Abstract
The effects of ethylene oxide (EO), vaporized hydrogen peroxide (VHP), gamma (γ) radiation, and electron-beam (E-beam) on the physiochemical and morphological properties of medical device polymers are investigated. Polymers with ether, carbonate, carboxylic acid, amide and ester functionalities are selected from a family of poly(ethylene glycol) (PEG) containing tyrosine-derived polycarbonates (TyrPCs) to include slow, medium, fast, and ultrafast degrading polymers. Poly(lactic acid) (PLA) is used for comparison. Molecular weight (Mw) of all tested polymers decreases upon gamma and E-beam, and this effect becomes more pronounced at higher PEG content. Gamma sterilization increases the glass transition temperature of polymers with high PEG content. EO esterifies the carboxylic acid groups in desaminotyrosol-tyrosine (DT) and causes significant degradation. VHP causes hydroxylation of the phenyl ring, and hydrolytic degradation. This study signifies the importance of the chemical composition when selecting a sterilization method, and provides suggested conditions for each of the sterilization methods.
Keywords: biodegradable, biomaterials, poly(ethylene glycol), sterilization, structure–property relations
Graphical Abstract
1. Introduction
The most widely used biodegradable polymers in medical applications are poly(hydroxy acid)s such as poly(lactic acid) (PLA), poly(d-lactic acid) (PDLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), and poly(caprolactone) (PCL). Poly(ethylene glycol) (PEG) containing polymers are also widely used.[1,2] Recently, several medical devices were commercialized that incorporate derivatives of the natural amino acid l-tyrosine.[3–5] However, unlike devices made from metals and ceramics, devices made from polymers can undergo physicochemical changes during sterilization that could compromise their clinical performance. Materials and devices intended for in vivo applications are either aseptically processed or terminally sterilized before use to avoid any implant-related infection. Aseptic processing of medical devices is rarely practical or economical.[6] Therefore, terminal sterilization, where a device is sterilized within its final sterile barrier package, is preferred for medical device sterilization.[6] Clinical success of polymeric biomaterials thus depends on the ability of the polymers to be sterilized while retaining their essential physical and chemical characteristics.
In the recent years, various terminal sterilization techniques have been explored to study the effects of sterilization on polymers for biomedical applications. Chemical-based (vaporized hydrogen peroxide (VHP) and ethylene oxide (EO)) and radiation-based sterilization methods (gamma and electron-beam (E-beam)) are the most commonly used sterilization tools for a wide variety of polymers and polymeric scaffolds. For example, the effects of EO, gamma, and E-beam sterilizations have been investigated for electrospun PLA fiber membranes,[7] silk fibroin sponges,[8] PCL nerve conduits,[9] cyclodextrin-hexamethyldiisocynate crosslinked polymers[10] and most recently on a proprietary hydrogel (Cyborgel).[11] VHP is a low-temperature alternative to EO and has mostly been used for sterilizing polyurethane scaffolds,[12] polyethylene (LDPE) tubing, polyvinylchloride (PVC) tubing, and PVC films.[13]
Typically, in the hospitals, steam sterilization or autoclave is used for sterilizing surgical instruments and medical equipment that are not heat sensitive. For heat-sensitive medical devices and instruments made from polymers, historically hospitals have relied on EO-based sterilization systems. However, due to its environmental and safety concerns, VHP sterilization (Sterrad) is filling the gap between steam sterilization and EO.[14] Both radiation-based (E-beam and gamma) and chemical-based (VHP and EO) terminal sterilization methods are effective in sterilizing biomaterials. However, each of these methods is known to affect the physicochemical properties of biomaterials. For instance, gamma radiation causes chain scission in PLA, PGA, and PLGA, which decreases the molecular weight and adversely affects the mechanical properties of the polymers.[15–18] The chain scission process occurs due to free radical formation. These free radicals can break the polymer chains, and can result in the deterioration of the polymer’s mechanical properties. Depending on the dose of radiation and the chemical structure of the polymers, gamma irradiation can also cause crosslinking of the polymer chains, which generally results in increased tensile strength.[18,19] E-beam radiation, like gamma, also causes molecular weight degradation and crosslinking in the polymers.[18] While E-beam radiation is generally regarded as less damaging than gamma radiation since it does not penetrate materials as deeply as gamma radiation, E-beam radiation may not be suitable for thicker or denser materials.[20]
Chemical sterilization using EO and VHP may be suitable for polymers that can withstand a short exposure to slightly elevated temperatures under moist conditions. But, both of these chemical sterilization techniques have potential disadvantages. Ethylene oxide sterilization can leave harmful residues in the polymer and its packaging.[15,20] This requires the inclusion of a lengthy aeration phase, which must be part of any validated EO sterilization process. EO exposure can also have a dramatic effect on the morphology of the polymeric implants. For example, Holy et al. found that under EO sterilization, PLGA scaffolds shrank to 60% of their initial volume.[21] Phillip et al. also reported that exposing electrospun mats and porous scaffolds fabricated from fast degrading tyrosine-derived polycarbonates (TyrPCs) to high doses of EO can cause the electrospun fiber mats to fuse together and the pores of porous scaffolds to collapse.[22] These detrimental effects can potentially be circumvented with VHP, which was not found to alter the morphology of porous scaffolds.[23] A study comparing the morphology of PLGA scaffolds sterilized by EO and VHP showed that VHP-sterilized PLGA scaffolds had a smoother appearance than the EO-sterilized PLGA scaffolds. However, VHP is an oxidizing agent that increases the concentration of free radicals on polymer surfaces, which in turn can have a profound effect on polymer properties.[16] Thus, currently there is no generally applicable method for the sterilization of medical implants made of degradable polymers.
The goal of this work was to evaluate four common industry sterilization techniques (EO, VHP, gamma radiation, and E-beam) by measuring their effect on the physicochemical properties of a series of bioresorbable model polymers with varying degradation profiles and chemical compositions. Each of the tested sterilization modalities has associated processing parameters such as temperature, relative humidity, amount of sterilization agent, radiation dose, time, and post-sterilization treatment. We selected three parameter sets for each modality (based on commonly used industrial sterilization conditions), giving them generalized classifications of mild, medium, and severe. In EO, increasing the level of severity corresponds to increasing temperature and relative humidity. For VHP, the severity of the sterilization conditions from mild to medium to severe corresponds to the increased dwell time for VHP. In E-beam and gamma, severity increases with increasing radiation dose. In general, the mild condition is expected to have a minimal impact on the physiochemical and morphological properties and the severe condition is expected to have a maximal impact. Medium conditions are expected to show effects between these two extremes. It is noted that no claim is made to the efficacy of any of these conditions to reduce or eliminate the microbial bioburden; only an assessment of changes in the physicochemical and morphological properties of the test polymers is described. Furthermore, it is required by any medical device manufacturer to validate a terminal sterilization process in accordance with applicable national and international standards and guidelines.
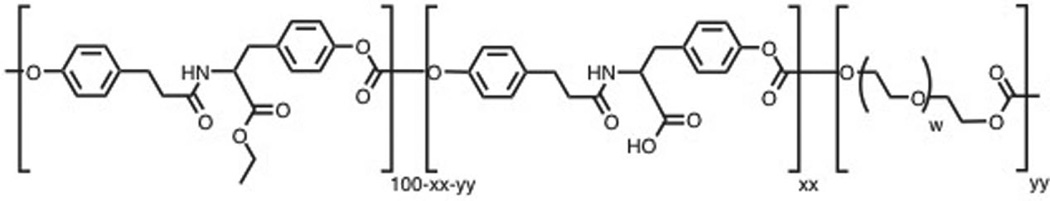
Here, we studied a family of tyrosine-derived poly(DTE-co-DT-co -PEG carbonate)s (TyrPCs), which are random terpolymers consisting of desaminotyrosyl-tyrosine ethyl ester (DTE), desaminotyrosyl-tyrosine (DT), and blocks of low molecular weight poly(ethylene glycol) (PEG) (Figure 1). We selected TyrPCs as model polymers since they contain carbonate, ester, amide, and ether bonds within their structure, which represent the most prevalent backbone bonds present in degradable polymers. This may enable other researchers to apply the results obtained from this work to numerous other polymers with similar functionalities. Furthermore, the presence of PEG in our TyrPC model polymers makes the findings of this study applicable to other widely used biodegradable PEG-containing polymers.
Figure 1.
Poly(DTE-co-DT-co-PEG carbonate) is abbreviated as Exxyy(nk). DTE: desaminotyrosyl-tyrosine ethyl ester; DT: desaminotyrosyl tyrosine, present at xx mol%; and PEG: polyethylene glycol) of molecular weight nk (n is an integer and k = 1000 Da) present at yy mol%. For example, in E1001(1k): xx is 10% DT and yy is 1% PEG (1k).
