Abstract
Background
Published appropriate use criteria (AUC) for Mohs micrographic surgery (MMS) for melanoma are based on consensus opinion.
Objective
To evaluate whether published AUC identify melanomas for which MMS may benefit patients by detecting subclinical spread or confirming clear microscopic margins prior to flap or graft reconstruction.
Materials and methods
Retrospective cohort study of 591 melanomas in 556 patients evaluating the correlation between current AUC (anatomic location, recurrent status, and tumor stage) and subclinical spread or reconstruction with a flap or graft.
Results
Anatomic location on the head, neck, genitalia, hands, feet, or pretibial leg was significantly associated with a higher frequency of subclinical spread (OR 1.89, p=0.0280) and flap or graft reconstruction (OR 10.3, p=0.0001). Compared to primary lesions, recurrent melanomas had a higher frequency of subclinical spread (OR 1.78, p=0.0104) and reconstruction with a flap or graft (OR 1.67, p=0.0217). The frequencies of subclinical spread and flap or graft reconstruction did not differ between in situ and invasive melanomas.
Conclusion
Anatomic location and recurrent status are useful criteria to identify melanomas that may benefit from MMS. Tumor stage is not a useful criterion, as MMS has similar benefits for subsets of both invasive and in situ melanomas.
Introduction
Based on the consensus opinion of expert dermatologists from the American Academy of Dermatology, the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery, MMS is considered to be appropriate for primary melanoma in situ and lentigo maligna located on the head and neck, acral sites, genitalia, and pretibial leg and for locally recurrent melanoma in situ or lentigo maligna in any location. The guidelines do not comment on invasive melanomas.(1) AUC are useful if they identify melanomas that are at increased risk for suboptimal outcomes with conventional excision and that will benefit from superior outcomes by being treated with MMS.
Suboptimal outcomes after conventional excision may result when melanomas have indistinct clinical margins or reconstruction is performed prior to confirmation of clear margins. Melanomas with indistinct clinical margins are at increased risk for local recurrence or positive pathologic margins with conventional excision.(2, 3) If reconstruction is performed immediately after conventional excision and prior to confirming clear margins, patients may require additional excisions and more complex reconstruction procedures.
Mohs micrographic surgery (MMS) with melanoma antigen recognized by T cells 1 (MART-1) immunostaining addresses these practical challenges.(4) Complete peripheral and deep microscopic margin evaluation of the sections of the Mohs layers aided by immunostains assures local clearance of more than 99% of tumors and allows for immediate reconstruction in a reliably tumor-free skin.(4–7)
This retrospective study of a large cohort of both in situ and invasive melanomas treated with MMS evaluates the correlation between published AUC (anatomic location, recurrent status, and tumor stage) and the frequency of subclinical spread (a surrogate for indistinct clinical margins) or reconstruction with a flap or graft. These data may help to refine AUC and treatment guidelines for surgery of melanoma.
Methods
Experimental Design
Institutional review board (IRB) approval was obtained prior to initiating this retrospective cohort study. Five hundred and ninety-one consecutive primary or locally recurrent cutaneous melanomas without clinical evidence of in-transit, regional, or distant metastasis at the time of surgery treated at the University of Pennsylvania with MMS aided by MART-1 immunostaining from March 2006 to September 2012 were included from a prospectively updated MMS database (see Figure 1).
Figure 1.
