Abstract
Purpose of review
Notch signaling is an evolutionary conserved pathway critical for cardiovascular development and angiogenesis. More recently, the contribution of Notch signaling to the homeostasis of the adult vasculature has emerged as an important novel paradigm, but much remains to be understood.
Recent findings
Recent findings shed light on the impact of Notch in vascular and immune responses to microenvironmental signals as well as on the onset of atherosclerosis. In the past year, studies in human and mice explored the role of Notch in the maintenance of a nonactivated endothelium. Novel pieces of evidence suggest that this pathway is sensitive to environmental factors, including inflammatory mediators and diet-derived by-products.
Summary
An emerging theme is the ability of Notch to respond to changes in the microenvironment, including glucose and lipid metabolites. In turn, alterations in Notch enable an important link between metabolism and transcriptional changes, thus this receptor appears to function as a metabolic sensor with direct implications to gene expression.
Keywords: atherosclerosis, dietary by-products, endothelium, inflammation, signaling pathway
INTRODUCTION
Endothelial cells provide a selective and highly responsive barrier that offers, under physiological conditions, an optimal ratio between vessel integrity and permeability. The endothelium also prevents thrombosis and regulates the trafficking of cells from the blood to adjacent tissues. Coordination of leukocyte trafficking in particular is of critical importance to inflammation and, in fact, the endothelium is the first line of regulatory control during the inflammatory response. In the absence of disease, endothelial cells maintain a closed, anti-inflammatory status, by preventing binding and extravasation of leukocytes from the circulation. In contrast, during the response to inflammatory stimuli, endothelial cells become ‘activated’ by expressing a subset of leukocyte-adhesion molecules and facilitating the exit of leukocytes from the circulation into tissues. During this process, endothelial junctions become weakened promoting leakage of plasma proteins and solutes. Thus, the endothelium provides a critical barrier that regulates the inflammatory response and breakage of its homeostasis is a major determinant of vascular disorders, including hypertension, atherosclerosis, and thrombosis [1].
As a primary barrier between blood and tissue, the endothelium also differentially responds to hemodynamic patterns that define atherosusceptible versus atheroprotected sites of the arterial tree. In fact, laminar shear stress has been found to protect against the disease through the induction in endothelial cells of anti-inflammatory, antioxidant, and antithrombotic genes. In contrast endothelium exposed to disturbed blood flow exhibits biological changes such as increased permeability and chronic low-inflammation, which in the presence of additional risk factors favors the emergence of atherosclerosis lesions [2,3]. The activation of endothelial cells toward a prolonged proinflammatory and atherogenic phenotype could be driven by cell surface receptors in response to chemokines, like tumor necrosis factor (TNF) and IL-1β [4,5], and downstream signaling cascades that are fairly well understood. Importantly, endothelial activation can also be promoted and strengthened by a cohort of lipid mediators, a process that holds special significance to the onset and progression of atherosclerosis. In fact, it is fair to state that atherosclerosis is a lipid-driven, chronic inflammatory disease that develops as a result of lipid accumulation, followed by lipid modifications and subsequent growth of the intra-arterial atherosclerotic plaque. Oxidative modification of lipid products such as LDLs and derived phospholipids have been designated as a critical step in the initiation of atherosclerosis [6,7]. These oxidized lipid by-products trapped in the subendothelial space impact numerous cell types, including immune, smooth muscle, and endothelial cells. It has become clear that a large number of signaling pathways are altered in endothelial cells as the result of exposure to oxidized lipids, leading to endothelial activation, inflammation, and atherosclerosis [6,8]. Less known, however, are the intrinsic pathways essential for the maintenance of arterial endothelium integrity, involved in sensing lipid products, interpreting microenvironment cues, and transducing these readouts into transcriptional changes that counteract the effect of lipid by-products. Here, we are reviewing the recent literature that links deregulation of the Notch pathway to inflammatory processes and atherosclerosis, with a focus on its response to lipid by-products and subsequent pathophysiological impact.
NOTCH SIGNALING PATHWAY
Notch signaling together with the WNT, sonic hedgehog, and bone morphogenetic protein/transforming growth factor β pathways are evolutionary conserved mechanisms involved in the development and homeostasis of most tissues. In mammals, expression of four Notch receptors (Notch1–4) and five canonical ligands [Delta-like ligand (Dll) 1, 3, 4 and Jagged (Jag)1, 2] coordinate activation of this signaling pathway. Canonical transactivation of the pathway occurs after binding of a receptor (signal-receiving cell) with a ligand presented on an adjacent cell (signal-sending cell). Endocytosis of the ligand exerts mechanical forces on the receptor that become accessible to proteases [9,10] enabling successive cleavage of Notch extracellular domain and intramembrane domain by a-disintegrin and metalloprotease (ADAM) family members [11] and the γ-secretase complex, respectively [12,13]. These events ultimately release Notch intracellular domain (NICD) that is translocated to the nucleus where it interacts with a transcriptional complex, RBP-jκ (recombination signal-binding protein for immunoglobulin κ J region)/CSL (CBF1, suppressor of hairless, Lag-1), and MAML (mastermind-like), to induce target genes. In addition, Notch contributes to the regulation of cellular mechanisms through noncanonical pathways [14] (Fig. 1A).