TyrPCs exhibit a wide range of mechanical properties (from soft to hard), and degradation times (from resorbing within a few hours to being stable for over 4 years) depending on their composition. Previously published reports describe the successful use of these TyrPCs in the fabrication of scaffolds for bone regeneration,[24] pins for the stabilization of bone fractures,[25] cardiovascular stents,[25] nerve guidance tubes for peripheral nerve regeneration,[26,27] electrospun fiber mats for wound healing,[28] and ultrafast degrading coating materials for neural probes.[29] PLA, which is widely used in the manufacture of sutures, orthopedic fixation devices, dental implants, tissue staples and skin covering devices,[1] was also included in our study.
2. Experimental Section
2.1. Polymers
TyrPCs with the chemical structure poly((100-xx-yy)%DTE-co-xx%DT-co-yy%PEG carbonate) (Figure 1) were synthesized using previously published procedures.[30] Brief y, the DTE and desaminotyrosyl-tyrosine tert -butyl ester (DTtBu) were copolymerized with a predetermined molar equivalent of PEG under anhydrous conditions using bis(trichloromethyl) carbonate (triphosgene), followed by selective and quantitative removal of the tert -butyl ester protecting groups using trifluoroacetic acid (TFA).[30] These terpolymers are identified as Exxyy(nk) where E is the ethyl ester, xx is the mole percent of DT, yy is the mole percent of PEG and nk is the average molecular weight of PEG in kDa (Figure 1).[30] As an example, poly(89%DTE-co-10%DT-co-1%PEG1K carbonate) is designated as E1001(1k). The following TyrPC terpolymers were used in this study: E0000, E1001(1k), E2502(2k) and E5005(2k). In addition, we used commercially-available PLA (NAT 40430; Nature Works, Nebraska, USA).
2.2. Preparation of Polymer Films
2.2.1. Compression Molding
Dry PLA, E0000, and E1001(1k) polymer pellets or powders were molded at Tg + 50 °C using a previously described procedure.[31] Briefly, a pre-weighed amount of polymer (300–400 mg) was placed between stainless steel plates lined with Kapton film (American Durafilm, Holliston, MA) and spaced apart by 250 µm thick custom stainless steel shims. The mold plates were placed between the platens of a Carver Press (model 4122, Carver Inc., Wabash, IN) that were preheated to the molding temperature of Tg + 50 °C. All compression-molded films, ≈250 µm thick, were cut into four quadrants, packaged appropriately for each sterilization condition and stored at −20 °C prior to sterilization.
2.2.2. Solvent Casting
E2502(2k) and E5005(2k) films degrade upon exposure to heat during compression molding and hence were prepared by solvent casting. 1.25 g of E2502(2k) and E5005(2k) polymer was dissolved in 65 mL anhydrous tetrahydrofuran (THF). The solution was filtered through a 0.45 µm pore diameter Teflon syringe filter and poured into a glass mold. All films were dried under ambient conditions for 24 h followed by vacuum drying at 40 °C for 48 h to evaporate the residual solvent. The films were then cut into four quadrants, packaged appropriately for each sterilization condition and finally stored at −20 °C prior to sterilization.
2.3. Sterilization
Polymer films were processed at Johnson and Johnson Sterility Assurance (JJSA) facility following the conditions summarized in Table 1. The samples for radiation treatment (gamma, E-beam) were transferred from −20 °C to ambient conditions, and the EO and VHP samples were first transferred to 4 °C to prevent condensation and then to ambient conditions. The samples were equilibrated at ambient temperatures prior to processing. The chosen sterilization conditions are commonly used in industrial settings and are known to be effective by JJSA.
Table 1.
Sterilization methods and processing conditions.
| Sterilization modality |
General classification |
Temperature [°C] |
Relative humidity [%] |
EO conc. [mg L−1] |
|---|---|---|---|---|
| Ethylene Oxide (100% EO) |
Mild | 35 | 35 | 850 |
| Medium | 54 | 50 | 712 | |
| Severe | 54 | 75 | 597 | |
|
Sterilization modality |
General classification |
VHP [mg L1] |
Dwell time [min] |
Total cycle time [h:m:s] |
| Vaporized hydrogen peroxide |
Mild | 1315.9, 2189.6 | 7.5 + 7.5 | 0:47:03 |
| Medium | 1995.2, 2939.4 | 14.0 + 14.0 | 0:48:59 | |
| Severe | 2963.2, 7167.2 | 22.5 + 22.5 | 1:18:05 | |
| Sterilization modality | General classification | Pre-processing equilibration | Delivered dose range [kGy] | |
| Gamma (γ) | Mild | 20 h @ 21 °C | 14.5–16.0 | |
| Medium | 20 h @ 21 °C | 24.0–26.7 | ||
| Severe | 20 h @ 21 °C | 50.0–54.6 | ||
| Electron beam | Mild | 20 h @ 21 °C | 14.0–14.1 | |
| Medium | 20 h @ 21 °C | 24.0–24.7 | ||
| Severe | 20 h @ 21 °C | 49.7–52.3 | ||
2.3.1. Gamma (γ) Radiation Processing
Gamma sterilization was performed using a cobalt-60 source (MDS Nordion Gamma Cell 220E Research irradiator at JJSA), with a central dose rate of ≈0.003 kGy s−1. The temperature during exposure was controlled via a Vortex Tube cooler (Model 328) and monitored using an Omega Temperature Recorder (Model No. RD-MV112-1-2-1D). The polymer film samples for the gamma irradiation (see Table 1) were centrally positioned in the research irradiator sample chamber along with FWT-60-IP radia-chromic dosimeters (Batch: 1114). Post irradiation, the dosimeters were evaluated using a Genesys 20 spectrophotometer to determine the delivered dose range. The irradiated samples were stored at −20 °C.
2.3.2. E-Beam Radiation Sterilization
E-beam irradiations were conducted in the Mevex 5 MeV, 2 kW electron beam linear accelerator. The beam current (0.40 mA) was held constant, and the conveyor speed was adjusted to deliver the target dose (≈12.5 kGy s−1). The polymer film samples for the E-beam irradiation (Table 1) were placed horizontally in a single layer onto irradiation totes along with B3 DoseStix radiachromic dosimeters (Batch: BE). Postirradiation, the dosimeters were evaluated using a Genesys 20 spectrophotometer to determine the delivered dose range. The irradiated samples were stored at −20 °C.
2.3.3. Vaporized Hydrogen Peroxide Sterilization
Samples were treated with VHP (see Table 1 for processing conditions) in a STERRAD 100NX sterilizer, which uses 59% hydrogen peroxide. The peroxide was concentrated in the sterilizer to 96–98% before injection into the sterilizer chamber. Hydrogen peroxide naturally decomposes over time into water and oxygen. This process reduces the peroxide concentration during the dwell phase. The sterilization cycle was divided into two separate injections and dwell periods (half of the total target time) to ensure adequate exposure of the samples.
2.3.4. Ethylene Oxide Sterilization
Samples were transferred from the refrigerator (4 °C) to ambient temperature one day prior to EO sterilization for preprocessing equilibration. Sterilization (see Table 1 for processing conditions) was carried out in a 75 cubic foot rectangular single-door sterilizer with vacuum drying capacity (Environmental Tectronics Corporation, PA). The film samples were placed in a perforated metal tray, which was transferred to the sterilization cart. Sterilizer chamber pressure was reduced to 3.75 mmHgA (1 mmHgA = 1 Torr), followed by injection with steam to achieve the desired relative humidity, followed by 30 min of moisture conditioning. 100% EO (sterilant) was then injected into the chamber. After EO injection, an inert gas (nitrogen) was introduced into the chamber to bring the pressure to 701 mmHgA. The chamber was held at this pressure for the required EO exposure time. Post EO exposure, the chamber pressure was reduced to 0.04 mmHgA followed by injection of nitrogen to ambient pressure to remove EO.
2.4. Characterization
Processed film samples were examined visually for any changes in their color or morphology. 6 mm diameter disks were punched out of the films (using Precision Brand metric “10” TRU punch, IL, USA). Individual disks were used for nuclear magnetic resonance (NMR), gel permeation chromatography (GPC), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), thermal gravimetric analysis (TGA), and differential scanning calorimetry (DSC). Additionally, films were cut into 5 mm wide, 35–40 mm long strips for mechanical tensile testing. Total of 45 samples (3 per 12 sterilization conditions) were obtained. Control (pre-sterilization) polymer film samples were also prepared in a similar way.
2.4.1. Scanning Electron Microscopy
The effect of sterilization on the surface morphology of the films was examined using SEM (Amray 1830I, acceleration potential of 20 kV). 6 mm diameter disks of unsterilized and sterilized films (TyrPCs and PLA) were mounted on Al studs with an adhesive, sputter coated (SCD 004, 30 milliamps for 120 s with gold/ palladium (Au/Pd)) and SEM images were obtained at 20× and 1000× magnification.
