Molecular Ecology. 2013b;22:215–228. doi: 10.1111/mec.12106. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Offspring sex ratio varies with clutch size for female house wrens induced to lay supernumerary eggs. Behavioral Ecology. 2014a;25:165–171. [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, Sakaluk SK. Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behavioral Ecology. 2014b;25:1485–1493. [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014c;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. Journal of Animal Ecology. 2015;84:473–486. doi: 10.1111/1365-2656.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Within-female plasticity in sex allocation in associated with a behavioural polyphenism in house wrens. Journal of Evolutionary Biology. 2016 doi: 10.1111/jeb.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Albon SD, Guinness FE. Maternal dominance, breeding success and birth sex ratios in red deer. Nature. 1984;308:358–360. [Google Scholar]
- Cockburn A, Legge S, Double MC. Sex-ratios in birds and mammals: can the hypotheses be disentangled? In: Hardy ICW, editor. Sex-ratios: concepts and research methods. Cambridge: Cambridge University Press; 2002. pp. 266–286. [Google Scholar]
- Cordero PJ, Viñuela J, Aparicio JM, Veiga JP. Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. Journal of Evolutionary Biology. 2001;14:829–834. [Google Scholar]
- DeMory ML, Thompson CF, Sakaluk SK. Male quality influences male provisioning in house wrens independent of attractiveness. Behavioral Ecology. 2010;21:1156–1164. [Google Scholar]
- Dijkstra C, Daan S, Buker JB. Adaptive seasonal variation in the sex ratio of kestrel broods. Functional Ecology. 1990;4:143–147. [Google Scholar]
- Dobbs RC, Styrsky JD, Thompson CF. Clutch size and the costs of incubation in the house wren. Behavioral Ecology. 2006;17:849–856. [Google Scholar]
- Douhard M, Gaillard JM, Delorme D, Capron G, Duncan P, Klein F, Bonenfant C. Variation in adult body mass of roe deer: early environmental conditions influence early and late body growth of females. Ecology. 2013;94:1805–1814. doi: 10.1890/13-0034.1. [DOI] [PubMed] [Google Scholar]
- Drilling NA, Thompson CF. Natal and breeding dispersal in house wrens (Troglodytes aedon) Auk. 1988;105:480–491. [Google Scholar]
- Drummond H, Ancona S. Observational field studies reveal wild birds responding to early-life stresses with resilience, plasticity, and intergenerational effects. Auk. 2015;132:563–576. [Google Scholar]
- Eckerle KP, Thompson CF. Mate choice in house wrens: nest cavities trump male characteristics. Behaviour. 2006;143:253–271. [Google Scholar]
- Finke MA, Milinkovich DJ, Thompson CF. Evolution of clutch size: an experimental test in the house wren (Troglodytes aedon) Journal of Animal Ecology. 1987;56:99–114. [Google Scholar]
- Fisher RA. The genetical theory of natural selection. Oxford: Clarendon Press; 1930. [Google Scholar]
- Gaukler SM, Ruff JS, Potts WK. Paroxetine exposure skews litter sex ratios in mice suggesting a Trivers-Willard process. Behavioral Ecology. 2016 doi: 10.1093/beheco/arw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana SC, Sakaluk SK, Bowden RM, Doellman MA, Vogel LA, Thompson CF. Reproductive allocation in female house wrens is not influenced by experimentally altered male attractiveness. Behavioral Ecology and Sociobiology. 2012;66:1247–1258. [Google Scholar]
- Gustafsson L, Sutherland WJ. The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature. 1988;355:813–815. [Google Scholar]
- Harmsen R, Cooke F. Binomial sex-ratio distribution in the lesser snow goose: a theoretical enigma. American Naturalist. 1983;121:1–8. [Google Scholar]
- Harrison XA, Blount JD, Inger R, Norris RD, Bearhop S. Carry-over effects as drivers of fitness differences in animals. Journal of Animal Ecology. 2011;80:4–18. doi: 10.1111/j.1365-2656.2010.01740.x. [DOI] [PubMed] [Google Scholar]
- Haywood S, Perrins CM. Is clutch size in birds affected by environmental conditions during growth? Proceedings of the Royal Society of London B. 1992;249:195–197. doi: 10.1098/rspb.1992.0103. [DOI] [PubMed] [Google Scholar]