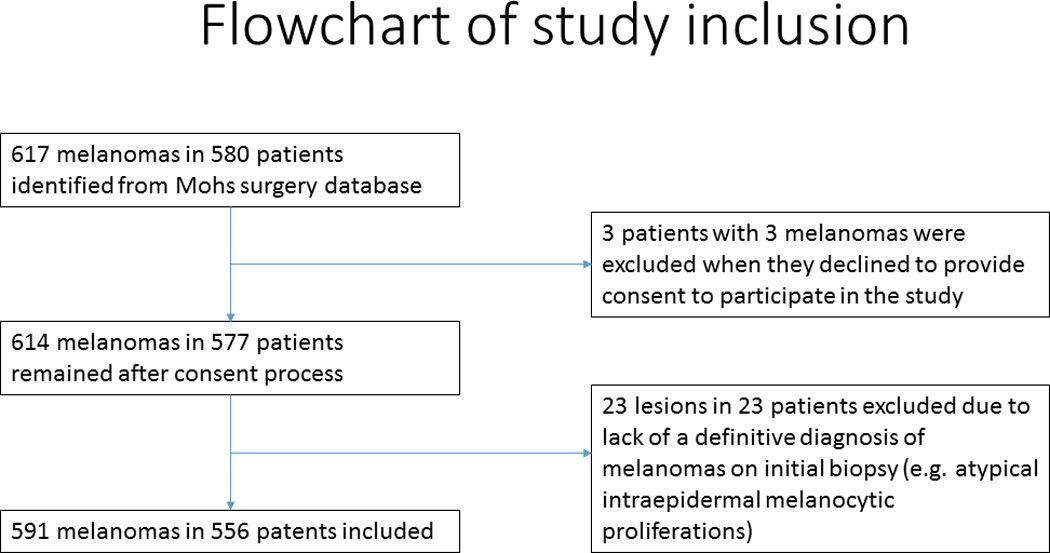
Flowchart of study inclusion
Data for all tumors had been prospectively entered at the time of MMS in an electronic database that includes the following: demographics, preoperative diagnosis and tumor stage (based on original biopsy), postoperative diagnosis and tumor stage (based on pathologic review of the entire tumor), tumor location, number of stages of MMS, and type of reconstruction. All data were verified by a search through the electronic and/or physical medical records. All diagnoses were verified by examination of the biopsy reports from both the preoperative diagnostic biopsy and from the formalin-fixed paraffin-embedded (FFPE) sections of the debulking excision or any other sections with invasive disease removed at the time of MMS. Only lesions with a definitive preoperative diagnosis of either in situ or invasive melanoma were included in this retrospective study. Melanocytic tumors were excluded if they had an indeterminate preoperative diagnosis, such as atypical intraepidermal melanocytic proliferation.
Surgical Procedure
All patients were treated under local anesthesia with a similar protocol, which has been previously described and illustrated.(4) A few key points merit emphasis. The clinically visible melanoma was first removed with a debulking excision, which was sent for FFPE breadloaf sections for purposes of tissue archiving and confirmation of pathologic staging. The Mohs layer was then excised for immediate complete peripheral and deep microscopic margin evaluation with frozen section hematoxylin and eosin stains and MART-1 immunostains. The peripheral margin of the Mohs excision was a minimum of 5–6 mm from the clinically visible edge of the melanoma for nearly all cases, and a 1 cm margin was excised whenever possible for melanomas with a tumor stage of T1b or greater.
The Mohs surgeon immediately reconstructed most wounds after confirming clear margin status. The few cases reconstructed by other surgical subspecialists were usually sent at the request of the referring surgical subspecialist and did not necessarily represent increased complexity of the case.
Data Analysis
The following outcomes were assessed for each melanoma: presence of subclinical spread (defined as greater than one stage of MMS required to achieve tumor-free margins) and reconstruction with a flap or graft. Reconstruction data were unavailable for 10 cases, which were all located on the head and neck and were referred to outside physicians for repair. For these 10 cases, reconstruction was classified as not being performed with a flap or graft.
The frequency of subclinical spread or reconstruction with a flap or graft was correlated with anatomic location, recurrent status, and tumor stage. Anatomic location was classified as AUC-appropriate (head and neck, acral sites, genitalia, and pretibial leg) versus AUC-inappropriate (all other locations). Recurrent status was classified as primary (no previous treatment) versus recurrent (previously treated with surgical excision, laser, liquid nitrogen, cautery or curettage, chemical peels, or topical imiquimod). Tumor stage was classified as in situ versus invasive based on the most aggressive tumor noted on pre-operative biopsy(ies). Odds ratios and 95% confidence intervals (CI) are reported. Fisher's exact test was used to calculate a two-tailed p-value, and a p < 0.05 was considered significant.