2.4.2. Gel Permeation Chromatography
Molecular weights (weight and number averages) and polydispersity indices (PDI) were measured using GPC (Waters Corporation, Milford, MA) following the previously described procedures.[30–33] Briefly, 6 mm diameter disks were punched from TyrPC film samples, either pre or post-sterilization. Each disk weighing ≈10 mg was dissolved in 1 mL of the mobile phase (DMF + 0.1%TFA), the sample solution was filtered through a 0.45 µm Teflon filter (13 mm diameter, Whatman Inc., Florham Park, NJ), and analyzed at room temperature by GPC consisting of two PL gel columns (103 and 105 Å pore size, 30 cm in length, Polymer Laboratories, Amherst, MA) at a flow rate of 1 mL min−1. PLA films were not evaluated since they do not dissolve in the mobile phase available to us. The molecular weight of all samples was measured in triplicate (n = 3 per sterilization condition) for statistical analysis.
2.4.3. Mechanical Testing
Tensile properties of unsterilized and sterilized polymer (TyrPC and PLA) films (≈5 mm wide × 35–40 mm long strips) were measured at ambient temperature using a Sintech 5/D mechanical tester (MTS Systems Corp.). Testing was performed at a rate of 10 mm min−1, with a pre-load of 0.1 N, using a 100 N load cell (D82942). The tensile modulus (Young’s modulus) was calculated from the slope of the initial linear segment of the stress–strain curve, typically 0.5 to 2%. Stress at yield and % elongation was also recorded. All measurements were taken for an average of three film samples per condition (unsterilized and sterilized) unless otherwise specified.
2.4.4. Thermal Analysis
Polymer glass transition temperature (Tg) was determined using a DSC Model 823e with STARe software (Mettler-Toledo Inc., Columbus, OH). Unsterilized and sterilized polymer films (TyrPCs and PLA) ≈5–10 mg in weight were sealed in an aluminum pan. The samples were heated from room temperature to 225 °C at a rate of 10 °C per minute, and then kept at 225 °C for 1 min to erase the thermal history of the polymer (first heat cycle). The sample was cooled to 15 °C and then heated again from 15 to 225 °C at a rate of 10 °C per minute (second heat cycle). The Tg of the polymers was determined in the second heat cycle as the midpoint (ASTM) of the transition.[34]
The amount of residual solvent in the polymers was measured using a TGA (model TGA/SDTA851e) with STARe software version 19.10 (Mettler-Toledo Inc., Columbus, OH). Unsterilized and sterilized film samples (TyrPC and PLA) ≈5–10 mg in weight were heated from 25 to 225 °C at a rate of 10 °C per minute in an aluminum oxide crucible. Percent mass loss due to water (up to 100 °C) and due to organics (100 to 200 °C) was determined.
2.4.5. Proton Nuclear Magnetic Resonance
The effects of different sterilization modalities on the chemical composition of the polymers were investigated using 1H NMR data. The 1H NMR spectra were obtained using Varian VNMRS 400 or 500 MHz spectrometer (Varian Inc., Palo Alto, CA).[30,31] The 6 mm diameter TyrPC film samples pre and post-sterilization, ≈10 mg, were dissolved in DMSO-d6 and 64 scans were collected at room temperature. The molar ratios of DTR, DT, and PEG were calculated by integrating the respective 1H NMR peaks. PLA films were not analyzed due to their insolubility in DMSO-d6. All samples were analyzed in triplicate (n = 3 per sterilization condition) for statistical analysis.
2.4.6. X-ray Photoelectron Spectroscopy
Surface elemental composition of polymer film samples were examined using X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha), consisting of a monochromatic Al Kα X-ray source with an energy of 1486.6 eV and an angle of incidence of 30°. Measurements were performed with a standard X-ray beam spot of 400 × 400 µm at an electron take off angle of 90 degrees. Survey scans were collected at 0.5 eV resolution, and high-resolution scans were recorded with a step size of 0.1 eV. In order to minimize the build-up of electrical charge during XPS measurements, charge compensation was performed using a dualbeam flood source of low-energy Ar+ ions and 1 eV electrons. Samples were not exposed to X-rays until the start of the measurements to minimize the possibility of polymer degradation.
2.4.7. Statistical Analysis
All data are represented as means ± standard deviations. GraphPad software was used to analyze the statistical variation through ordinary two-way ANOVA with Tukey’s multiple comparisons post-hoc tests at a significant level of p ≤ 0.05.
3. Results and Discussion
3.1. Polymer Selection
In this study, we selected four TyrPC terpolymers (Table 2) as our model polymers to evaluate the influence of various terminal sterilization conditions on their physicochemical properties. These terpolymers, which are composed of two tyrosine-derived monomers and PEG, are non-cytotoxic, biocompatible, and biodegradable. The DTE segments provide mechanical strength while the more hydrophilic DT segments control the rate of polymer resorption. PEG blocks modify the protein adsorption, enhance drug release, water uptake and accelerate polymer degradation and erosion. The mol% of the three components can be varied to obtain polymers with a broad range of resorption rates as shown in Table 2. PLA was used as a reference polymer.
Table 2.
Approximate periods of resorption for all tested polymers.
|
Exxyy [nk] |
DTE [mol%] |
DT [mol%] |
PEG [mol%] Mw in [Da] |
Resorption period |
|---|---|---|---|---|
| E0000 | 100 | 0 | 0 | ≈4 years |
| E1001(1k) | 89 | 10 | 1(1k) | ≈18 months |
| E2502(2k) | 73 | 25 | 2(2k) | ≈4 d |
| E5005(2k) | 45 | 50 | 5(2k) | ≈<4 h |
3.2. Effect of Sterilization on Morphology
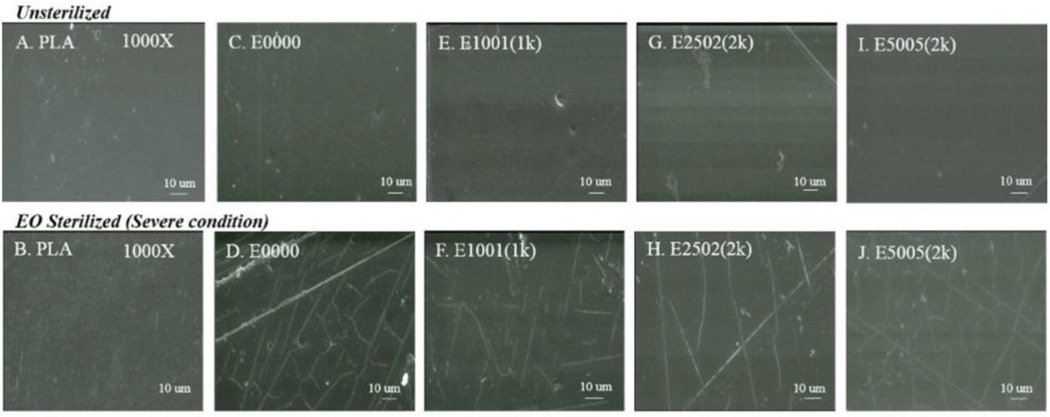
In compression - molded PLA films, none of the tested sterilization protocols caused any obvious change in the visual appearance or the surface morphology by SEM. Likewise, films made of TyrPC polymers showed no obvious changes in visual appearance or surface morphology by SEM after sterilization, except when EO was applied under severe conditions. Only in that case, the sterilized TyrPC films exhibited microscopic surface cracks which were clearly visible by SEM (Figure 2).
Figure 2.
SEM micrographs of A,B) PLA, C,D) E0000, E,F) E1001 (1k), G,H) E2502 (2k), I,J) E5005 (2k) films before sterilization (upper panel) and after EO sterilization under severe condition (lower panel). Surface cracks were observed only on TyrPC films. [Scale bar = 10 µm].
3.3. Effect of Sterilization on Molecular Weight
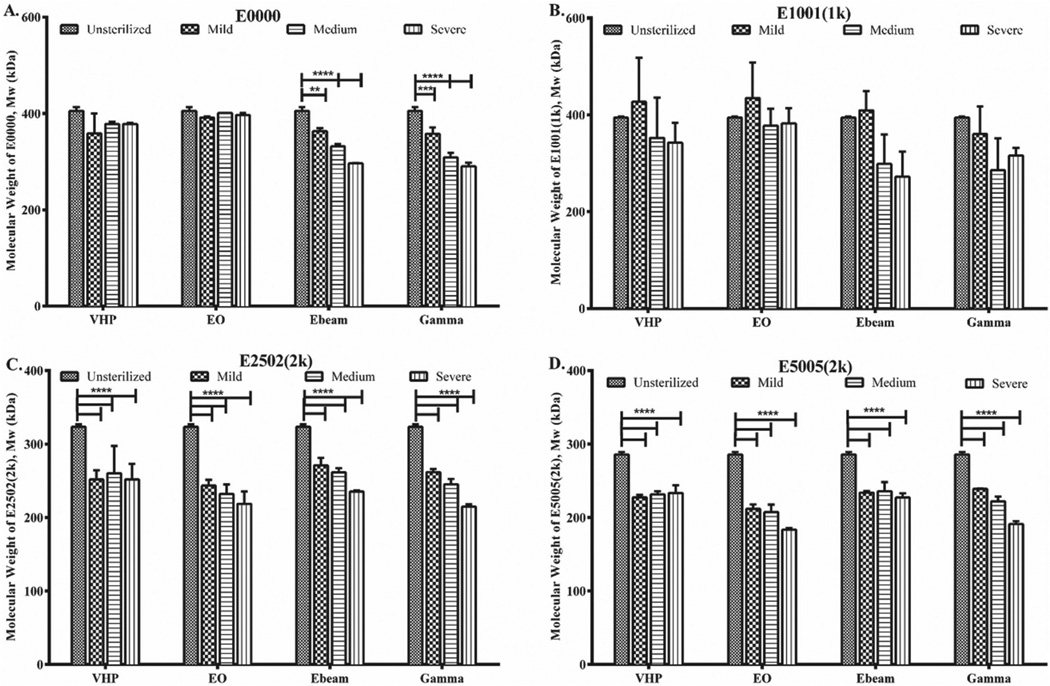
The molecular weight of degradable polymers, particularly TyrPCs, can be sensitive to sterilization conditions because of their hydrolytic degradability. We investigated the effect of various sterilization conditions on the weight average molecular weight (Mw) of slow, medium, fast and ultra-fast degrading TyrPCs (Figure 3).
