Abstract
Background
Erectile dysfunction (ED) is common in older men with chronic kidney disease. Magnesium is essential for metabolism of nitric oxide which helps in penile erection. There is little information available about the influence of serum magnesium on ED. The aim of the study was to assess the influence of hypomagnesemia on ED in elderly chronic kidney disease patients.
Subjects and methods
A total of 372 patients aged 65–85 years, with an estimated glomerular filtration rate of 60–15 mL/min/1.73 m2, were divided into two groups according to serum magnesium levels: hypomagnesemia, n=180; and normomagnesemia, n=192. ED was assessed through the International Index of Erectile Function-5. Hypomagnesemia is defined as serum magnesium <1.8 mg/dL.
Results
The prevalence of ED was higher among hypomagnesemic subjects compared to that among normomagnesemics (93.3% vs 70.8%, P<0.001). Severe ED (62.8% vs 43.8%, P=0.037), mild-to-moderate ED (12.2% vs 5.2%, P=0.016), abdominal obesity (37.2% vs 22.9%, P=0.003), metabolic syndrome (38.4% vs 19.2%, P=0.026), proteinuria (0.83±0.68 vs 0.69±0.48 mg/dL, P=0.023), and C-reactive protein (6.1±4.9 vs 4.1±3.6 mg/L, P<0.001) were high; high-density lipoprotein cholesterol (48.8±14.0 vs 52.6±13.5 mg/dL, P=0.009), and albumin (4.02±0.53 vs 4.18±0.38 g/dL, P=0.001) were low in the hypomagnesemia group. Serum magnesium ≤1.85 mg/dL was the best cutoff point for prediction of ED. Hypomagnesemia (relative risk [RR] 2.27), age ≥70 (RR 1.74), proteinuria (RR 1.80), smoking (RR 21.12), C-reactive protein (RR 1.34), abdominal obesity (RR 3.92), and hypertension (RR 2.14) were predictors of ED.
Conclusion
Our data support that ED is related to hypomagnesemia in elderly patients with moderately to severely reduced kidney function.
Keywords: erectile dysfunction, elderly, chronic kidney disease, magnesium
Video abstract
Introduction
Longer lifespans create new health-related problems. Epidemiologic studies clearly show an increasing age-related prevalence and severity of erectile dysfunction (ED) among elderly men.1–4 ED is a condition in which a man is persistently unable to attain and/or maintain penile erection sufficient for sexual intercourse and is the most common sexual dysfunction among men worldwide.2,5 Increasing comorbidities and pathological changes in the erectile tissue and the supplying vessels result in a high prevalence of ED in geriatric population.2,3 ED affects only 4% of men in their 50s but almost 50% over the age of 75, and up to 90% of elderly men with chronic kidney disease (CKD) have ED.2,6–8
ED may result from psychologic, neurologic, hormonal, arterial, or cavernosal impairment or from a combination of these factors.1,9 Sexuality is a significant quality-of-life consideration for all individuals, including older adults.10 The identification of novel risk factors for ED may improve our understanding of the pathogenesis of ED and allow the development of new prevention strategies for ED. There is a close relationship between ED, endothelial dysfunction, and decreased production of nitric oxide (NO).11,12 Hypomagnesemia inhibits NO release from endothelium and therefore associated with endothelial dysfunction.13,14 Hypomagnesemia, endothelial dysfunction, and lower serum levels of NO are common in patients with CKD, especially among elderly.14–17 Therefore, hypomagnesemia may be linked to ED in these subjects. We hypothesize that hypomagnesemia would increase the ED rates. In the literature, there have been few attempts to assess the possible relationship between serum magnesium level and ED. The present study was the first to determine the prevalence and severity of ED in elderly hypomagnesemic patients with moderately to severely reduced kidney function.
Subjects and methods
Study population
A single-center, prospective cross-sectional study was performed in non-diabetic male patients with stage 3 and 4 CKD, with age 65–85 years between November 2014 and August 2015 in Department of Medicine, Division of Nephrology, Balikesir University School of Medicine. All included patients in the study had a regular partner. A total of 372 patients met the inclusion criteria. They were divided, according to the serum magnesium levels, into two groups: hypomagnesemia (n=180) and normomagnesemia (n=192). ED was assessed in the study patients. The study complied with the Declaration of Helsinki and was approved by the Ethical Committee of Balikesir University School of Medicine. All patients gave written informed consent.
Assays
Blood samples were collected from an antecubital vein of patients by venipuncture into vacutainer tubes after an 8 h overnight fasting, and the samples were centrifuged and stored at −80°C until analysis. Serum magnesium levels were analyzed by the colorimetric method, with a clinical chemistry autoanalyzer. Calcium, urea, and urinary protein concentrations were also measured by the colorimetric method. Serum creatinine and urinary creatinine concentrations were determined enzymatically on an autoanalyzer using the Jaffé method. Uric acid levels were determined by uricase–peroxidase method and glucose levels by glucose oxidase method. Lipid profiles were analyzed by enzymatic methods using the Beckman Coulter AU680 Analyzer (Beckman Coulter, Inc., Brea, CA, USA) with commercially available kits. Albumin and C-reactive protein (CRP) were determined in serum by nephelometric method. Parathyroid hormone was measured by an enzyme-linked immunosorbent assay.