Results
The characteristics of 591 melanomas in 556 patients were analyzed. 38.7% (229/591) were in female patients, and the mean age at time of treatment was 65.6 years with a standard deviation of 13.1 years. 512 (86.6%) cases were located in AUC-appropriate locations, and 79 (13.4%) were located in AUC-inappropriate locations, 95% CI [83.9%, 89.4%] and [10.6%, 16.1%], respectively. 101 (17.1%) lesions were recurrent (Table 1), and 490 (82.9%) melanomas were primary, 95% CI [14.1%, 20.1%] and [79.9%, 85.9%], respectively. AUC for location or recurrent status were not met in 10.3% (61/591) tumors, 95% CI [7.9%, 12.8%]. Pre-operative tumor stage was in situ in 452 (76.5%) lesions and invasive in 139 (23.5%) lesions, 95% CI [73.1%, 79.9%] and [20.1%, 26.9%], respectively.
Table 1.
Breakdown of prior treatment for lesions classified as recurrent.
| Prior treatment | Number of melanomas |
|---|---|
| Conventional excision* | 75 |
| Liquid nitrogen | 14 |
| Laser | 4 |
| Mohs surgery | 3 |
| Cautery or curettage | 2 |
| Liquid nitrogen, cautery, and chemical peels | 1 |
| Topical imiquimod | 1 |
| Unknown | 1 |
Of the 75 melanomas with a history of prior conventional excision, 18 had undergone multiple attempts at conventional excision prior to Mohs surgery.
Subclinical spread
Subclinical spread was present in 32.5% (192/591) of tumors, 95% CI [28.7%, 36.3%]. Subclinical spread was statistically significantly more likely for melanomas that were in AUC-appropriate anatomic locations or that had recurred after previous treatment (Table 2). The likelihood of subclinical spread was similar for in situ and invasive melanomas (Table 2).
Table 2.
The frequency of and odds ratios for subclinical spread relative to anatomic location, recurrent status, and tumor stage.
| Appropriate use criteria | Frequency of subclinical spread |
Odds ratio [95% CI] |
P value | |
|---|---|---|---|---|
| Anatomic location | H or M* | 34.2% (175/512) | 1.89 [1.07, 3.34] | 0.0280 |
| L** | 21.5% (17/79) | 1 (ref) | ||
| Recurrent status | Recurrent | 43.6% (44/101) | 1.78 [1.15,1.76] | 0.0104 |
| Primary | 30.2% (148/490) | 1 (ref) | ||
| Tumor stage | In situ | 31.6% (143/452) | 0.85 [0.57, 1.27] | 0.4687 |
| Invasive | 35.3% (49/139) | 1 (ref) | ||
H or M locations are the head and neck, acral sites, genitalia, and pretibial leg.
L locations are all other anatomic sites. ref = reference variable for calculation of odds ratios.
Frequency of reconstruction with a flap or graft
Flap or graft reconstruction was utilized in 47.9% (283/591) of tumors, 95% CI [43.9%, 51.9%]. Flap or graft reconstruction was statistically significantly more likely for melanomas that were in AUC-appropriate anatomic locations or that had recurred after previous treatment (Table 3). The frequency of reconstruction with a flap or graft was similar for in situ and invasive melanomas (Table 3).
Table 3.
The frequency of and odds ratios for flap or graft reconstruction relative to anatomic location, recurrent status, and tumor stage.
| Appropriate use criteria | Frequency of flap or graft reconstruction |
Odds ratio [95% CI] |
P value | |
|---|---|---|---|---|
| Anatomic location |
H or M* | 53.7% (275/512) | 10.3 [4.86, 21.8] | 0.0001 |
| L** | 10.1% (8/79) | 1 (ref) | ||
| Recurrent status | Recurrent | 58.4% (59/101) | 1.67 [1.08, 2.57] | 0.0217 |
| Primary | 45.7% (224/490) | 1 (ref) | ||
| Tumor stage | In situ | 48.0% (217/452) | 1.02 [0.70, 1.49] | 0.9230 |
| Invasive | 47.5% (66/139) | 1 (ref) | ||
H or M locations are the head and neck, acral sites, genitalia, and pretibial leg.
L locations are all other anatomic sites. ref = reference variable for calculation of odds ratios.