Figure 3.
Bar graphs showing the changes in Mw of E0000 (slow), E1001(1k) (medium), E2502(2k) (fast), and E5005(2k) (ultrafast degrading) polymers. Data are presented as mean ± SD for n = 3. Significant difference from the unsterilized control is indicated as (** p≤ 0.01, *** p≤ 0.001, **** p ≤ 0.0001).
The slow degrading TyrPC (E0000) shows no change in the Mw upon VHP and EO sterilization conditions. E-beam irradiation, however, decreases the Mw of E0000 by 10, 18, and 27% in mild, medium, and severe conditions, respectively. Similarly, gamma irradiation also decreases the Mw of E0000 by 12, 24, and 29% in mild, medium, and severe conditions, respectively. As the content of DT increases, the changes in Mw also increase. Mw of E1001(1k), a medium degrading polymer with 10 mol% DT, is more susceptible to medium and severe conditions of E-beam and gamma irradiation. Hence, there is a decrease in the Mw of E1001(1k) upon E-beam irradiation (mild: 0%; medium: 24% and severe: 31% Mw loss) and gamma irradiation (mild: 9%; medium: 28%; severe: 30% Mw loss). Although the Mw of E1001(1k) remains unaffected by EO, upon VHP sterilization, its Mw decreases by 0 to 13% under mild to severe conditions.
The Mw of E2502(2k) (fast degrading) and E5005(2k) (ultra-fast degrading) polymers decreases upon all the sterilization methods. Under EO, the Mw loss measured from mild to severe condition was 25 to 32% for E2502(2k) and 26 to 36% for E5005(2k). Upon VHP sterilization, the Mw of E2502(2k) and E5005(2k) decreased by ≈21% but did not change from mild to severe conditions. Gamma and E-beam severe conditions dramatically affect the Mw of E2502(2k) and E5005(2k) compared to the unsterilized control samples, decreasing it by 33% and 27%, respectively. This is attributed to the increase in PEG and DT content in both E2502(2k) and E5005(2k).
In summary, the Mw of all TyrPCs is affected by gamma and E-beam irradiation. It appears these polymers are susceptible to radiation-induced chain scission of the poly mer backbone, more so than by the chemical methods. Quantitative values of % Mw retention are presented in Table S1 (Supporting Information). Increase in PEG content in TyrPCs increases the rate of degradation of the polymer backbone by increasing the availability of water within the polymer matrix.[32,35,36] Increase in the carboxylic acid content also has a similar effect.
3.3.1. Radiation Chemical Yields
The molecular weight profile of the polymers before and after radiation sterilization can provide valuable information regarding polymer response to radiation in terms of polymer chain scission or cross-linking. The radiation chemical yield, generally given as G values, represents the extent of chain scission (scission yield, Gs) or cross-linking (cross-linking yield, Gx) per 100 eV energy absorbed during irradiation. The values of Gs and Gx can be determined using Equations (1) and (2) for radiation doses within the range 0 to 100 kGy: [37]
| (1) |
| (2) |
where Mw0 and Mn0 are the weight- and number-average molecular weight before irradiation, Mw and Mn are the corresponding values after irradiation, and D kGy is the dose of radiation.
A summary of the calculated Gs and Gx values are presented in Table S2 (Supporting Information). In general, for all the polymers that were evaluated, in the soluble fractions, the scission yield was higher than the cross-linking yield. There were no significant differences in the scission yields with polymer composition in both E-beam and gamma irradiation.
3.4. Effect of Sterilization on Mechanical Properties
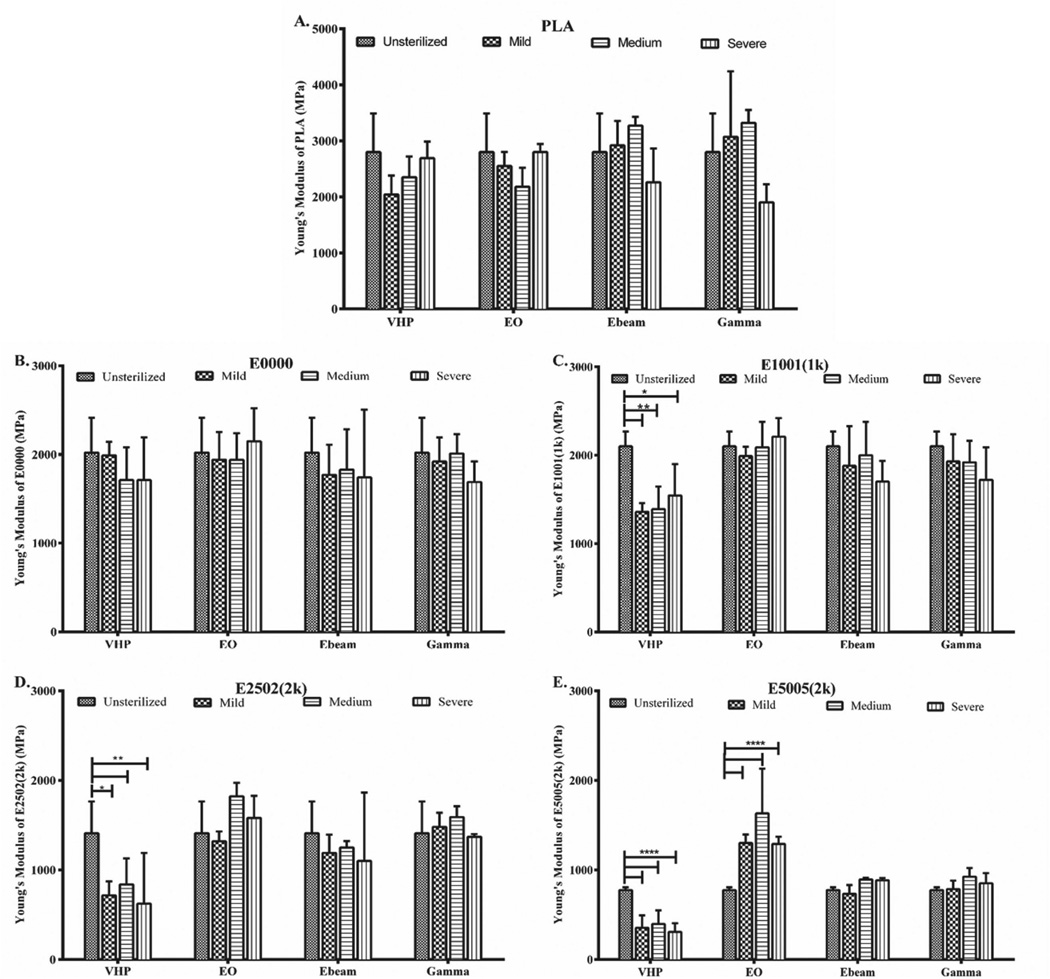
The mechanical properties of a polymer were evaluated by measuring Young’s modulus, tensile strength and % elongation. Figure 4 shows the Young’s modulus of PLA, E0000, E1001(1k), E2502(2k) and E5005(2k) polymers before and after sterilization. As seen in Figure 4 A, PLA’s Young’s modulus remains largely unaffected by sterilization; the decrease seen after severe gamma irradiation is not statistically significant (p > 0.05). Similarly, the Young’s modulus of E0000 also does not change significantly when any of the sterilization methods are employed. Despite the Mw loss observed in all TyrPCs upon E-beam and gamma irradiation (Figure 3), the Young’s moduli of the TyrPCs remain unaffected (Figure 4). A similar observation was reported by Ertel et al.,[38] where no change was observed in the tensile modulus of E0000, upon gamma and E-beam irradiation.
Figure 4.