Analytical methods and measurements
Blood samples for measurement of serum glucose, creatinine, urea, uric acid, parathyroid hormone, CRP, albumin, magnesium, calcium, phosphorus, lipids (triglyceride, high-density lipoprotein cholesterol [HDL-C], total cholesterol, low-density lipoprotein cholesterol), and hemoglobin were drawn after an 8 h overnight fasting conditions in a standing position. Urine samples were taken for measurement of the urine protein-to-creatinine (P/C) ratio. Anthropometric measurements, such as weight, height, waist circumference, and body mass index (BMI), were performed with the subjects wearing light clothing and without shoes. Weight and height were measured using a fixed scale with a stadiometer (Nan DR-MOD-85, Istanbul, Turkey). Each patient’s BMI was calculated as their weight (kg) divided by the square of their height (m2). Waist circumference was measured to the nearest 0.1 cm using a flexible metric measuring tape with the subject in a standing position. Glomerular filtration rate (GFR) were estimated using the Chronic Kidney Disease Epidemiology Collaboration.18 Systolic and diastolic blood pressures were measured in the sitting position, after a rest period of more than 5 min. Patient’s medical history was reviewed, and comorbidities, risk factors, and medications with the potential to affect ED and magnesium levels were recorded. Genital and systemic examinations were conducted with attention to any genital abnormalities. A urinary system ultrasound was done by an urologist for any surgical manipulations or injuries that affect the penis, prostate, or bladder.
Clinical definitions
Hypomagnesemia was defined by serum magnesium level <1.8 mg/dL;19 obesity as a BMI ≥30 kg/m2; abdominal obesity as a waist circumference >102 cm; diabetes was defined as fasting plasma glucose ≥126 mg/dL, which was confirmed by repeated testing, previous diagnosis of diabetes, or current use of antidiabetic medications. Metabolic syndrome, conformed to the National Cholesterol Education Program Adult Treatment Panel III guidelines, is defined as the presence of three or more of these conditions: abdominal obesity (waist circumference >102 cm), high blood pressure (130 mmHg systolic or 85 mmHg diastolic), hypertriglyceridemia (≥150 mg/dL), low HDL-C (<40 mg/dL), and high fasting blood glucose (≥110 mg/dL).20 The metabolic syndrome score was defined as the number of constituents of metabolic syndrome. Cigarette smoking was defined as if a patient reported smoking 100 cigarettes in their lifetime and currently smoking cigarettes every day or some days.21 According to the National Kidney Foundation Clinical Practice Guidelines, CKD stage 3 (moderately reduced kidney function) was defined as an estimated GFR (eGFR) of 30–59 mL/min/1.73 m2, and CKD stage 4 (severely reduced kidney function) was defined as an eGFR of 15–29 mL/min/1.73 m2.22 Patients on antihypertensive medication or with a blood pressure of ≥140/90 were regarded as having hypertension. The causes of CKD in patients were hypertension (41.7% vs 35.9%), glomerulonephritis (12.2% vs 14.4%), polycystic kidney disease (6.1% vs 8.8%), myeloma kidney (4.4% vs 3.6%), pyelonephritis (13.3% vs 10.4%), and unknown conditions (22.3% vs 26.9%) in hypomagnesemia and normomagnesemia groups, respectively.
Assessment of ED
Each participant completed a self-administered questionnaire. The Turkish translated and validated version of the International Index of Erectile Function-5 (IIEF-5) was used to assess ED.23 Patients completed the IIEF-5 questionnaire themselves under the supervision of trained medical staff. This consisted of five questions, each scored on a 5-point ordinal scale (1–5), where higher values indicated better sexual function. Patients with a score of ≥22 were considered to have normal erectile function, and subjects with a score of <22 were considered to have ED. With a total IIEF-5 score ranging from 1 to 25, the degree of ED was classified into the following five categories: severe (5–7), moderate (8–11), mild-to-moderate (12–16), mild (17–21), and no ED (22–25).
Exclusion criteria
Exclusion criteria included a diagnosis or a prior history of hypogonadism; genital malformations; penile implant or deformities; and surgical manipulations or injuries of urogenital system, spinal cord, or brain. Other exclusion criteria included a history of or current treatments for bladder, prostate, or testicle diseases or any cancer types; Parkinson’s disease, multiple sclerosis, stroke, diabetes mellitus, hepatic impairment, respiratory failure, peripheral vascular disease, connective tissue diseases, congestive heart failure stage 3 and 4, uncontrolled hypertension or hypotension, use of antihistamines and illegal drugs, and mental impairment leading to inability to cooperate; diagnosis or current treatments for depression and anxiety; subjects who had no regular partner; concurrent use of magnesium-containing supplements, inflammatory bowel disease, malabsorptions, and alcoholism.