Discussion
This study demonstrates that current AUC(1) of anatomic location and recurrent status, but not tumor stage, identify melanomas that are more likely to have subclinical spread or to require reconstruction with a flap or graft. Compared to conventional excision, MMS offers the advantages of immediate detection and removal of subclinical spread and confirmation of clear margins prior to flap or graft reconstruction.(4)
“Subclinical spread” describes the microscopic extension of tumor beyond the clinically visible margins. MMS is especially useful to treat tumors with subclinical spread, since conventional surgery poses a higher risk for positive pathologic margins.(8). 32.5% (192/591) of tumors in our cohort exhibited subclinical spread, measured by the requirement for more than one stage of MMS. This rate is comparable to previous cohorts of MMS for melanoma.(5, 7, 9–12) Subclinical spread was statistically significantly more likely for melanomas in AUC-appropriate anatomic locations and for recurrent melanomas (Table 2), supporting the use of MMS for lesions located on the head and neck, acral sites, genitalia, and pretibial leg and for recurrent melanomas in any anatomic location. The likelihood of subclinical spread was similar for in situ (31.6%) and invasive melanomas (35.3%), indicating that MMS is useful to detect and remove subclinical spread for both invasive and in situ melanomas (Table 2).
Melanomas requiring flap or graft reconstruction present a particular challenge for conventional surgery. If a positive margin is detected after performing flap or graft reconstruction, subsequent excision, pathologic margin assessment, and reconstruction are often more challenging. Anatomic location correlated strongly with the likelihood of reconstructing with a flap or graft. More than half (53.7%) of melanomas located on the head and neck, acral sites, genitalia, and pretibial leg required reconstruction with a flap and/or graft, compared to only 10.1% of melanomas located on the trunk and proximal extremities (p = 0.0001).
Compared to primary melanomas, recurrent lesions were statistically significantly more likely to require flap or graft reconstruction (Table 3). 17.1% of the melanomas in our cohort had recurred after previous treatment. Our data suggest that these patients may have benefited from simpler reconstruction procedures of their primary melanomas. Patients place high value on restoring a normal appearance after reconstruction of facial defects,(13–15) so optimizing outcomes by confirming clear margins prior to reconstruction is especially important for melanomas in cosmetically or functionally sensitive locations. The incidence of flap or graft reconstruction did not vary between melanoma in situ (48.0%) and invasive melanoma (47.5%) [p = 0.9230].
Our study has limitations. Our clinic has a referral bias for large or ill-defined melanomas, and the frequency of melanomas in AUC-appropriate locations was higher than the frequency seen in the general population,(16) so our findings may not be generalizable for all melanomas. We have previously published our criteria for taking additional stages of MMS;(4) however, since there is no consensus on what constitutes a positive margin, our results may not be generalizable to the practice of other Mohs surgeons.
In summary, current AUC of anatomic location and recurrent status help to identify melanomas that have an increased risk for subclinical spread and that require flap or graft reconstruction. The risk of subclinical spread or reconstruction with a flap or graft does not differ for in situ or invasive melanoma, so consideration should be given to expand AUC to include invasive melanomas. While AUC provide helpful guidelines, clinical judgment remains important to determine the utility of MMS for melanoma. For example, MMS may be useful for some poorly defined primary melanomas on the trunk and proximal extremities, as demonstrated by the fact that 21.5% (17/79) trunk and proximal extremity melanomas in our cohort had subclinical spread. On the other hand, conventional excision may be sufficient for well-demarcated, small melanomas of the head and neck. Future research will be useful to refine AUC and optimize triage of melanomas to MMS versus conventional excision.
Acknowledgments
This work was generously supported by an American Society for Dermatologic Surgery Cutting Edge Research Grant. JFS was supported by a Dermatology Foundation Clinical Career Development Award in Dermatologic Surgery. EYC was supported by a Dermatology Foundation Dermatopathology Research Career Development Award. JMG was supported by NIAMS grant K24AR064310.
Footnotes
No authors involved in the production of this manuscript have any commercial associations that might create or pose a conflict of interest with information presented herein. Such associations include consultancies, stock ownership, or other equity interests, patent licensing arrangements, and payments for conducting or publicizing a study described in the manuscript.
Contributor Information
Jeremy Robert Etzkorn, University of Pennsylvania Health System, Assistant Professor, Department of Dermatology.