Change in Young’s modulus of A) PLA, B) E0000 (slow), C) E1001(1k) (medium), D) E2502(2k) (fast) and E) E5005 (2k) (ultrafast degrading) polymers. Data are presented as mean ± SD for n = 3. Significant difference from the unsterilized control is indicated as (* p ≤ 0.05, ** p ≤ 0.01, **** p ≤ 0.0001).
However, with the increase in carboxylic acid and PEG, TyrPCs become more susceptible to hydrolytic degradation, thus affecting their Young’s modulus.[39] The Young’s moduli of E2502(2k) and E5005(2k) which are faster degrading and have a higher PEG and carboxylic acid content, increase upon EO sterilization. This change which could be due to esterification of DT was more significant (p ≤ 0.0001) in E5005(2k).[22] VHP sterilization also decreased the Young’s modulus TyrPCs with higher DT and PEG. A significant decrease in the Young’s modulus of E1001(1k), E2502(2k), and E5005(2k) was observed upon VHP sterilization.
3.5. Effect of Sterilization on Thermal Properties
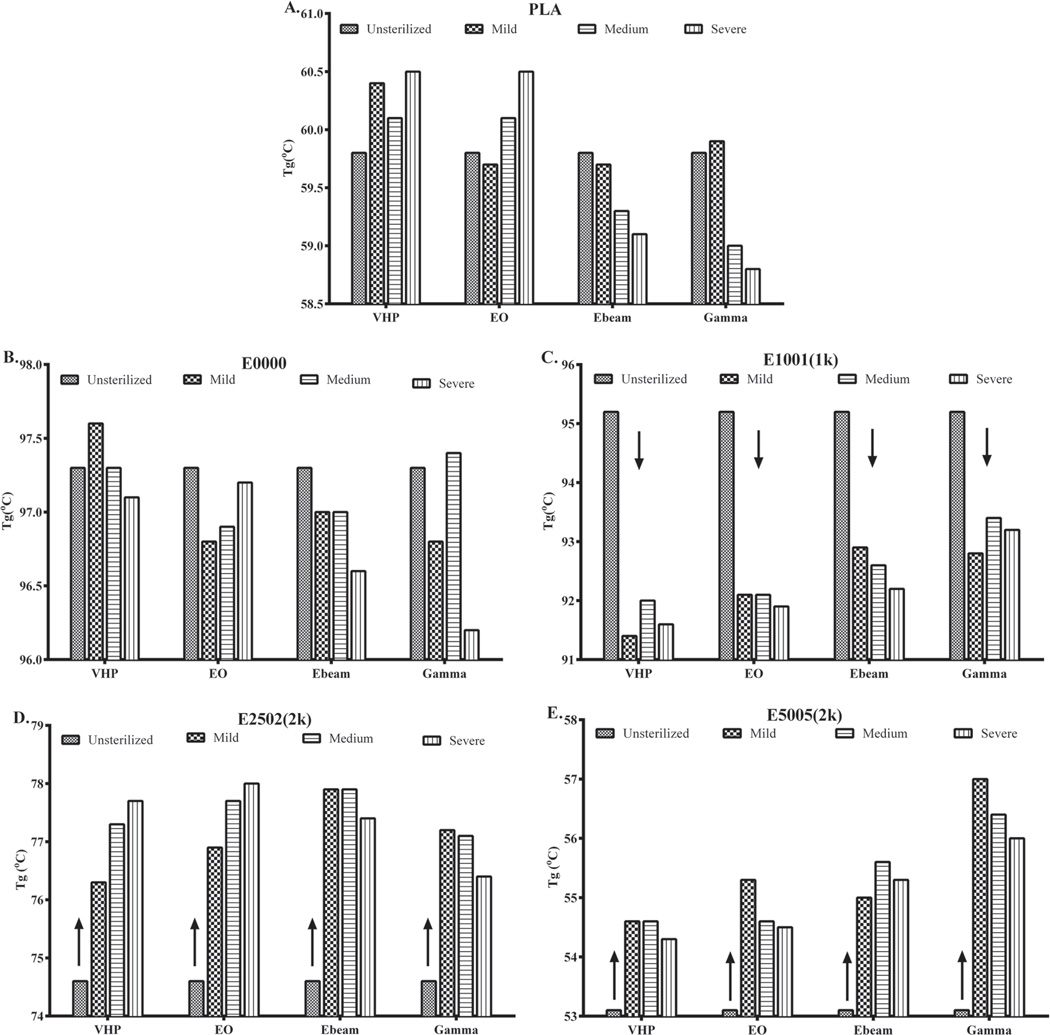
We evaluated the effect of sterilization on the thermal properties by measuring Tg. Changes in Tg predict how chemical and structural changes due to sterilization can affect the chain mobility and thus the polymer properties. Figure 5 shows the changes in Tg for all tested polymers. Under all sterilization conditions, PLA and E0000 show a small change in their Tg (changes <3 °C). For E1001(1k), the Tg decreases after any of the sterilization methods were employed. The chemical sterilization method (VHP and EO) had an even stronger effect and reduced Tg even more. This result is consistent with an earlier report by Phillip et al.[22] where it was shown that the Tg of E1001(1k) compression molded films decreased upon high-temperature EO condition.
Figure 5.
Change in glass transition temperature of A) PLA, B) E0000 (slow), C) E1001 (1k) (medium), D) E2502 (2k) (fast), and E) E5005(2k) (ultrafast degrading) polymers. Experimental SD calculated from changes in Tg of PLA = 0.5 °C.
Interestingly, in polymers with higher PEG and DT, E2502(2k) (fast degrading polymers) and E5005(2k) (ultra-fast degrading polymers), all sterilization methods resulted in an increase in Tg. There may be two competing reactions occurring in high PEG/DT polymers: loss of rigid segments (at low concentrations of DT) that would decrease the Tg, and increase in crosslinking (at high concentration of DT) that would increase the Tg.
3.6. Compositional Changes
3.6.1. 1H NMR Analysis
1H NMR was used to analyse the chemical composition of the polymer backbone. Three samples of each polymer (E0000, E1001(1k), E2502(2k), and E5005(2k)) were tested before and after each of the sterilization processes. In the following discussion, peak position and peak integration will be used to identify new chemical species and to quantify the compositional change, respectively.
1H NMR Analysis of TyrPCs Upon Radiation Sterilization (E-beam and Gamma)
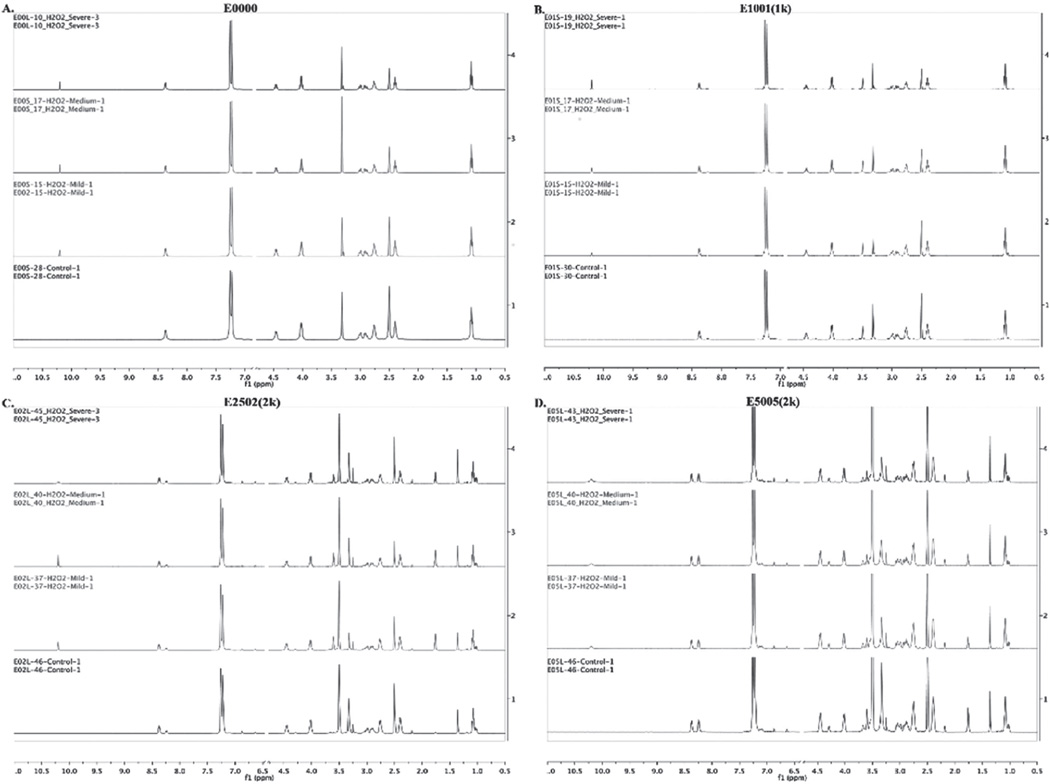
There was no significant change in the bulk chemical composition of TyrPCs after E-beam and gamma irradiation even at higher doses of radiation. The resulting spectra can be found in Supporting Information. Figures S3, S4, S5, and S6 (Supporting Information) show the stacked 1H NMR spectra of E0000, E1001(1k), E2502(2k), and E5005(2k) respectively, pre-and post E-beam sterilization. Figures S7, S8, S9, and S10 (Supporting Information) show the 1H NMR spectra of E0000, E1001(1k), E2502(2k), and E5005(2k) respectively, pre- and post-gamma sterilization.