Statistical analysis
Assuming an 80% prevalence of ED in the hypomagnesemia group and 65% prevalence in the normomagnesemia group, we found that a sample size of 368 (184 per group) patients would be required to detect a statistically significant difference with power of 90% (α=0.05). The primary study end point was the prevalence of ED. Secondary end points included severity and risk factors of ED. Comparisons of continuous variables between groups were made using Student’s t-test for normally distributed data and the Mann–Whitney U-test for non-normally distributed data. Within-subject comparisons of continuous variables were made using a paired t-test or the Wilcoxon signed-rank test for normally and non-normally distributed data, respectively. Categorical variables were analyzed using the chi-square test or alternatively Fisher’s exact test. We performed logistic regression with the presence of ED as the dependent variable and the following 11 parameters as potential covariates: presence of hypomagnesemia, age ≥70 years, hypertension, smoking, high urine P/C ratio (≥500 mg/dL), high CRP (>5 mg/L), abdominal obesity, metabolic syndrome, eGFR ≤30 mL/min/1.73 m2, low HDL-C (<40 mg/dL), and serum albumin levels. Variables that were statistically significant on univariate analysis were included in the multivariate model to identify predictors of ED. A two-sided 95% confidence interval (CI) was constructed around the point estimate of the relative risk (RR). Receiver operating characteristic (ROC) curve analyses of serum magnesium levels for the prediction of ED and the positive and negative predictive values of magnesium were performed. All tests were two-sided, and a P-value of <0.05 was considered to be statistically significant. Continuous data are reported as mean ± standard deviation. Categorical data are presented as absolute values and percentages. Analyses were performed using IBM SPSS Statistics Version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient population and baseline characteristics
A total of 3,810 patients were screened for inclusion and exclusion criteria, of that 3,438 patients were excluded from the study because they did not fulfill the inclusion or exclusion criteria. Ultimately, the study was carried out with 372 patients with a mean age of 72.52±5.43 years. The baseline demographic and clinical characteristics of the study patients are shown in Table 1. Patients with hypomagnesemia had higher urine P/C ratio (0.83±0.68 vs 0.69±0.48 mg/dL, P=0.023) and CRP levels (6.13±4.98 vs 4.13±3.66 mg/L, P<0.001), whereas there were significantly lower serum magnesium (1.52±0.13 vs 2.27±0.18 mg/dL, P<0.001) and albumin (4.02±0.53 vs 4.18±0.38 g/dL, P=0.001) levels among patients with hypomagnesemia.
Table 1.
Characteristics of the study patients
| Characteristics | All patients (n=372) |
Hypomagnesemia (n=180) |
Normomagnesemia (n=192) |
P-value |
|---|---|---|---|---|
| Age, year | 72.52±5.43 | 72.96±4.70 | 72.11±6.02 | 0.134 |
| Weight, kg | 73.66±11.62 | 74.11±11.81 | 72.68±11.39 | 0.092 |
| SBP, mmHg | 132.07±18.01 | 132.33±16.85 | 131.82±19.08 | 0.785 |
| DBP, mmHg | 69.50±9.88 | 68.67±7.61 | 70.29±11.57 | 0.114 |
| Hypertension | 145 (39%) | 75 (41.7%) | 69 (35.9%) | 0.215 |
| Hemoglobin, mg/dL | 12.35±1.49 | 12.27±1.49 | 12.43±1.50 | 0.315 |
| CRP, mg/L | 5.10±4.46 | 6.13±4.98 | 4.13±3.66 | <0.001 |
| Albumin, g/dL | 4.10±0.46 | 4.02±0.53 | 4.18±0.38 | 0.001 |
| Smoking | 42 (11.3%) | 24 (13.3%) | 18 (9.4%) | 0.229 |
| ARB or ACE-I usage | 30 (8.1%) | 16 (8.8%) | 14 (7.2%) | 0.572 |
| CCB usage | 31 (8.4%) | 14 (7.7%) | 17 (8.8%) | 0.732 |
| Beta-blocker usage | 37 (9.9%) | 17 (9.4%) | 20 (10.4%) | 0.755 |
| Diuretic usage | 18 (4.8%) | 10 (5.5%) | 8 (4.1%) | 0.543 |
| Alpha-blocker usage | 20 (5.4%) | 11 (6.1%) | 9 (4.6%) | 0.543 |
| eGFR, mL/min/1.73 m2 | 30.46±11.80 | 31.64±12.09 | 29.35±11.44 | 0.061 |
| Urine P/C, mg/dL | 0.76±0.59 | 0.83±0.68 | 0.69±0.48 | 0.023 |
| PTH, pg/mL | 159.38±108.36 | 151.86±95.45 | 166.44±119.02 | 0.195 |
| Magnesium, mg/dL | 1.91±0.41 | 1.52±0.13 | 2.27±0.18 | <0.001 |
| Calcium, mg/dL | 9.37±0.54 | 9.41±0.49 | 9.34±0.58 | 0.196 |
| Phosphorus, mg/dL | 3.79±0.77 | 3.74±0.82 | 3.84±0.72 | 0.231 |
Note: Values expressed as mean ± standard deviation or n (%).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactive protein; ARB, angiotensin receptor blocker; ACE-I, angiotensin-converting enzyme inhibitor; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone; P/C, protein-to-creatinine; CCB, calcium-channel blocker.