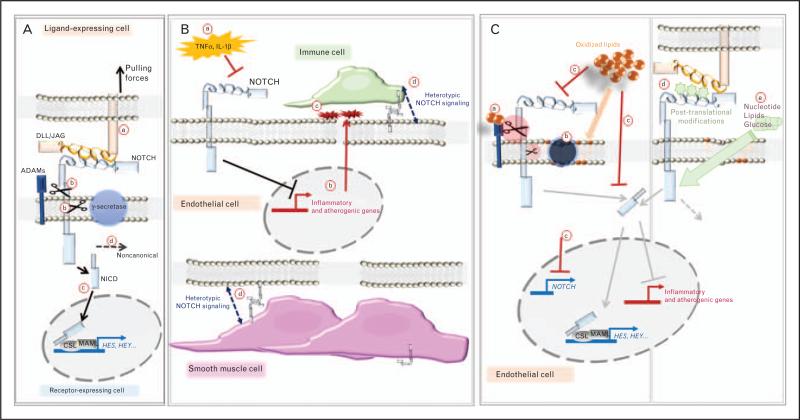
FIGURE 1. Endothelial Notch signaling pathway and interactions with the microenvironment.
(A) After dimerization of a NOTCH receptor with a DLL/JAG ligand (a), proteolytic cleavage of NOTCH by ADAM family proteases and the γ-secretase complex occurs (b), releasing NOTCH intracellular domain (NICD) that translocates to the nucleus, interacts with the MAML/CSL complex to induce the transcription of target genes (c; HES, HEY family). Non-canonical effects of NOTCH have also been described (d). (B) Inflammatory stimuli suppress NOTCH expression and activity in endothelial cells (a); this results in the expression of inflammatory and atherogenic mediators (b) favoring the recruitment of immune cells (c). Notch signaling can involve bi-directional heterotypic communication between endothelial cells and immune or smooth muscle cells (d). (C) Changes in protease activity secondary to oxidized lipids exposure that can (a) bind to and increase activity of ADAM proteases and (b) change the lipid microenvironment altering γ-secretase activity. Oxidized phospholipids repress NOTCH expression and activity, a mechanism that promotes endothelial activation (c). NOTCH signaling is regulated by post-translational changes that may reflect biosynthetic activity of the cell (d). Nutrients impact NOTCH expression and activity (e). ADAM, a-disintegrin and metalloprotease; CSL, CBF1, Suppressor of Hairless, Lag-1; Dll, Delta-like ligand; MAML, mastermind-like; NICD, Notch intracellular domain.
The outcome of Notch activation is cell type and context dependent with multiple combinations of receptors and ligands that transduce different biological effects [15,16]. In addition, progress made in characterizing structural features of ligands and receptors has allowed us to understand the critical impact of post-translational modifications of the Notch receptors in ligand binding and activation [17,18■■,19■].
The biological relevance of Notch signaling to developmental processes is highlighted by the broad number of anomalies and disorders arising when the pathway is deregulated. This includes immune [20–22], skeletal [23–25], hepatic [26,27], vascular [28] defects and cardiac malformations [26,27,29]. More recently large studies examining genome-wide association study for coronary artery disease identified genetic signals enriched in Notch-related pathways [30,31■■] and polymorphisms near the HEY2 gene (canonical target of Notch signaling) associated with Brugada syndrome [32].
Although the role of Notch has been extensively studied in the context of development and cancer [14,33,34], recent experiments using in vitro assays and mouse models also showed that changes in Notch activity can impact organ homeostasis in adults. Blocking Notch signaling is known to initiate sprouting angiogenesis [35–37], but this postulate should now be refined to include tissue-specific differences [38]. In endothelial cells, Notch signaling protects against apoptosis in a rat allograft model [39,40] and in response to laminar blood flow [41,42]. This review will focus on findings describing the impact of Notch deregulation in the initiation and progression of atherosclerosis.
NOTCH SIGNALING PATHWAY AND INFLAMMATION
Inflammation constitutes a major player in multiple steps of atherosclerosis. Intriguingly, Notch signaling has been shown to contribute to and be modulated by inflammatory signals. Importantly both pro and anti-inflammatory roles have been attributed to the pathway depending on the specific cell types.
In immune cells, including T lymphocytes and macrophages, blockade of Notch signaling often results in the repression of the inflammatory response [43] although differences are noted depending on the heterodimer engaged and the cell type studied [44,45]. Thus, during atherosclerosis, activated macrophages recruited to the vascular wall express the Notch ligand Dll4, which is increased in response to inflammatory stimuli. In particular, Dll4 participates to homotypic activation of Notch signaling and promotes expression of M1-type molecules [46]. Reduction of Notch signaling in macrophages with a broad spectrum inhibitor (γ-secretase inhibitor) or with antibodies against Dll4 attenuates atherosclerosis in mice [47,48]. Blocking Dll4 also reduces the development of vein graft lesion in LDL receptor-null animals. Interestingly when using cell-type specific nanoparticles to deliver short hairpin RNA (siRNA) targeting Dll4 in vivo, the authors observed that the protective effect was exclusive to macrophages but was absent when endothelial cells were targeted [49■]. Although Dll4 is highly expressed in capillaries during development, mature arterial endothelium express Dll4 at low levels [50–52].