1H NMR Analysis of TyrPCs Upon EO Sterilization
E0000 and E1001(1k) show no change in their overall peak integrations upon EO sterilization, (Figures S11 and S12, Supporting Information) indicating that the bulk chemical compositions of these polymers remained unaffected by the tested conditions of EO sterilization. In contrast, there were changes in solvent-cast E2502(2k) and E5005(2k) films upon EO sterilization as indicated by the appearance of a new peak at 3.38 ppm (Figures S13 and S14, Supporting Information). This peak arises from the hydroxyl group formed by the reaction of the free carboxylic acid with EO.[22] Averaging the peak integrations from three separate samples in each condition of sterilization showed that the concentrations of the hydroxyl groups present in the polymer backbone were about 7 to 11 % in E2502(2k) and 14 to 20 mol% in E5005(2k), depending upon the sterilization conditions.
EO is a highly strained three-member cyclic ether that can participate in addition reactions with a number of functional groups such as amines, carboxylic acids, hydroxyl, and sulfhydryl groups. Since each DT unit in the polymer backbone has a free carboxylic acid pendent group, EO can react with these carboxylic acids to form an ester during sterilization. It has previously been reported that this side reaction was indeed detected in tyrosine-derived polymers with high free carboxylic acid content.[22]
1H NMR Analysis of TyrPCs Upon VHP Sterilization
Figure 6 shows the NMR spectra of the TyrPCs pre- and post-VHP sterilization. VHP sterilization led to hydroxylation of the phenyl ring in the polymer backbone as indicated by the appearance of the peak at 10.2 ppm. Hydrogen peroxide sterilization mainly occurs through the formation of hydroxyl radicals that react with biomole cules and polymers. The average hydroxylation was calculated by comparing the integrated areas of the peak at 10.2 ppm (−OH) against the ≈8.3 ppm amide peaks (−NH−) of DTE and DT, and the peak of PEG protons at ≈3.5 ppm (−CH2−).
Figure 6.
Stacked 1H NMR spectra of A) E0000, B) E1001(1k), C) E2502 (2k), and D) E5005(2k) films pre- and post VHP sterilization.
In E0000 compression molded films the degree of hydroxylation was ≈11 mol% for all VHP treatment conditions. No other change was observed in the overall chemical composition of the polymer backbone. Interestingly, the degree of hydroxylation was not dependent on the dose of VHP or the dwell time. E1001(1k) films were affected by VHP sterilization in a similar way as E0000. However, unlike E0000, the percent hydroxylation in E1001(1k) after VHP sterilization was dependent on the dosage and dwell time. Average % hydroxylation was 15, 22, and 24 mol% under mild, medium and severe conditions, respectively.
In solvent cast E2502(2k), the hydroxylation of the phenyl ring was 16 mol% in mild conditions and 26 mol% in medium and severe conditions of VHP sterilization. The degree of hydroxylation was higher in E5005(2k) films: ≈38 mol% under mild and medium conditions and 47 mol% under severe VHP conditions (Table S3, Supporting Information). Polymers with higher mol% of PEG are more susceptible to hydroxylation. As the content of PEG increases, such as in E5005(2k), the polymer becomes more hydrophilic. This results in an increased infiltration of vaporized hydrogen peroxide into the polymer backbone, resulting in increased reactivity with the phenyl rings.
3.6.2. XPS Analysis
Surface elemental composition of the pre- and post-sterilized polymers films was investigated using XPS high-resolution scans. High-resolution C1s and O1s scans provided detailed information regarding the oxidative destruction and concomitant degradation of the polymers samples during the sterilization evaluation. High-resolution C1s scan can be resolved into various components, corresponding to C−C and C−H bonds at 284.7 eV, C−OH and C−O−C bonds at 286.5 eV, C=O bond at 287.9 eV, O−C=O bond at 289.2 eV, and −COOH bond at 290.5 eV binding energies.[40,41] Among these various components, we selected C−OH/C−O−C peak intensities from C1s scans at 286.5 eV and independently analyzed the variations in their intensities with the sterilization conditions. A summary of the results is presented in Table 3. Overall, both gamma and E-beam resulted in an increase in peak intensity at 286.5 eV for E0000 and E1001(1k) polymers and a reduction in the peak intensity for the PEG-rich E2502(2k) and E5005(2k) polymers (Figure 7). The increase in the 286.5 eV peak intensity observed in PEG-free E0000 and low PEG E1001(1k) polymers could be due to the formation of hydroxyl end groups as a result of the cleavage of the carbonate bonds caused by sterilization.
Table 3.
Carbon at% present as C−O−C (286.5 eV) determined from high-resolution XPS scans.
| Unsterilized | Mild | Medium | Severe | |
|---|---|---|---|---|
| E-beam | ||||
| E0000 | 7.7 | 13.5 | 8.8 | 9.8 |
| E1001(1k) | 6.6 | 9.4 | 14.2 | 10.2 |
| E2502(2k) | 15.1 | 14.7 | 11.1 | 11.1 |
| E5005(2k) | 16.2 | 7.7 | 16.2 | 5.6 |
| Gamma | ||||
| E0000 | 7.7 | 6.1 | 10.1 | 10.9 |
| E1001(1k) | 6.6 | 9.7 | 7.9 | 13.8 |
| E2502(2k) | 15.1 | 13.7 | 9.0 | 9.8 |
| E5005(2k) | 16.2 | 10.9 | 10.8 | 10.9 |
| Ethylene oxide | ||||
| E0000 | 7.7 | 9.4 | 12.5 | 10.7 |
| E1001(1k) | 6.6 | 12.6 | 13.7 | 13.4 |
| E2502(2k) | 15.1 | 10.1 | 15.0 | 18.1 |
| E5005(2k) | 16.2 | 10.9 | 11.1 | 20.7 |
| VHP | ||||
| E0000 | 7.7 | 13.4 | 11.8 | 11.5 |
| E1001(1k) | 6.6 | 13.7 | 11.5 | 12.9 |
| E2502(2k) | 15.1 | 12.4 | 17.6 | 13.5 |
| E5005(2k) | 16.2 | 17.6 | 11.0 | 16.7 |
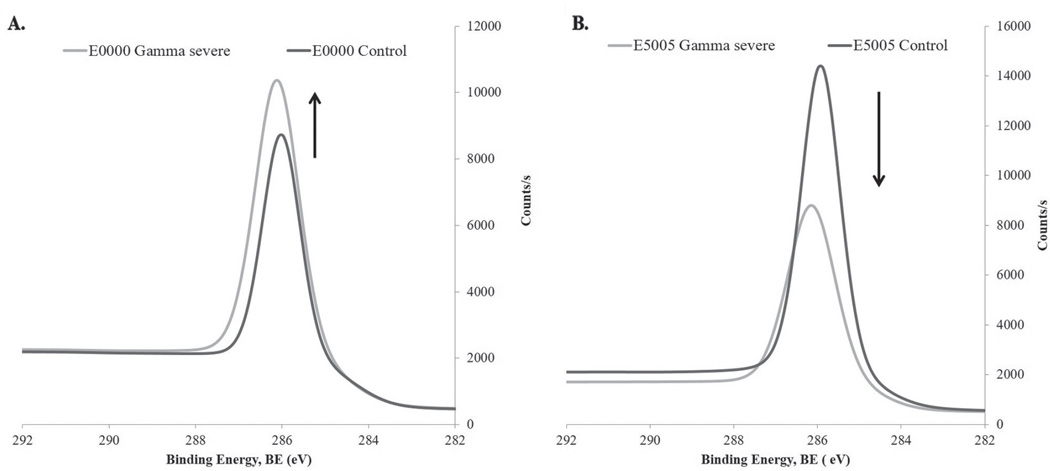
Figure 7.
Representative XPS high-resolution scans at binding energy 286.5 eV showing changes in peak intensities as a function of change in C−OH or C−O−C concentration after radiation sterilization. A) E0000 peak intensity before and after gamma severe irradiation. B) E5005(2k) peak intensity before and after gamma severe irradiation.
Research has shown that the intensity of the C−O−C peak at 286.5 eV, which is also the binding energy of C−OH, can be directly correlated to the PEG concentration in a matrix.[42] Thus, any change in this particular peak intensity after sterilization treatment will reflect the extent of oxidation, polymer degradation, and cleavage of the PEG segment from the polymer matrix that result in breaking of C−O−C bonds.