Metabolic parameters of the study patients
A significantly greater proportion of patients with hypomagnesemia had abdominal obesity (37.2% vs 22.9%, P=0.003), metabolic syndrome (38.44% vs 19.27%, P=0.026), and increased waist circumference (98.22±13.27 vs 95.73±10.26 cm, P=0.043) compared to those with normomagnesemia. HDL-C was significantly lower in the hypomagnesemia group (48.88±14.03 vs 52.65±13.56 mg/dL, P=0.009) compared with normomagnesemia. Other metabolic parameters were not significant between the groups (Table 2).
Table 2.
Metabolic parameters of the study patients
| Characteristics | All patients (n=372) |
Hypo-Mg (n=180) |
Normo-Mg (n=192) |
P-value |
|---|---|---|---|---|
| Body mass index, kg/m2 | 27.41±4.11 | 27.19±3.67 | 27.71±4.54 | 0.226 |
| Waist circumference, cm | 96.78±11.78 | 98.22±13.27 | 95.73±10.26 | 0.043 |
| Obesity | 68 (18.3%) | 35 (19.4%) | 33 (17.18%) | 0.574 |
| Abdominal obesity | 111 (29.8%) | 67 (37.2%) | 44 (22.9%) | 0.003 |
| Metabolic syndrome | 108 (29%) | 71 (38.44%) | 37 (19.27%) | 0.026 |
| Metabolic syndrome score | 1.69±1.13 | 1.79±1.15 | 1.59±1.11 | 0.089 |
| Total cholesterol, mg/dL | 194.05±50.09 | 191.10±52.73 | 196.82±47.45 | 0.271 |
| HDL-C, mg/dL | 50.83±13.90 | 48.88±14.03 | 52.65±13.56 | 0.009 |
| LDL-C, mg/dL | 116.42±40.49 | 115.32±41.31 | 117.45±39.78 | 0.614 |
| Triglyceride, mg/dL | 134.45±73.06 | 135.32±70.15 | 133.63±75.86 | 0.824 |
| Uric acid, mg/dL | 7.00±1.77 | 7.00±1.97 | 6.99±1.56 | 0.986 |
| Glucose, mg/dL | 105.42±12.40 | 104.38±12.57 | 106.39±12.20 | 0.119 |
Note: Values expressed as mean ± standard deviation or n (%).
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Hypo-Mg, hypomagnesemia; normo-Mg, normomagnesemia.
ED prevalences, ED scores, and ED severities in study groups
The prevalence of ED was 81.7% in the entire survey population, and a significantly greater proportion of patients with hypomagnesemia had ED compared to those with normomagnesemia (93.3% vs 70.8%, P<0.001; Figure 1). The mean IIEF-5 score was significantly lower in hypomagnesemic patients compared with normomagnesemics (8.97±5.87 vs 12.97±8.51, P<0.001). A significantly greater proportion of patients with hypomagnesemia had severe ED and mild-to-moderate ED compared to those with normomagnesemia (12.2% vs 5.2%, P=0.016 and 62.8% vs 43.8%, P=0.037, respectively; Table 3).
Figure 1.

Incidence of ED in the study groups.
Note: Values are expressed as percentage of ED (ED-developed patients/total number of patients in the group).
Abbreviations: ED, erectile dysfunction; Hypo-Mg, hypomagnesemia; Normo-Mg, normomagnesemia; RR, relative risk.
Table 3.
Comparison of the ED severities and mean IIEF-5 score in the study patients
| All patients (n=372) |
Hypomagnesemia (n=180) |
Normomagnesemia (n=192) |
P-value | |
|---|---|---|---|---|
| IIEF-5 scores (mean ± SD) | 11.03±7.61 | 8.97±5.87 | 12.97±8.51 | <0.001 |
| No ED, n (%) | 68 (18.3%) | 12 (6.7%) | 56 (29.2%) | <0.001 |
| Mild ED, n (%) | 28 (7.5%) | 14 (7.8%) | 14 (7.3%) | 0.859 |
| Mild-to-moderate ED, n (%) | 32 (8.6%) | 22 (12.2%) | 10 (5.2%) | 0.016 |
| Moderate ED, n (%) | 47 (12.6%) | 19 (10.6%) | 28 (14.6%) | 0.243 |
| Severe ED, n (%) | 197 (53%) | 113 (62.8%) | 84 (43.8%) | 0.037 |
Notes: Values expressed as mean ± standard deviation or n (%). No ED score: 22–25, mild ED score: 17–21, mild-to-moderate ED score: 12–16, moderate ED score: 8–11, severe ED score: 5–7.
Abbreviations: ED, erectile dysfunction; IIEF-5, International Index of Erectile Function-5.
ROC curve analysis and predictive values
Using the ROC curve analysis, we found that serum magnesium ≤1.85 mg/dL was the best cutoff point for the prediction of ED with a sensitivity of 73.0% and a specificity of 15.3%. Area under the curve was 0.842, 95% CI: 0.800–0.883, P<0.001 (Figure 2). The cutoff point of magnesium levels that we found in ROC curve analysis for the prediction of ED was almost the same as that we used for the definition of hypomagnesemia in our study. With an 81.7% prevalence of ED, we found that the positive predictive value of serum magnesium ≤1.8 mg/dL was 87.8%, and that the negative predictive value of serum magnesium ≤1.8 mg/dL was 66.7%.
Figure 2.