Modulation of Notch signaling by inflammatory signals also occurs in endothelial cells from various vascular beds. In human umbilical vein endothelial cells (HUVEC), a pulse of TNFα was shown to increase JAG1 through activation of NF-κB but a concurrent decrease of Notch4 and target genes Hairy and Enhancer of Split 1 (HES1) and Hairy and Enhancer of Split-Related 1 (HESR1) were also observed [53] resulting from a possible negative regulatory loop [54,55]. In human aortic endothelial cells (HAEC), JAG1 expression was upregulated by a short treatment with inflammatory cytokines, including IL-1β and TNFα, whereas NOTCH1 and targets HES1, Hairy/Enhancer-of-Split Related with YRPW motif-Like (HEYL) were strongly repressed through a mechanism involving Signal Transducer and Activator of Transcription 3 (STAT3) activation [56■■]. Therefore, despite the increase in ligand, the overall signaling pathway was also suppressed upon exposure to inflammatory mediators. Others also reported that in endothelial cells in vitro and from small coronary vessels in a model of heart transplant in rat, inflammatory stimuli differentially impact Notch2 and Notch4, with the first being upregulated, whereas the latest was repressed [40,57].
In addition to a direct impact of inflammatory cytokines on Notch signaling, repression of the Notch pathway promotes endothelial cell activation. For example, repression of endothelial Notch4 triggered an increase in Vascular Cell Adhesion Molecule 1 at the cell surface [40]. In endothelial cells from the bone marrow, the canonical effector of Notch signaling RBP-jκ inhibits MicroRNA-155, NF-κB activation and subsequent production of proinflammatory cytokines [58].
Inflammatory activation of the endothelium is also linked to disturbed hemodynamic shear stress in the arterial tree. Although relevant mechanosensors have been investigated, the Notch signaling pathway has emerged as both sensitive to and a mediator of shear stress. In fact, data collected in vitro and in vivo have shown that the pathway could be activated by shear stress [54,59–63] while it is required for downstream biological processes such as endothelial cells alignment [64], arterial specification [59–61], repair of the endothelium [65] and repression of inflammatory and osteogenic genes [63,66■■]. In particular in aortic valve leaflet, Notch activity levels are lower in the aortic side compared with the ventricular side, the first being more prone to the emergence of calcific and inflammatory events [67].
Consistent with a molecular impact of differential Notch activity in endothelial cells, work by Theodoris and colleagues have recently uncovered that mutations in NOTCH1, that have been known to cause aortic valve disease [29], were also responsible for profound changes in epigenetic landscape. The authors showed that NOTCH1 haploinsufficiency in aortic valve endothelial cells disrupts antiosteogenic and anti-inflammatory networks normally induced by protective hemodynamic shear stress [66■■]. Although mechanical dysfunction was proposed to be a strong determinant of the disease, their findings shed light on a more direct role for Notch1 in the maintenance of endothelial cell fate and its critical contribution in repressing intrinsic expression of inflammatory mediators. In agreement with these findings knockdown of NOTCH1 in HAEC led to the upregulation of pro-inflammatory and proatherogenic molecules that promote binding of monocytes in vitro. These results using human cells were corroborated in mice carrying heterozygous deletion of endothelial Notch1. Inactivation of a single allele in the endothelium resulted in the accumulation of C-X-C Motif Chemokine Ligand 1 (CXCL1) in the luminal side of the aorta and significant recruitment of CD45 Positive (immune) cells. Furthermore, inducible endothelial deletion of Notch1, even in adult uninjured arteries, supported a critical role for Notch1 in the maintenance of a nonactivated endothelium [56■■]. Finally, Notch3 expressed by vascular smooth muscle cells is essential to protect against their inflammatory activation and transdifferentiation induced by inflammatory stimuli, such as IL-1β [68,69].
To note, because Notch is involved in close range cell–cell communication heterotypic activation in addition to homotypic activation must be considered. For example, in the liver, Dlls expressed by synovial endothelial cells activate Notch signaling in Th1 lymphocytes, inducing the expression of IL-10 to blunt the inflammatory response [70■]. Interactions between macrophage Notch and Dll4 expressed on tip-cells are important for retinal sprouting angiogenesis [71] and recently an in vitro model of angiogenesis integrated Notch/ligand interactions in macrophages, mural cells, and endothelial cells [72■]. Finally, Notch activation in smooth muscle cells, essential for their fate decision and maintenance, is driven in part by ligands expressed by endothelial cells [73] (Fig. 1B).