It has been shown that radiation sterilization of various biomedical polymeric materials, such as high-molecular-weight polyurethane, polyethylene, and polypropylene [43–45] under atmospheric conditions can lead to oxidative degradation of the polymer. This oxidative degradation in TyPC results in the cleavage of the carbonate linkages leading to the formation of terminal hydroxyl groups with a reduction in their molecular weights. Consequently, within the experimental constraints and uncertainty, the observed net increase in the peak intensity at the 286.5 eV could be attributed to a rise in the C−OH content in E0000 and E1001(1k) polymers (Figure 7A).
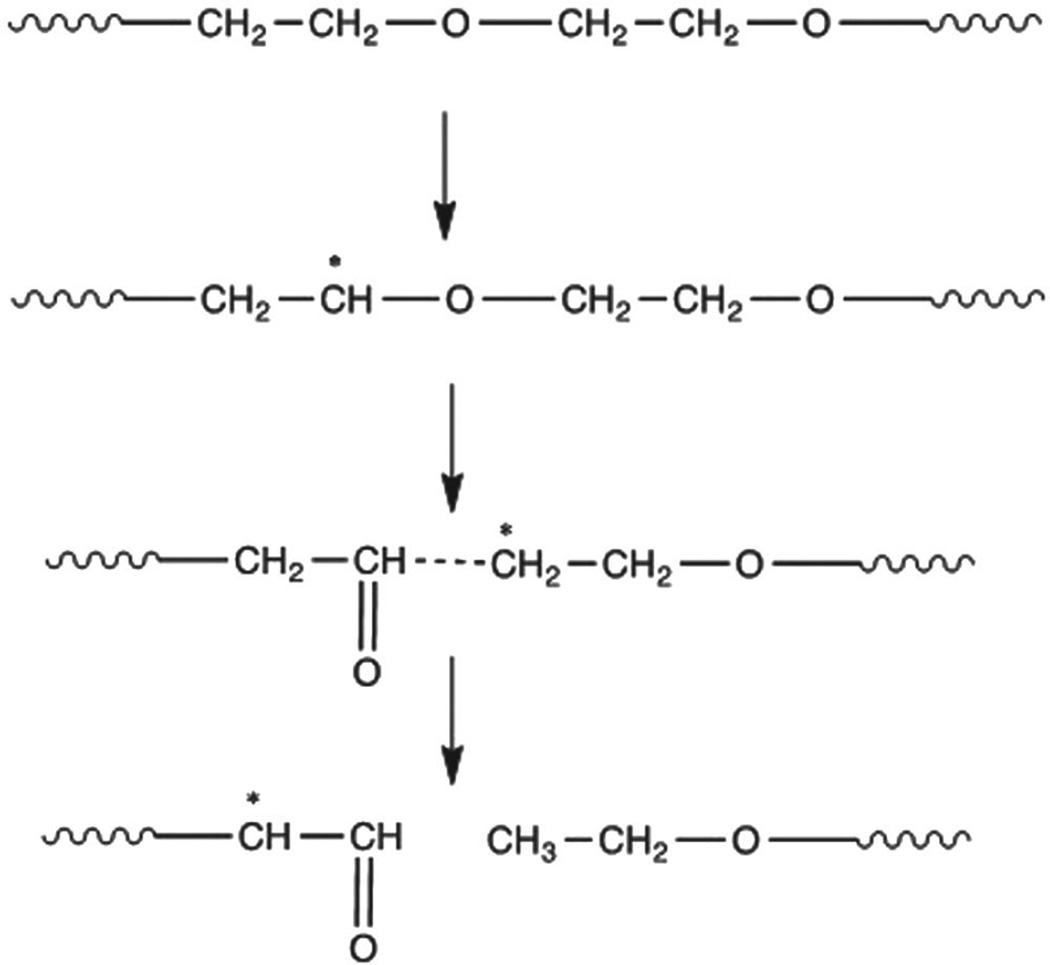
In contrast to the above observation in PEG-poor TyrPCs, in PEG-rich E2502(2k) and E5005(2k) irradiation with gamma and E-beam results in radiolytic degradation of the PEG segment parallel to the cleavage of carbonate linkages. Solid-state radiolysis of PEG can initiate the formation of −CH*−O-free radicals at room temperature. Formation of these radicals leads to ether bond (C−O−C) scission with the formation of aldehyde radicals, which are relatively stable up to several months at room temperature (Figure 8).[46] The extent of the radiolytic degradation of PEG increases as both the PEG concentration and radiation dosages increase. Additionally, the radiolytic degradation of the PEG results in breaking of the softer segment of the polymer backbone, and consequently we observed an increase in the Tg values for E2502(2k) and E5005(2k) polymers after these radiation sterilizations.
Figure 8.
Decrease in C−O−C content due to PEG degradation caused by radiolysis.
In the case of EO and VHP sterilizations, the reactive EO and hydrogen peroxide (H2O2) species generated additional hydroxyl groups in the polymer backbone caused by EO esterification and H2O2 oxidation. Consequently, there is a net increase in C−OH peak intensity for all polymers sterilized under these treatment conditions. With an increasing free carboxyl content for E2502(2k) and E5005(2k) polymers, a significant increase in the 286.5 eV peak intensity was observed with the increasing dose of EO content. With the introduction of additional polar hydroxyl groups on the polymer backbone, as evident from these XPS data, the interchain hydrogen bonding between the polymer chains increases after both EO and VHP sterilization and consequently leads to an increased Tg values for all these polymers after sterilization.
4. Conclusions
Our studies show that the properties of bioresorbable polymers, like TyrPC and PLA, can be potentially altered by any of the most commonly used sterilization techniques (EO, VHP, gamma radiation, and E-beam) by choosing an inappropriate sterilization condition. When considering the sterilization of degradable polymers containing ether, ester, amide or carbonate bonds, and/or free carboxylic acid groups, our results provide a framework that can assist in the selection of a suitable sterilization protocol:
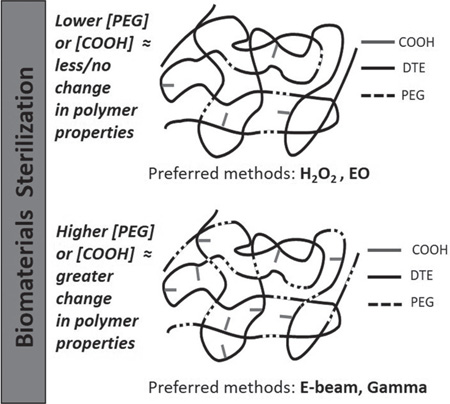
PEG and free carboxylic acid groups are the most sterilization-sensitive components tested in our work. Therefore, the susceptibility of our test polymers to sterilization damage (in terms of Mw degradation, changes in mechanical, morphological, and thermal properties) can be roughly correlated with the proportion of PEG and carboxylic acid groups contained within their composition. The two polymers without either PEG or carboxylic acid groups (E0000 and PLA) were not generally affected by either radiation or chemical sterilization, even under severe conditions; the only exception was the change in Mw of E0000 upon E-beam and gamma sterilizations.
The TyrPC polymer denoted as E1001(1k) has a low ratio of PEG and carboxylic acid groups. This polymer can be sterilized using mild and medium conditions of E-beam, gamma radiation, and EO without significant changes of its properties.
Polymers that contain a high proportion of PEG and carboxylic acid groups require careful optimization of the sterilization protocol. Radiation sterilization methods (E-Beam and gamma) resulted in the radiolytic degradation of PEG in E2502(2k) and E5005(2k). Radiolytic degradation can also result in breaking of the softer segments in the polymer backbone, increasing the Tg. These polymers can only be sterilized when using mild conditions. Likewise, the tendency of highly degradable polymers (those with high PEG and carboxylic acid content) to undergo hydrolytic cleavage during EO and VHP exposure, limits the use of these chemical sterilization methods.
Overall, VHP sterilization was found to be the least desirable of all tested methods since it causes hydroxylation of the phenyl ring in all TyrPC polymers. It is likely that VHP will similarly react with any polymer containing phenyl rings.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. David Devore for his valuable discussions and Joseph Marchica, Ramyata Upmaka and Kishan Patel for their help in sample preparation. Funding for this work was provided by RESBIO – The National Resource for Polymeric Biomaterials supported by the National Institutes of Health (NIH grant EB001046), and by the New Jersey Centre for Biomaterials at Rutgers University. D.B. is an affiliated fellow of the postdoctoral training program “Translational research in regenerative medicine” and acknowledges the matching support of Rutgers University.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Divya Bhatnagar, NJ Centre for Biomaterials, Rutgers-The State University of New Jersey, 145 Bevier Road, Piscataway, NJ 08854, USA.
Koustubh Dube, NJ Centre for Biomaterials, Rutgers-The State University of New Jersey, 145 Bevier Road, Piscataway, NJ 08854, USA.
Vinod B. Damodaran, NJ Centre for Biomaterials, Rutgers-The State University of New Jersey, 145 Bevier Road, Piscataway, NJ 08854, USA
Ganesan Subramanian, NJ Centre for Biomaterials, Rutgers-The State University of New Jersey, 145 Bevier Road, Piscataway, NJ 08854, USA.