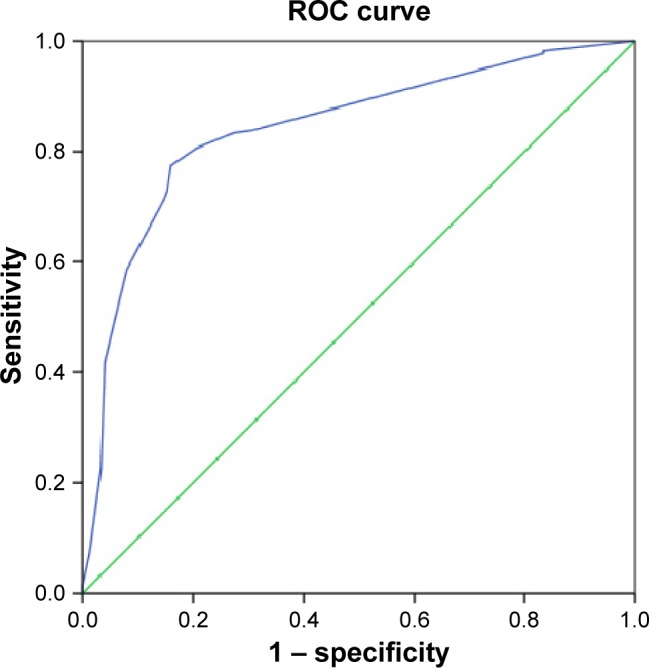
ROC curve analysis of serum magnesium levels for the prediction of ED.
Notes: Cutoff value of serum magnesium: ≤1.85 mg/dL. Sensitivity =73.0%, specificity =15.3%. Area under the ROC curve: 0.842, 95% CI: 0.800–0.883, P<0.001.
Abbreviations: ROC, receiver operating characteristic; ED, erectile dysfunction; CI, confidence interval.
Univariate and multivariate variables associated with ED
We used univariate analysis to study 11 different possible risk factors for developing ED. Univariate variables associated with ED were hypomagnesemia, age ≥70 years, hypertension, smoking, urine P/C ≥500 mg/dL, CRP >5 mg/L, and abdominal obesity (>112 cm). These variables were confirmed after a multivariate analysis. After adjusting for potential confounding factors by multivariable logistic regression, ED was associated with hypomagnesemia (RR =2.27, P=0.001), age ≥70 years (RR =1.74, P=0.001), urine P/C ≥500 mg/dL (RR =1.80, P=0.001), smoking (RR =21.12, P=0.001), CRP >5 mg/L (RR =1.34, P=0.003), abdominal obesity (RR =3.92, P=0.001), and hypertension (RR =2.14, P=0.031; Table 4).
Table 4.
Risk factors for ED by logistic regression
| Variable | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P-value | RR | 95% CI | P-value | |
| Hypomagnesemia | 2.40 | 1.57–3.80 | 0.001 | 2.27 | 1.50–3.56 | 0.001 |
| Age ≥70 years | 1.85 | 1.16–3.03 | 0.001 | 1.74 | 1.06–2.72 | 0.001 |
| Urine P/C ≥500 mg/dL | 1.69 | 0.83–2.93 | 0.001 | 1.80 | 1.01–2.91 | 0.001 |
| Smoking | 19.63 | 18.82–20.64 | 0.001 | 21.12 | 20.30–22.45 | 0.001 |
| CRP >5 mg/L | 1.46 | 0.63–2.94 | 0.002 | 1.34 | 0.57–2.60 | 0.003 |
| Abdominal obesity | 3.93 | 1.39–11.08 | 0.010 | 3.92 | 1.75–8.75 | 0.001 |
| Hypertension | 2.39 | 1.10–5.19 | 0.027 | 2.14 | 1.07–4.29 | 0.031 |
| eGFR <30 mL/min/1.73 m2 | 0.77 | 0.34–1.76 | 0.544 | |||
| Metabolic syndrome | 0.95 | 0.29–3.06 | 0.934 | |||
| HDL-C <40 mg/dL | 2.02 | 0.65–6.25 | 0.219 | |||
| Albumin levels | 1.00 | 0.36–2.73 | 0.995 | |||
Abbreviations: ED, erectile dysfunction; RR, relative risk; CI, confidence interval; eGFR, estimated glomerular filtration rate; P/C, protein-to-creatinine; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol.
Discussion
The main novel findings of this study are that we found a 93.3% prevalence of ED in hypomagnesemic non-diabetic elderly patients with stage 3 and 4 CKD. The present study was the first which reported the prevalence of ED in hypomagnesemic CKD patients. Second, individuals with a serum magnesium level of <1.8 mg/dL were 1.31 times more likely to develop ED than those in magnesium level >1.8 mg/dL (93.3% vs 70.8%). Third, hypomagnesemia is a risk for ED with a RR of 2.27. Fourth, this study shows a significant positive association between hypomagnesemia and the ED severities among the patients. Patients with hypomagnesemia have high prevalence of severe (62.8%) ED. Fifth, high prevalence of abdominal obesity, proteinuria, and increased CRP levels in hypomagnesemic patients may explain the high risk of ED. Finally, we found that serum magnesium level ≤1.85 mg/dL is the best cutoff point for prediction of ED.