REGULATION OF NOTCH SIGNALING PATHWAY BY LIPID PRODUCTS
Lipid by-products are major contributors to atherosclerosis as they promote inflammatory-related events as well as endothelial dysfunction. For example, in endothelial cells, Notch signaling is blocked by oxidized lipids. A follow-up consequence of Notch suppression is the emergence of a pro-inflammatory transcriptional signature that includes upregulation of CXCL1, IL-8 and E-Selectin [56■■]. In vivo, exposure of wild-type mice to high-fat diet led to a decrease in endothelial Notch1 expression and activity, which was rescued when circulating cholesterol levels were reduced. The findings demonstrate that by-products of high-fat diet, currently used to induce atherosclerosis in mice, rapidly impact endothelial Notch pathway and promote inflammation. Consistent with this observation, NOTCH1 expression and activity were also repressed by high dose of oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylc holine (Ox-PAPC) in HAEC, through a mechanism involving the activation of STAT3 [56■■]. Ox-PAPC also triggers a strong and rapid downregulation of JAG1 and target genes HES1 and HEYL. Repression of NOTCH1 by Ox-PAPC participated in endothelial cell activation downstream of oxidized phospholipids. Importantly, the degree of NOTCH1 suppression by Ox-PAPC was variable across 147 donors and associated with specific polymorphisms [56■■] previously linked to HDL levels in approximately 100 000 individuals [74,75]. Therefore, endothelial Notch signaling appears to be a sensor of oxidized-PAPC.
Data from different systems support such a sensor function of Notch and provide potential mechanisms, including modulation of plasma membrane lipid composition and protein post-translational modification. Oxidized phospholipids, in particular Ox-PAPC bind to and activate ADAM10 [76]. ADAM10 contributes to the cleavage of Notch receptor; this protease is also able to shave the ligand JAG1 from the membrane [77] an event that is expected to affect close range ligand-dependent activation of the pathway that requires transcytosis of Notch extracellular domain by the ligand expressing cells [10]. However, the net effect of excessive ADAM10 activity on Notch signaling remains to be explored, as the biological impact of soluble Notch ligand is unclear [78] and this enzyme also participates in the cleavage of the receptor itself. Another critical enzyme in Notch activation is γ-secretase, a complex of transmembrane proteins, which cleaves the intramembrane domain of Notch receptor (among other substrates) to release its transcriptionally active form. Interestingly, the activity of γ-secretase is highly sensitive to local membrane lipid composition. In fact, cholesterol raft-like membrane structures were proposed to be optimal to sustain high activity [79–82]. Although the precise mechanisms are not yet elucidated, changes in membrane lipid composition and fluidity occur in response to oxidized phospholipids such as oxidized LDL [83], an event that may affect γ-secretase function and Notch activity. In tumor cell lines, exosomal lipids were shown to affect Notch1 signaling likely through changes in membrane lipid microenvironment [84]. In addition, a recent study in HUVEC suggested that the protective effect of epigallocatechin gallate, a natural polyphenol that can inhibit metalloproteases, toward ox-LDL was mediated by Jag1/Notch signaling [85]. Thus lipid by-products might also affect endothelial Notch signaling through changes in membrane composition and regulation of protease activity, but more direct validation is needed to support these conclusions.
Another mechanism by which lipids may interfere with Notch signaling relate to potential lipid–ligand interactions. In fact, a C2 phospholipid recognition domain in N-terminal region of Jag1 was recently uncovered. This domain also present in Dll1 does not impact dimerization with the receptor but it regulates levels of Notch activation [86]. More recently, ligand-independent induction of Notch was observed in response to shingosine-1-phosphate (S1P) and S1P receptor 3 engagement in cancer stem cells [87]. A protective role for S1P bound to HDL has been previously shown in the onset of atherosclerosis [88] with a recent study uncovering molecular mechanisms involved in their anti-inflammatory and antiatherogenic function in endothelial cells [89■■]. Although the molecular events are likely to differ from cancer stem cells, it would be interesting to determine whether regulation of Notch pathway by S1P may also occur in adult aortic endothelial cells.
Together, the findings converge on the idea that this evolutionary conserved pathway may be considered as a signaling hub between circulating factors and cell homeostasis. A role for Notch in ‘sensing’ the systemic metabolic status is further supported by elegant studies in different systems. In the developing mouse heart, hyperglycemia abolishes left–right axis formation and affects heart morphogenesis. The resulting condition that resembles congenital heart defects associated with pregestational diabetes was shown to be secondary to a reduction in Notch pathway activity [90■■]. In the nematodes Notch [abnormal Germ Line Proliferation (GLP-1] signaling is modulated by nucleotide levels and proposed to be part of a sensing mechanism to adapt their reproductive program to environmental and nutritional clues [91■]. Finally, recent advances in the characterization of structural features of the oligomerization of Notch and its ligands through O-fucose and O-glucose provided additional clues supporting that the activity of the pathway may reflect the metabolic state of the cell [18■■] (Fig. 1C).