- Helle H, Koskela E, Mappes T. Life in varying environments: experimental evidence for delayed effects of juvenile environment on adult life history. Journal of Animal Ecology. 2012;81:573–582. doi: 10.1111/j.1365-2656.2011.01937.x. [DOI] [PubMed] [Google Scholar]
- Hewison AJM, Gaillard JM. Successful sons or advantaged daughters? The Trivers-Willard model and sex-biased maternal investment in ungulates. Trends in Ecology & Evolution. 1999;14:229–234. doi: 10.1016/s0169-5347(99)01592-x. [DOI] [PubMed] [Google Scholar]
- Hodges CJ, Bowers EK, Thompson CF, Sakaluk SK. Cascading costs of reproduction in female house wrens induced to lay larger clutches. Journal of Evolutionary Biology. 2015;28:1383–1393. doi: 10.1111/jeb.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JP. Decision rules for site fidelity in a migratory bird, the prothonotary warbler. Ecology. 2003;84:416–430. [Google Scholar]
- Janiszewski T, Minias P, Wojciechowski Z. Occupancy reliably reflects territory quality in a long-lived migratory bird, the white stork. Journal of Zoology. 2013;291:178–184. [Google Scholar]
- Johnson LS. House wren (Troglodytes aedon) In: Poole A, editor. The birds of North America online. 2. Ithaca, NY: Cornell Lab of Ornithology and American Ornithologists’ Union; 2014. [DOI] [Google Scholar]
- Johnson LS, Kermott LH. Possible causes of territory takeovers in a north-temperate population of house wrens. Auk. 1990;107:781–784. [Google Scholar]
- Johnson LS, Searcy WA. Nest site quality, female mate choice, and polygyny in the house wren. Ethology. 1993;95:265–277. [Google Scholar]
- Kendeigh SC. Territorial and mating behavior of the house wren. Illinois Biological Monographs. 1941;18:1–120. [Google Scholar]
- Komdeur J. Sex allocation. In: Royle NJ, Smiseth PR, Kölliker M, editors. The evolution of parental care. Oxford: Oxford University Press; 2012. pp. 171–188. [Google Scholar]
- Krist M. Should mothers in poor condition invest more in daughter than in son? Ethology Ecology & Evolution. 2006;18:241–246. [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, Barba E, Bouvier J-C, Camprodon J, Cooper CB, Dawson RD, Eens M, Eeva T, Faivre B, Garamszegi LZ, Goodenough AE, Gosler AG, Grégoire A, Griffith SC, Gustafsson L, Johnson LS, Kania W, Keišs O, Llambías PE, Mainwaring MC, Mänd R, Massa B, Mazgajski TD, Møller AP, Moreno J, et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithologica. 2010;45:1–26. [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends in Ecology & Evolution. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Leimar O. Life-history analysis of the Trivers and Willard sex-ratio problem. Behavioral Ecology. 1996;7:316–325. [Google Scholar]
- Merkling T, Welcker J, Hewison AJM, Hatch SA, Kitaysky AS, Speakman JR, Danchin E, Blanchard P. Identifying the selective pressures underlying offspring sex-ratio adjustments: a case study in a wild seabird. Behavioral Ecology. 2015;26:916–925. [Google Scholar]
- Millon A, Petty SJ, Little B, Lambin X. Natal conditions alter age-specific reproduction but not survival or senescence in a long-lived bird of prey. Journal of Animal Ecology. 2011;80:968–975. doi: 10.1111/j.1365-2656.2011.01842.x. [DOI] [PubMed] [Google Scholar]
- Myers JH. Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? American Naturalist. 1978;112:381–388. [Google Scholar]
- Naguib M, Gil D. Transgenerational effects on body size caused by early developmental stress in zebra finches. Biology Letters. 2005;1:95–97. doi: 10.1098/rsbl.2004.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib M, Nemitz A, Gil D. Maternal developmental stress reduces reproductive success of female offspring in zebra finches. Proceedings of the Royal Society B. 2006;273:1901–1905. doi: 10.1098/rspb.2006.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Pärt T, Gustafsson L. Breeding dispersal in the collared flycatcher (Ficedula albicollis): possible causes and reproductive consequences. Journal of Animal Ecology. 1989;58:305–320. [Google Scholar]
- Peig J, Green AJ. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. 2009;118:1883–1891. [Google Scholar]
- Pen I, Weissing FJ, Daan S. Seasonal sex ratio trend in the European kestrel: an evolutionarily stable strategy analysis. American Naturalist. 1999;153:384–397. doi: 10.1086/303183. [DOI] [PubMed] [Google Scholar]