The self-administered questionnaire of the IIEF-5 has been used widely in studies to detect the presence and the severity of ED in elderly and in CKD patients.5,24 Therefore, we used IIEF-5 for assessment of ED in the present study. ED is currently one of the most common sexual dysfunctions in the elderly CKD population. People aged ≥65 years comprise the fastest growing segment of the world. Sexual function is an important component of quality of life.1–3 Our study population include elderly CKD persons who are at high risk for ED. If we can decrease the incidence of ED among elderly CKD males, quality of life of these patients would be improved. Several studies confirm the high prevalence and severity of ED among elderly men with CKD.1,2,4,10 ED affects only 2%–4% of men in their 40s but almost 80%–90% of all men over the age of 80.1,9,10,25 The prevalence of ED among male CKD patients is estimated to be ~80%, and up to 90% of elderly men with CKD have ED.6–8,26 Mesquita et al reported that the prevalence rates of ED in CKD outpatients with stages 3, 4, and 5 were 72.3%, 81.5%, and 85.7%, respectively.26 In our study, we have excluded most of the comorbidities that could contribute to ED because of the observed real effect of hypomagnesemia on ED. Therefore, the prevalence of ED in our study might be lower than in the non-selected patient population. However, the high prevalence (81.7%) and severity (53% severe ED) of ED among all elderly CKD patients in our study are compatible with the published reports.
Several mechanisms have been suggested as etiologic factors for ED in elderly patients with CKD, most of which are associated with age, hypertension, obesity, hyperlipidemia, diabetes mellitus, cardiovascular disease, neurological and hormonal disorders, alcohol abuse, and smoking.2–4,27–31 CKD has also gained attention as a risk factor for ED.17,25,26,32 In the present study, we found that in addition to the well-known classical risk factors, hypomagnesemia, high CRP levels, abdominal obesity, and proteinuria are predictors of ED. Some essential minerals, such as zinc, magnesium, and selenium, may have role in erectile function.17,33–36 Hypomagnesemia may lead to ED can be explained by an association between hypomagnesemia and decreased NO.13 NO is important in initiating and maintaining erection, and ED is associated with reduced plasma NO levels and endothelial dysfunction.11,33 NO, the key element in endothelial function, needs magnesium for its synthesis.13 Hypomagnesemia decreases the NO levels and decreases the blood circulation in penis by penile vasoconstriction. In addition, lack of magnesium affects the production of testosterone.1,17,37 Some small studies in patients with normal kidney function postulate a role of decreased seminal magnesium in premature ejaculation. In a case–control study by Aloosh et al,38 it was demonstrated that there was a significant relationship between seminal plasma magnesium and premature ejaculation. The same results were found in a case–control study of 38 patients by Nikoobakht et al.36 However, these studies have not found any relation between serum magnesium and premature ejaculation.
Inflammation, metabolic syndrome and its components were independently associated with hypomagnesemia and ED.4,29,39,40 Compatible with the literature, in our study we documented that high CRP levels, abdominal obesity, and hypertension were risk factors for ED.28,29,31,39–42 However, we have not found any association between metabolic syndrome, low HDL-C, and ED in multivariate analysis. Similar to other studies, we found that smoking is a strong risk factor for ED.26,28,32 Several studies have shown that proteinuria is associated with ED, especially in diabetic patients.30,43–45 In a cross-sectional study of 455 men with type 2 diabetes, ED had a strong association with macroalbuminuria than microalbuminuria.46 In the present study, we found that there is a strong relation between urine P/C ≥500 mg/dL and ED in non-diabetic subjects. The possible explanation why we have found a higher prevalence of ED in hypomagnesemic subjects may be related to high levels of CRP, proteinuria, and abdominal obesity, which all may reflect endothelial dysfunction.12 In multivariate analyses, we found that all these three conditions were associated with ED, and that all were high in hypomagnesemic patients.
Strength and study limitations
The strength of this study includes that this is the first report to investigate the correlation between ED and hypomagnesemia in elderly men with CKD. The present study has several limitations: First, we did not measure levels of gonadotropins, testosterone, prolactin, NO, and seminal plasma magnesium which are important causes of ED. Second, we have not assessed blood flow of the penis. Third, the possible reasons for hypomagnesemia in our study may be secondary to reduced dietary magnesium intake or renal loss of magnesium. We have not measured the dietary intake or renal loss of magnesium. Finally, this study included only Turkish men, and thus, cultural and sociodemographic differences might have affected our results. How can we adapt the findings of the present study to the clinical practice? Current findings suggest that ED is highly prevalent in men with hypomagnesemia. Therefore, elderly patients with ED should be screened for hypomagnesemia. Correction of hypomagnesemia may lead to improvement in erectile function.