CONCLUSION
Progress have been made in understanding the role of Notch signaling in the adult vasculature and current studies converge toward a protective role of the pathway in endothelial cells from large vessels. In addition, evidence from different models support that the Notch activity may be considered as a component of the cell ‘sensing’ machinery, transducing microenvironment and metabolic clues to transcriptional changes. This includes shear stress, inflammatory signals, and dietary by-products but also close range activation through homo and heterotypic contact with cells residing in blood vessels. Although the current studies show a rapid impact of these stimuli on endothelial Notch, the long-term effect of chronic exposure to inflammatory mediators and lipid byproducts together with heterotypic communication within the plaque deserves additional investigations. Despite the complexity of the plaque microenvironment this will contribute to improve our understanding of the role and regulation of endothelial Notch in the disease progression and stabilization.
As several therapeutic strategies aim at inhibiting Notch signaling, for example, to reduce tumor angiogenesis, a better characterization of this pathway in adult vessels is important. Although most studies on endothelial Notch are focused on cancer setting and cardiovascular diseases, it is also critical to consider its potential role in the microvasculature of highly metabolic organs that are constantly challenged by inflammatory molecules and metabolic by-products.
KEY POINTS.
Notch signaling is critical for the maintenance of vascular homeostasis.
Notch contributes to and is modulated by inflammation in various cell types.
Notch activity is impaired by dietary by-products, including oxidized lipids.
Repression of Notch signaling in arterial endothelial cells unlocks proinflammatory and proatherogenic signals that contribute to the initiation of atherosclerosis.
Acknowledgements
None.
Financial support and sponsorship
The work is supported by funds from INSERM (A. Briot and A. Bouloumié), aviesan ITMO Physiopathology, Metabolism and Nutrition (A. Briot) and National Institutes of Health HL030568 (M.L.I.A.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Baeyens N, Nicoli S, Coon BG, et al. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. eLife. 2015;4:e04645. doi: 10.7554/eLife.04645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies PF, Civelek M, Fang Y, et al. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99:315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branen L, Hovgaard L, Nitulescu M, et al. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 5.Qamar A, Rader DJ. Effect of interleukin 1β inhibition in cardiovascular disease. Curr Opin Lipidol. 2012;23:548–553. doi: 10.1097/MOL.0b013e328359b0a6. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Birukov KG, Romanoski CE, et al. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111:778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiolino G, Rossitto G, Caielli P, et al. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediators Inflamm. 2013;2013:714653. doi: 10.1155/2013/714653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linton MF, Yancey PG, Davies SS, et al. The role of lipids and lipoproteins in atherosclerosis. In: De Groot LJ, Beck-Peccoz P, Chrousos G, et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA: 2000. [Google Scholar]
- 9.Meloty-Kapella L, Shergill B, Kuon J, et al. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev Cell. 2012;22:1299–1312. doi: 10.1016/j.devcel.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musse AA, Meloty-Kapella L, Weinmaster G. Notch ligand endocytosis: mechanistic basis of signaling activity. Semin Cell Dev Biol. 2012;23:429–436. doi: 10.1016/j.semcdb.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Tetering G, Vooijs M. Proteolytic cleavage of Notch: ‘HIT and RUN’. Curr Mol Med. 2011;11:255–269. doi: 10.2174/156652411795677972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 13.Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 14.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedito R, Roca C, Sorensen I, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Kangsamaksin T, Murtomaki A, Kofler NM, et al. NOTCH decoys that selectively block DLL/NOTCH or JAGG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer Discov. 2014;5:182–197. doi: 10.1158/2159-8290.CD-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi H, Haltiwanger RS. Significance of glycosylation in Notch signaling. Biochem Biophys Res Commun. 2014;453:235–242. doi: 10.1016/j.bbrc.2014.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18■■.Luca VC, Jude KM, Pierce NW, et al. Structural biology. Structural basis for Notch1 engagement of Delta-like 4. Science. 2015;347:847–853. doi: 10.1126/science.1261093. [In this study, the authors reported the crystal structure of two interaction interfaces, with O-linked glycan, between Notch and DLL4; with one interface being less conserved, the authors proposed that posttranslational modifications of Notch provide a way of regulating the pathway depending on the cell biosynthetic state. In addition, this study provides new structural clues to develop efficient and specific therapeutic targeting of the Notch signaling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19■.Weisshuhn PC, Sheppard D, Taylor P, et al. Non-linear and flexible regions of the human Notch1 extracellular domain revealed by high-resolution structural studies. Structure. 