- Poirier NE, Whittingham LA, Dunn PO. Males achieve greater reproductive success through multiple broods than through extrapair mating in house wrens. Animal Behaviour. 2004;67:1109–1116. [Google Scholar]
- Postma E, Heinrich F, Koller U, Sardell RJ, Reid JM, Arcese P, Keller LF. Disentangling the effect of genes, the environment and chance on sex ratio variation in a wild bird population. Proceedings of the Royal Society B. 2011;278:2996–3002. doi: 10.1098/rspb.2010.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A, Ambrosini R, Caprioli M, Bonisoli-Alquati A, Saino N. Secondary sex ratio covaries with demographic trends and ecological conditions in the barn swallow. Evolutionary Ecology. 2012;26:1041–1053. [Google Scholar]
- Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. pp. 231–244. [Google Scholar]
- Rutkowska J, Koskela E, Mappes T, Speakman JR. A trade-off between current and future sex allocation revealed by maternal energy budget in a small mammal. Proceedings of the Royal Society B. 2011;278:2962–2969. doi: 10.1098/rspb.2010.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaluk SK, Wilson AJ, Bowers EK, Johnson LS, Masters BS, Johnson BGP, Vogel LA, Forsman AM, Thompson CF. Genetic and environmental variation in condition, cutaneous immunity, and haematocrit in house wrens. BMC Evolutionary Biology. 2014;14:242. doi: 10.1186/s12862-014-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution. 2010;1:103–113. [Google Scholar]
- Schwanz LE, Robert KA. Proximate and ultimate explanations of mammalian sex allocation in a marsupial model. Behavioral Ecology and Sociobiology. 2014;68:1085–1096. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3. New York: W. H. Freeman and Company; 1995. [Google Scholar]
- Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Uller T. Sex-specific sibling interactions and offspring fitness in vertebrates: patterns and implications for maternal sex ratios. Biological Reviews. 2006;81:207–217. doi: 10.1017/S1464793105006962. [DOI] [PubMed] [Google Scholar]
- Uller T, Olsson M. Offspring size and timing of hatching determine survival and reproductive output in a lizard. Oecologia. 2010;162:663–671. doi: 10.1007/s00442-009-1503-x. [DOI] [PubMed] [Google Scholar]
- Warner DA, Uller T, Shine R. Transgenerational sex determination: the embryonic environment experienced by a male affects offspring sex ratio. Scientific Reports. 2013;3:2709. doi: 10.1038/srep02709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SA. Sex allocation. Princeton: Princeton University Press; 2009. [Google Scholar]
- West SA, Sheldon BC. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- Whittingham LA, Valkenaar SM, Poirier NE, Dunn PO. Maternal condition and nestling sex ratio in house wrens. Auk. 2002;119:125–131. [Google Scholar]
- Wild G, West SA. A sex allocation theory for vertebrates: combining local resource competition and condition-dependent allocation. American Naturalist. 2007;170:E112–E128. doi: 10.1086/522057. [DOI] [PubMed] [Google Scholar]
- Wilkin TA, Sheldon BC. Sex differences in the persistence of natal environmental effects on life histories. Current Biology. 2009;19:1998–2002. doi: 10.1016/j.cub.2009.09.065. [DOI] [PubMed] [Google Scholar]
- Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. American Naturalist. 1966;100:687–690. [Google Scholar]
- Williams GC. The question of adaptive sex ratio in outcrossed vertebrates. Proceedings of the Royal Society of London B. 1979;205:567–580. doi: 10.1098/rspb.1979.0085. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Nussey DH. What is individual quality? An evolutionary perspective. Trends in Ecology and Evolution. 2010;25:207–214. doi: 10.1016/j.tree.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Wong JWY, Kölliker M. Effects of food restriction across stages of juvenile and early adult development on body weight, survival and adult life history. Journal of Evolutionary Biology. 2014;27:2420–2430. doi: 10.1111/jeb.12484. [DOI] [PubMed] [Google Scholar]
- Zedrosser A, Pelletier R, Bischof R, Festa-Bianchet M, Swenson JE. Determinants of lifetime reproduction in female brown bears: early body mass, longevity, and hunting regulations. Ecology. 2013;94:231–240. doi: 10.1890/12-0229.1. [DOI] [PubMed] [Google Scholar]