Conclusion
ED is common among elderly patients with hypomagnesemia. Serum magnesium measurement is an easily available and inexpensive marker. Therefore, detecting the serum magnesium level in non-diabetic elderly men with CKD seems to be a useful guide in assessing the risk of ED.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, Spera G. Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol. 2010;17(1):38–47. doi: 10.1111/j.1442-2042.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 2.Gareri P, Castagna A, Francomano D, Cerminara G, De Fazio P. Erectile dysfunction in the elderly: an old widespread issue with novel treatment perspectives. Int J Endocrinol. 2014;2014:878670. doi: 10.1155/2014/878670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalra G, Subramanyam A, Pinto C. Sexuality: desire, activity and intimacy in the elderly. Indian J Psychiatry. 2011;53(4):300–306. doi: 10.4103/0019-5545.91902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupelian V, Shabsigh R, Araujo AB, O’Donnell AB, McKinlay JB. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study. J Urol. 2006;176(1):222–226. doi: 10.1016/S0022-5347(06)00503-9. [DOI] [PubMed] [Google Scholar]
- 5.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 6.Collaborative Depression and Sexual Dysfunction in Hemodialysis Working Group. Vecchio M, Palmer S, De Berardis G, et al. Prevalence and correlates of erectile dysfunction in men on chronic haemodialysis: a multinational cross-sectional study. Nephrol Dial Transplant. 2012;27(6):2479–2488. doi: 10.1093/ndt/gfr635. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulou E, Varouktsi A, Lazaridis A, Boutari C, Doumas M. Erectile dysfunction in chronic kidney disease: from pathophysiology to management. World J Nephrol. 2015;4(3):379–387. doi: 10.5527/wjn.v4.i3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vecchio M, Palmer SC, Tonelli M, Johnson DW, Strippoli GF. Depression and sexual dysfunction in chronic kidney disease: a narrative review of the evidence in areas of significant unmet need. Nephrol Dial Transplant. 2012;27(9):3420–3428. doi: 10.1093/ndt/gfs135. [DOI] [PubMed] [Google Scholar]
- 9.Maas R, Schwedhelm E, Albsmeier J, Böger RH. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med. 2002;7(3):213–225. doi: 10.1191/1358863x02vm429ra. [DOI] [PubMed] [Google Scholar]
- 10.Hyde Z, Flicker L, Hankey GJ, et al. Prevalence and predictors of sexual problems in men aged 75–95 years: a population-based study. J Sex Med. 2012;9(2):442–453. doi: 10.1111/j.1743-6109.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 11.Burnett AL. The role of nitric oxide in erectile dysfunction: implications for medical therapy. J Clin Hypertens (Greenwich) 2006;8(12 Suppl 4):S53–S62. doi: 10.1111/j.1524-6175.2006.06026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gökçen K, Kılıçarslan H, Coşkun B, Ersoy A, Kaygısız O, Kordan Y. Effect of ADMA levels on severity of erectile dysfunction in chronic kidney disease and other risk factors. Can Urol Assoc J. 2016;10(1–2):E41–E45. doi: 10.5489/cuaj.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson PJ, Evora PR, Seccombe JF, Schaff HV. Hypomagnesemia inhibits nitric oxide release from coronary endothelium: protective role of magnesium infusion after cardiac operations. Ann Thorac Surg. 1998;65(4):967–972. doi: 10.1016/s0003-4975(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 14.Chacko SA, Song Y, Nathan L, et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care. 2010;33(2):304–310. doi: 10.2337/dc09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berner YN, Stern F, Polyak Z, Dror Y. Dietary intake analysis in institutionalized elderly: a focus on nutrient density. J Nutr Health Aging. 2002;6(4):237–242. [PubMed] [Google Scholar]
- 16.Tin A, Grams ME, Maruthur NM, et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015;87(4):820–827. doi: 10.1038/ki.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki E, Nishimatsu H, Oba S, Takahashi M, Homma Y. Chronic kidney disease and erectile dysfunction. World J Nephrol. 2014;3(4):220–229. doi: 10.5527/wjn.v3.i4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas L. Clinical Laboratory Diagnostics: Use And Assessment Of Clinical Laboratory Results. Frankfurt: TH-Books Verlagsgesellschaft; 1998. pp. 231–241. [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Schoenborn CA, Adams PE. Health behaviors of adults: United States, 2005–2007. Vital Health Stat 10. 2010;(245):1–132. [PubMed] [Google Scholar]
- 22.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 23.Turunc T, Deveci S, Güvel S, Peşkircioğlu L. The assessment of Turkish validation with 5 question version of International Index of Erectile Function (IIEF-5) Turkish J Urol. 2007;33(1):45–49. [Google Scholar]
- 24.Rhoden EL, Telöken C, Sogari PR, Vargas Souto CA. The use of the simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. Int J Impot Res. 2002;14(4):245–250. doi: 10.1038/sj.ijir.3900859. [DOI] [PubMed] [Google Scholar]