2016;24:555–566. doi: 10.1016/j.str.2016.02.010. [Using nuclear magnetic resonance spectroscopy and X-ray crystallography of unmodified EGF domains of Notch, the authors showed that EGF10 modulates the sensitivity to Dll4 and Dll1 but not Jag1. This study provides new insight into the fine-tuning of the interaction of Notch receptor with a repertoire of ligands.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 21.Fabbri G, Rasi S, Rossi D, et al. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med. 2011;208:1389–1401. doi: 10.1084/jem.20110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparrow DB, Chapman G, Wouters MA, et al. Mutation of the LUNATIC FRINGE gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am J Hum Genet. 2006;78:28–37. doi: 10.1086/498879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparrow DB, Guillen-Navarro E, Fatkin D, et al. Mutation of Hairy-and-Enhancer-of-Split-7 in humans causes spondylocostal dysostosis. Hum Mol Genet. 2008;17:3761–3766. doi: 10.1093/hmg/ddn272. [DOI] [PubMed] [Google Scholar]
- 25.Whittock NV, Sparrow DB, Wouters MA, et al. Mutated MESP2 causes spondylocostal dysostosis in humans. Am J Hum Genet. 2004;74:1249–1254. doi: 10.1086/421053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniell R, Warthen DM, Sanchez-Lara PA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda T, Elkahloun AG, Pike BL, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 28.Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 29.Garg V, Muth AN, Ransom JF, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 30.Makinen VP, Civelek M, Meng Q, et al. Integrative genomics reveals novel molecular pathways and gene networks for coronary artery disease. PLoS Genet. 2014;10:e1004502. doi: 10.1371/journal.pgen.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31■■.Ghosh S, Vivar J, Nelson CP, et al. Systems genetics analysis of genome-wide association study reveals novel associations between key biological processes and coronary artery disease. Arterioscler Thromb Vasc Biol. 2015;35:1712–1722. doi: 10.1161/ATVBAHA.115.305513. [In this study, the authors identified 32 Reactome pathways replicated between seven genome-wide association study for coronary artery disease and nine additional meta-analyses from the CARDIoGRAM Consortium. Among these pathways they found genetic variation in a critical number of genes representing core biological processes, including Platelet-Derived Growth Factor, Notch, transforming growth factor β signaling with the strength of association comparable with those observed in lipid transport pathways. Their approach provided new insight into potential causal mechanisms of coronary atherosclerosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bezzina CR, Barc J, Mizusawa Y, et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling: are we there yet? Nat Rev Drug Discov. 2014;13:357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 34.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 35.Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 36.Lobov IB, Renard RA, Papadopoulos N, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suchting S, Freitas C, Eichmann A. [Angiogenesis under Delta-Notch couple control]. Med Sci (Paris) 2007;23:347–348. doi: 10.1051/medsci/2007234347. [DOI] [PubMed] [Google Scholar]
- 38.Ramasamy SK, Kusumbe AP, Wang L, et al. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacKenzie F, Duriez P, Wong F, et al. Notch4 inhibits endothelial apoptosis via RBP-Jkappa-dependent and -independent pathways. J Biol Chem. 2004;279:11657–11663. doi: 10.1074/jbc.M312102200. [DOI] [PubMed] [Google Scholar]
- 40.Quillard T, Coupel S, Coulon F, et al. Impaired Notch4 activity elicits endothelial cell activation and apoptosis: implication for transplant arteriosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2258–2265. doi: 10.1161/ATVBAHA.108.174995. [DOI] [PubMed] [Google Scholar]
- 41.Rostama B, Peterson SM, Vary CP, et al. Notch signal integration in the vasculature during remodeling. Vasc Pharmacol. 2014;63:97–104. doi: 10.1016/j.vph.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walshe TE, Connell P, Cryan L, et al. Microvascular retinal endothelial and pericyte cell apoptosis in vitro: role of hedgehog and Notch signaling. Invest Ophthalmol Vis Sci. 2011;52:4472–4483. doi: 10.1167/iovs.10-7061. [DOI] [PubMed] [Google Scholar]
- 43.Shang Y, Smith S, Hu X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell. 2016;7:159–174. doi: 10.1007/s13238-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kassner N, Krueger M, Yagita H, et al. Cutting edge: plasmacytoid dendritic cells induce IL-10 production in T cells via the Delta-like-4/Notch axis. J Immunol. 2010;184:550–554. doi: 10.4049/jimmunol.0903152. [DOI] [PubMed] [Google Scholar]
- 45.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Fung E, Tang SM, Canner JP, et al. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115:2948–2956. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 47.Aoyama T, Takeshita K, Kikuchi R, et al. gamma-Secretase inhibitor reduces diet-induced atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2009;383:216–221. doi: 10.1016/j.bbrc.2009.03.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukuda D, Aikawa E, Swirski FK, et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc Natl Acad Sci U S A. 2012;109:E1868–E1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49■.Koga J, Nakano T, Dahlman JE, et al. Macrophage Notch ligand delta-like 4 promotes vein graft lesion development: implications for the treatment of vein graft failure. Arterioscler Thromb Vasc Biol. 2015;35:2343–2353. doi: 10.1161/ATVBAHA.115.305516. [In a model of vein graft lesion in LDL-null mice, the authors used two approaches to block Notch activity (Dll4 blocking antibodies or cell-specific targeted siRNA). As they have previously observed in murine atherosclerosis models, blocking Dll4 in macrophages attenuated lesion formation and inflammatory macrophages accumulation. In contract, the use of Dll4 siRNA encapsulated in endothelial cell-targeted nanoparticles had no beneficial effects.