Joseph F. Sobanko, University of Pennsylvania Health System, Assistant Professor, Department of Dermatology.
Thuzar M. Shin, University of Pennsylvania Health System, Assistant Professor, Department of Dermatology.
Rosalie Elenitsas, University of Pennsylvania Health System, Professor, Department of Dermatology.
Emily Y. Chu, University of Pennsylvania Health System, Assistant Professor, Department of Dermatology.
Joel M. Gelfand, University of Pennsylvania Health System, Associate Professor, Department of Dermatology.
David J. Margolis, University of Pennsylvania Health System, Professor, Department of Dermatology.
Jason G. Newman, University of Pennsylvania Health System, Associate Professor, Department of Otorhinolaryngology.
Hayley Goldbach, University of Pennsylvania Health System, Intern, Department of Pediatrics.
Christopher J. Miller, University of Pennsylvania Health System, Assistant Professor, Department of Dermatology.
References
- 1.Ad Hoc Task F, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531–550. doi: 10.1016/j.jaad.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Christophel JJ, Johnson AK, McMurry TL, Park SS, et al. Predicting positive margins in resection of cutaneous melanoma of the head and neck. Laryngoscope. 2013;123:683–688. doi: 10.1002/lary.23799. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SR, Scott JR, Cole JK, Chi Y, et al. Head and neck malignant melanoma: margin status and immediate reconstruction. Ann Plast Surg. 2009;62:144–148. doi: 10.1097/SAP.0b013e31817dadc8. [DOI] [PubMed] [Google Scholar]
- 4.Etzkorn JR, Sobanko JF, Elenitsas R, Newman JG, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: Tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840–850. doi: 10.1016/j.jaad.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Bricca GM, Brodland DG, Ren D, Zitelli JA. Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol. 2005;52:92–100. doi: 10.1016/j.jaad.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Newman J, Beal M, Schram SE, Lee PK. Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma using Mel-5 immunostaining: an update from the University of Minnesota. Dermatol Surg. 2013;39:1794–1799. doi: 10.1111/dsu.12356. [DOI] [PubMed] [Google Scholar]
- 7.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012;66:438–444. doi: 10.1016/j.jaad.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Berdahl JP, Pockaj BA, Gray RJ, Casey WJ, et al. Optimal management and challenges in treatment of upper facial melanoma. Ann Plast Surg. 2006;57:616–620. doi: 10.1097/01.sap.0000235429.28182.f6. [DOI] [PubMed] [Google Scholar]
- 9.Zalla MJ, Lim KK, Dicaudo DJ, Gagnot MM. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg. 2000;26:771–784. doi: 10.1046/j.1524-4725.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 10.Albertini JG, Elston DM, Libow LF, Smith SB, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656–665. doi: 10.1046/j.1524-4725.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- 11.Bienert TN, Trotter MJ, Arlette JP. Treatment of cutaneous melanoma of the face by Mohs micrographic surgery. J Cutan Med Surg. 2003;7:25–30. doi: 10.1007/s10227-002-1161-7. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LM, McCall MW, Zax RH. Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. A follow-up study. Dermatol Surg. 1998;24:673–677. doi: 10.1111/j.1524-4725.1998.tb04226.x. [DOI] [PubMed] [Google Scholar]
- 13.Chuang GS, Leach BC, Wheless L, Lang PG, et al. Preoperative expectations and values of patients undergoing Mohs micrographic surgery. Dermatol Surg. 2011;37:311–319. doi: 10.1111/j.1524-4725.2011.01878.x. [DOI] [PubMed] [Google Scholar]
- 14.Borah GL, Rankin MK. Appearance is a function of the face. Plast Reconstr Surg. 2010;125:873–878. doi: 10.1097/PRS.0b013e3181cb613d. [DOI] [PubMed] [Google Scholar]
- 15.Sobanko JF, Sarwer DB, Zvargulis Z, Miller CJ. Importance of physical appearance in patients with skin cancer. Dermatol Surg. 2015;41:183–188. doi: 10.1097/DSS.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 16.Fisher SR. Cutaneous malignant melanoma of the head and neck. Laryngoscope. 1989;99:822–836. doi: 10.1288/00005537-198908000-00010. [DOI] [PubMed] [Google Scholar]