- 25.Navaneethan SD, Vecchio M, Johnson DW, et al. Prevalence and correlates of self-reported sexual dysfunction in CKD: a meta-analysis of observational studies. Am J Kidney Dis. 2010;56(4):670–685. doi: 10.1053/j.ajkd.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Mesquita JF, Ramos TF, Mesquita FP, Bastos Netto JM, Bastos MG, Figueiredo AA. Prevalence of erectile dysfunction in chronic renal disease patients on conservative treatment. Clinics (Sao Paulo) 2012;67(2):181–183. doi: 10.6061/clinics/2012(02)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javaroni V, Neves MF. Erectile dysfunction and hypertension: impact on cardiovascular risk and treatment. Int J Hypertens. 2012;2012:627278. doi: 10.1155/2012/627278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artom N, Pinna G, Musso NR, et al. Prevalence of erectile dysfunction in a cohort of Italian hypertensive subjects. Clin Exp Hypertens. 2016;38(2):143–149. doi: 10.3109/10641963.2015.1060994. [DOI] [PubMed] [Google Scholar]
- 29.Bal K, Oder M, Sahin AS, et al. Prevalence of metabolic syndrome and its association with erectile dysfunction among urologic patients: metabolic backgrounds of erectile dysfunction. Urology. 2007;69(2):356–360. doi: 10.1016/j.urology.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 30.Yu LW, Kong AP, Tong PC, et al. Evaluation of erectile dysfunction and associated cardiovascular risk using structured questionnaires in Chinese type 2 diabetic men. Int J Androl. 2010;33(6):853–860. doi: 10.1111/j.1365-2605.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- 31.Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol. 2004;171(6 Pt 1):2341–2345. doi: 10.1097/01.ju.0000125198.32936.38. [DOI] [PubMed] [Google Scholar]
- 32.Toorians AW, Janssen E, Laan E, et al. Chronic renal failure and sexual functioning: clinical status versus objectively assessed sexual response. Nephrol Dial Transplant. 1997;12(12):2654–2663. doi: 10.1093/ndt/12.12.2654. [DOI] [PubMed] [Google Scholar]
- 33.Blans MC, Visseren FL, Banga JD, et al. Infection induced inflammation is associated with erectile dysfunction in men with diabetes. Eur J Clin Invest. 2006;36(7):497–502. doi: 10.1111/j.1365-2362.2006.01653.x. [DOI] [PubMed] [Google Scholar]
- 34.Prasad AS, Mantzoros CS, Beck FW, Hess JW, Brewer GJ. Zinc status and serum testosterone levels of healthy adults. Nutrition. 1996;12(5):344–348. doi: 10.1016/s0899-9007(96)80058-x. [DOI] [PubMed] [Google Scholar]
- 35.Omu AE, Al-Bader AA, Dashti H, Oriowo MA. Magnesium in human semen: possible role in premature ejaculation. Arch Androl. 2001;46(1):59–66. doi: 10.1080/01485010150211164. [DOI] [PubMed] [Google Scholar]
- 36.Nikoobakht M, Aloosh M, Hasani M. Seminal plasma magnesium and premature ejaculation: a case-control study. Urol J. 2005;2(2):102–105. [PubMed] [Google Scholar]
- 37.Maggio M, De Vita F, Lauretani F, et al. The interplay between magnesium and testosterone in modulating physical function in men. Int J Endocrinol. 2014;2014:525249. doi: 10.1155/2014/525249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aloosh M, Hassani M, Nikoobakht M. Seminal plasma magnesium and premature ejaculation: a case-control study. BJU Int. 2006;98(2):402–404. doi: 10.1111/j.1464-410X.2006.06320.x. [DOI] [PubMed] [Google Scholar]
- 39.Karamanli H, Kizilirmak D, Akgedik R, Bilgi M. Serum levels of magnesium and their relationship with CRP in patients with OSA. Sleep Breath. 2016 Sep 6; doi: 10.1007/s11325-016-1402-4. Epub. [DOI] [PubMed] [Google Scholar]
- 40.Chiurlia E, D’Amico R, Ratti C, Granata AR, Romagnoli R, Modena MG. Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J Am Coll Cardiol. 2005;46(8):1503–1506. doi: 10.1016/j.jacc.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 41.Burchardt M, Burchardt T, Baer L, et al. Hypertension is associated with severe erectile dysfunction. J Urol. 2000;164(4):1188–1191. [PubMed] [Google Scholar]
- 42.Heidler S, Temml C, Broessner C, et al. Is the metabolic syndrome an independent risk factor for erectile dysfunction? J Urol. 2007;177(2):651–654. doi: 10.1016/j.juro.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 43.Siu SC, Lo SK, Wong KW, Ip KM, Wong YS. Prevalence of and risk factors for erectile dysfunction in Hong Kong diabetic patients. Diabet Med. 2001;18(9):732–738. doi: 10.1046/j.0742-3071.2001.00557.x. [DOI] [PubMed] [Google Scholar]
- 44.Hermans MP, Ahn SA, Rousseau MF. Erectile dysfunction, microangiopathy and UKPDS risk in type 2 diabetes. Diabetes Metab. 2009;35(6):484–489. doi: 10.1016/j.diabet.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Fukui M, Tanaka M, Okada H, et al. Five-item version of the International Index of Erectile Function correlated with albuminuria and subclinical atherosclerosis in men with type 2 diabetes. J Atheroscler Thromb. 2011;18(11):991–997. doi: 10.5551/jat.9316. [DOI] [PubMed] [Google Scholar]
- 46.Chuang YC, Chung MS, Wang PW, et al. Albuminuria is an independent risk factor of erectile dysfunction in men with type 2 diabetes. J Sex Med. 2012;9(4):1055–1064. doi: 10.1111/j.1743-6109.2011.02586.x. [DOI] [PubMed] [Google Scholar]