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briot A, Jaroszewicz A, Warren CM, et al. Repression of Sox9 by Jag1 is continuously required to suppress the default chondrogenic fate of vascular smooth muscle cells. Dev Cell. 2014;31:707–721. doi: 10.1016/j.devcel.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gale NW, Dominguez MG, Noguera I, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Limbourg A, Ploom M, Elligsen D, et al. Notch ligand delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007;100:363–371. doi: 10.1161/01.RES.0000258174.77370.2c. [DOI] [PubMed] [Google Scholar]
- 53.Sainson RC, Johnston DA, Chu HC, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borggrefe T, Liefke R. Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle. 2012;11:264–276. doi: 10.4161/cc.11.2.18995. [DOI] [PubMed] [Google Scholar]
- 55.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 56■■.Briot A, Civelek M, Seki A, et al. Endothelial NOTCH1 is suppressed by && circulating lipids and antagonizes inflammation during atherosclerosis. J Exp Med. 2015;212:2147–2163. doi: 10.1084/jem.20150603. [In this study, the authors reported that endothelial Notch1 expression and signaling was rapidly suppressed by atherogenic stimuli such as oxidized phospholipids in human and high-fat diet in mice. Repression of Notch1 participated to the activation of the endothelium, favored binding of inflammatory cells –an event that contributed to the early phases of atherosclerosis. These findings also suggested a role for Notch1 as a signaling hub between lipids by-products and the endothelial cell.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quillard T, Devalliere J, Coupel S, et al. Inflammation dysregulates Notch signaling in endothelial cells: implication of Notch2 and Notch4 to endothelial dysfunction. Biochem Pharmacol. 2010;80:2032–2041. doi: 10.1016/j.bcp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Zhang H, Rodriguez S, et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-κB-dependent manner. Cell Stem Cell. 2014;15:51–65. doi: 10.1016/j.stem.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jahnsen ED, Trindade A, Zaun HC, et al. Notch1 is pan-endothelial at the onset of flow and regulated by flow. PLoS One. 2015;10:e0122622. doi: 10.1371/journal.pone.0122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masumura T, Yamamoto K, Shimizu N, et al. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler Thromb Vasc Biol. 2009;29:2125–2131. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
- 61.Sivarapatna A, Ghaedi M, Le AV, et al. Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor. Biomaterials. 2015;53:621–633. doi: 10.1016/j.biomaterials.2015.02.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tu J, Li Y, Hu Z. Notch1 and 4 signaling responds to an increasing vascular wall shear stress in a rat model of arteriovenous malformations. Biomed Res Int. 2014;2014:368082. doi: 10.1155/2014/368082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White MP, Theodoris CV, Liu L, et al. NOTCH1 regulates matrix gla protein and calcification gene networks in human valve endothelium. J Mol Cell Cardiol. 2015;84:13–23. doi: 10.1016/j.yjmcc.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Godby R, Munjal C, Opoka A, et al. Cross talk between NOTCH signaling and biomechanics in human aortic valve disease pathogenesis. J Cardiovasc Dev Dis. 2014;1:237. doi: 10.3390/jcdd1030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schober A, Nazari-Jahantigh M, Wei Y, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66■■.Theodoris CV, Li M, White MP, et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160:1072–1086. doi: 10.1016/j.cell.2015.02.035. [Using induced pluripotent stem cell-derived endothelial cells from individuals with heterozygous mutations in NOTCH1, the authors demonstrated that the protective effect of shear stress (antiosteogenic, anti-inflammatory) were mediated by NOTCH1. Haploinsufficiency of NOTCH1 led to the dysregulation of numerous genes through epigenetics modifications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clement N, Gueguen M, Glorian M, et al. Notch3 and IL-1beta exert opposing effects on a vascular smooth muscle cell inflammatory pathway in which NF-kappaB drives crosstalk. J Cell Sci. 2007;120:3352–3361. doi: 10.1242/jcs.007872. [DOI] [PubMed] [Google Scholar]
- 69.Keuylian Z, de Baaij JH, Gueguen M, et al. The Notch pathway attenuates interleukin 1β (IL1β)-mediated induction of adenylyl cyclase 8 (AC8) expression during vascular smooth muscle cell (VSMC) trans-differentiation. J Biol Chem. 2012;287:24978–24989. doi: 10.1074/jbc.M111.292516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70■.Neumann K, Rudolph C, Neumann C, et al. Liver sinusoidal endothelial cells & induce immunosuppressive IL-10-producing Th1 cells via the Notch pathway. Eur J Immunol. 2015;45:2008–2016. doi: 10.1002/eji.201445346. [In this study, the authors explored the interaction of liver sinusoidal endothelial cells and inflammatory Th-1 lymphocytes. They showed that activation of Notch in inflammatory lymphocytes by ligand expression on liver sinusoidal endothelial cells induced the expression of the anti-inflammatory cytokine IL-10 and proposed that heterotypic activation of the pathway might thus limit liver inflammation.] [DOI] [PubMed] [Google Scholar]
- 71.Outtz HH, Tattersall IW, Kofler NM, et al. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118:3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72■.Tattersall IW, Du J, Cong Z, et al. In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment. Angiogenesis. 2016;19:201–215. doi: 10.1007/s10456-016-9501-1. [In this study, the authors combined endothelial cells, macrophages, and pericytes in an in vitro sprouting assay to study molecular interactions between endothelial cells and their cellular microenvironment. In particular they showed that Notch signaling contributed to the heterotypic interaction between the three cell types.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.High FA, Lu MM, Pear WS, et al. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Global Lipids Genetics Consortium. Willer CJ, Schmidt EM, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee S, Springstead JR, Parks BW, et al. Metalloproteinase processing of HBEGF is a proximal event in the response of human aortic endothelial cells to oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2012;32:1246–1254. doi: 10.1161/ATVBAHA.111.241257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He W, Hu J, Xia Y, et al. β-site amyloid precursor protein cleaving enzyme 1(BACE1) regulates Notch signaling by controlling the cleavage of Jagged 1 (Jag1) and Jagged 2 (Jag2) proteins. J Biol Chem. 2014;289:20630–20637. doi: 10.1074/jbc.M114.579862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D'Souza B, Meloty-Kapella L, Weinmaster G. Canonical and noncanonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fraering PC, Ye W, Strub JM, et al. Purification and characterization of the human gamma-secretase complex. Biochemistry. 2004;43:9774–9789. doi: 10.1021/bi0494976. [DOI] [PubMed] [Google Scholar]
- 80.Osenkowski P, Ye W, Wang R, et al. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wahrle S, Das P, Nyborg AC, et al. Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- 82.Wrigley JD, Schurov I, Nunn EJ, et al. Functional overexpression of gamma-secretase reveals protease-independent trafficking functions and a critical role of lipids for protease activity. J Biol Chem. 2005;280:12523–12535. doi: 10.1074/jbc.M413086200. [DOI] [PubMed] [Google Scholar]
- 83.Levitan I, Shentu TP. Impact of oxLDL on cholesterol-rich membrane rafts. J Lipids. 2011;2011:730209. doi: 10.1155/2011/730209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beloribi S, Ristorcelli E, Breuzard G, et al. Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 cells. PLoS One. 2012;7:e47480. doi: 10.1371/journal.pone.0047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin J, Huang F, Yi Y, et al. EGCG attenuates atherosclerosis through the Jagged-1/Notch pathway. Int J Mol Med. 2016;37:398–406. doi: 10.3892/ijmm.2015.2422. [DOI] [PubMed] [Google Scholar]
- 86.Chillakuri CR, Sheppard D, Ilagan MX, et al. Structural analysis uncovers lipid-binding properties of Notch ligands. Cell Rep. 2013;5:861–867. doi: 10.1016/j.celrep.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirata N, Yamada S, Shoda T, et al. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nat Commun. 2014;5:4806. doi: 10.1038/ncomms5806. [DOI] [PubMed] [Google Scholar]
- 88.Levkau B. HDL-S1P: cardiovascular functions, disease-associated alterations, and therapeutic applications. Front Pharmacol. 2015;6:243. doi: 10.3389/fphar.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89■■.Galvani S, Sanson M, Blaho VA, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8:ra79. doi: 10.1126/scisignal.aaa2581. [Using in vitro and in vivo models, the authors identified a mechanism by which the bioactive sphingolipid S1P present in HDL protects against inflammatory activation of the arterial endothelium. They reported that subcellular localization of endothelial S1P1 was modulated along the length of the aorta depending of hemodynamic shear stress and that changes in S1pr1 expression impacted atherosclerosis development. In their models HDL-S1P favors the maintenance of S1P1 at the plasma membrane where it interacted with β-arrestin 2 to inhibit cytokine-induced inflammatory response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90■■.Hachisuga M, Oki S, Kitajima K, et al. Hyperglycemia impairs left-right axis formation and thereby disturbs heart morphogenesis in mouse embryos. Proc Natl Acad Sci U S A. 2015;112:E5300–E5307. doi: 10.1073/pnas.1504529112. [In this study, the authors linked Notch signaling and glucose levels to the progression of congenital heart defects associated with pregestational diabetes. They showed that Notch pathway is repressed by high-glucose levels in the developing heart, which impede the expression of left–right axis determinant genes. These findings shed light on pathogenic mechanisms involved in cardiac malformation associated with hyperglycemia and subsequent deregulation of Notch signaling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chi C, Ronai D, Than MT, et al. Nucleotide levels regulate germline proliferation through modulating GLP-1/Notch signaling in C. elegans. Genes Dev. 2016;30:307–320. doi: 10.1101/gad.275107.115. [Studying C. elegans, the authors identified a novel nucleotide-sensing mechanisms involving GLP-1/Notch signaling to control germline proliferation. The authors proposed that modulation of GLP-1/Notch expression and signaling in response Uridine/Thymidine levels might be essential for the adaptation of the organism to nutrient availability.] [DOI] [PMC free article] [PubMed] [Google Scholar]