Kenneth Aston, Johnson & Johnson Sterility Assurance, 930, US 202, Raritan, NJ 08669, USA.
Frederick Halperin, Johnson & Johnson Sterility Assurance, 930, US 202, Raritan, NJ 08669, USA.
Meiyu Mao, Johnson & Johnson Sterility Assurance, 930, US 202, Raritan, NJ 08669, USA.
Kurt Pricer, Johnson & Johnson Sterility Assurance, 930, US 202, Raritan, NJ 08669, USA.
N. Sanjeeva Murthy, NJ Centre for Biomaterials, Rutgers-The State University of New Jersey, 145 Bevier Road, Piscataway, NJ 08854, USA.
Joachim Kohn, NJ Centre for Biomaterials, Rutgers-The State University of New Jersey, 145 Bevier Road, Piscataway, NJ 08854, USA.
References
- 1.Bernkopf M. Med. Device Technol. 2006;18:26. [PubMed] [Google Scholar]
- 2.Silindir M, Ozer Y. PDA J. Pharm. Sci. Technol. 2012;66:184. doi: 10.5731/pdajpst.2012.00774. [DOI] [PubMed] [Google Scholar]
- 3.Kohn J. Med. Device Dev. 2006:35. [Google Scholar]
- 4.Kohn J, Welsh WJ, Knight D. Biomaterials. 2007;28:4171. doi: 10.1016/j.biomaterials.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexy RD, Levi DS. BioMed Res. Int. 2013;2013:1. doi: 10.1155/2013/137985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert BJ, Mendelson TA, Craven MD. AAPS PharmSciTech. 2011;12:1116. doi: 10.1208/s12249-011-9644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valente TA, Silva DM, Gomes PS, Fernandes MH, Santos JD, Sencadas V. ACS Appl. Mater. Interfaces. 2016;8:3241. doi: 10.1021/acsami.5b10869. [DOI] [PubMed] [Google Scholar]
- 8.Rnjak-Kovacina J, DesRochers TM, Burke KA, Kaplan DL. Macromol. Biosci. 2015;15:861. doi: 10.1002/mabi.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliley JM, Sivak WN, Minteer DM, Tompkins-Rhoades C, Day J, Williamson G, Liao HT, Marra KG. ACS Biomater.-Sci. Eng. 2015;1:504. doi: 10.1021/ab5001518. [DOI] [PubMed] [Google Scholar]
- 10.Halpern JM, Gormley CA, Keech M, von Recum HA. J. Mater. Chem. B: Mater. Biol. Med. 2014;2:2764. doi: 10.1039/C4TB00083H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tohfafarosh M, Baykal D, Kiel JW, Mansmann K, Kurtz SM. J. Mech. Behav. Biomed. Mater. 2016;53:250. doi: 10.1016/j.jmbbm.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Bertoldi S, Fare S, Haugen HJ, Tanzi MC. J. Mater. Sci., Mater. Med. 2015;26:182. doi: 10.1007/s10856-015-5509-0. [DOI] [PubMed] [Google Scholar]
- 13.Lerouge S, Tabrizian M, Wertheimer MR, Marchand R, Yahia L. Bio-Med. Mater. Eng. 2002;12:3. [PubMed] [Google Scholar]
- 14.Patel M. Medical Sterilization Methods. Lemo USA Inc.; 2003. [accessed: June 2016]. http://www.digikey.com/short/329w5j. [Google Scholar]
- 15.Athanasiou KA, Niederauer GG, Agrawal CM. Biomaterials. 1996;17:93. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 16.Kanjickal D, Lopina S, Evancho-Chapman MM, Schmidt S, Donovan D. J. Biomed. Mater. Res. Part A. 2008;87:608. doi: 10.1002/jbm.a.31811. [DOI] [PubMed] [Google Scholar]
- 17.Premnath V, Harris W, Jasty M, Merrill E. Biomaterials. 1996;17:1741. doi: 10.1016/0142-9612(95)00349-5. [DOI] [PubMed] [Google Scholar]
- 18.Sintzel MB, Merkli A, Tabatabay C, Gurny R. Drug Dev. Ind. Pharm. 1997;23:857. [Google Scholar]
- 19.Cottam E, Hukins DW, Lee K, Hewitt C, Jenkins MJ. Med. Eng. Phys. 2009;31:221. doi: 10.1016/j.medengphy.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Nuutinen J-P, Clerc C, Virta T, Törmälä P. J. Biomater. Sci., Polym. Ed. 2002;13:1325. doi: 10.1163/15685620260449723. [DOI] [PubMed] [Google Scholar]
- 21.Holy C, Cheng C, Davies J, Shoichet M. Biomaterials. 2001;22:25. doi: 10.1016/s0142-9612(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 22.Phillip E, Murthy NS, Bolikal D, Narayanan P, Kohn J, Lavelle L, Bodnar S, Pricer K. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2013;101:532. doi: 10.1002/jbm.b.32853. [DOI] [PubMed] [Google Scholar]
- 23.Lee MH, Kim H-L, Kim CH, Lee SH, Kim JK, Lee SJ, Park J-C. Surf. Coat. Technol. 2008;202:5762. [Google Scholar]
- 24.Kim J, Magno MHR, Waters H, Doll BA, McBride S, Alvarez P, Darr A, Vasanji A, Kohn J, Hollinger JO. Tissue Eng., Part A. 2012;18:1132. doi: 10.1089/ten.TEA.2011.0582. [DOI] [PubMed] [Google Scholar]
- 25.Kohn J. Nat. Mater. 2004;3:745. doi: 10.1038/nmat1249. [DOI] [PubMed] [Google Scholar]
- 26.Ezra M, Bushman J, Shreiber D, Schachner M, Kohn J. Tissue Eng., Part A. 2013;20:518. doi: 10.1089/ten.tea.2013.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clements BA, Bushman J, Murthy NS, Ezra M, Pastore CM, Kohn J. J. Tissue Eng. 2016;7:1. doi: 10.1177/2041731416629471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meechaisue C, Dubin R, Supaphol P, Hoven VP, Kohn J. J. Biomater. Sci., Poly. Ed. 2006;17:1039. doi: 10.1163/156856206778365988. [DOI] [PubMed] [Google Scholar]
- 29.Lo M-C, Wang S, Singh S, Damodaran VB, Kaplan HM, Kohn J, Shreiber DI, Zahn JD. Biomed. Microdevices. 2015;17:1. doi: 10.1007/s10544-015-9927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magno MHR, Kim J, Srinivasan A, McBride S, Bolikal D, Darr A, Hollinger JO, Kohn J. J. Mater. Chem. 2010;20:8885. [Google Scholar]
- 31.Magno MHR. Optimization of Tyrosine-Derived Polycarbonate Terpolymers for Bone Regeneration Scaffolds. Rutgers, The State University of New Jersey, NJ; 2012. [Google Scholar]
- 32.Valenzuela LM, Michniak B, Kohn J. J. Appl. Polym. Sci. 2011;121:1311. [Google Scholar]
- 33.Hooper KA, Cox JD, Kohn J. J. Appl. Polym. Sci. 1997;63:1499. [Google Scholar]
- 34.Yu C, Kohn J. Biomaterials. 1999;20:253. doi: 10.1016/s0142-9612(98)00169-0. [DOI] [PubMed] [Google Scholar]
- 35.Bourke SL, Kohn J. Adv. Drug Delivery Rev. 2003;55:447. doi: 10.1016/s0169-409x(03)00038-3. [DOI] [PubMed] [Google Scholar]
- 36.Yu C, Kohn J. Biomaterials. 1999;20:253. doi: 10.1016/s0142-9612(98)00169-0. [DOI] [PubMed] [Google Scholar]
- 37.Dorati R, Colonna C, Serra M, Genta I, Modena T, Pavanetto F, Perugini P, Conti B. AAPS PharmSciTech. 2008;9:718. doi: 10.1208/s12249-008-9103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ertel SI, Kohn J. J. Biomed. Mater. Res. 1994;28:919. doi: 10.1002/jbm.820280811. [DOI] [PubMed] [Google Scholar]
- 39.Tangpasuthadol V, Pendharkar SM, Peterson RC, Kohn J. Biomaterials. 2000;21:2379. doi: 10.1016/s0142-9612(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 40.Damodaran VB, Fee CJ, Ruckh T, Popat KC. Langmuir. 2010;26:7299. doi: 10.1021/la9041502. [DOI] [PubMed] [Google Scholar]
- 41.Naumkin A, Kraut-Vass A, Gaarenstroom S, Powell C. NIST Standard Reference Database 20, Version 4.1 (web version) NIST: US Secretary of Commerce; 2012. [Google Scholar]
- 42.Damodaran VB, Fee CJ, Popat KC. Appl. Surf. Sci. 2010;256:4894. [Google Scholar]
- 43.Sudoł M, Czaja K, Cybo J, Duda P. Polimery. 2004;49:841. [Google Scholar]
- 44.Ishigaki I, Yoshii F. Int. J. Radiat. Appl. Instrum. Part C: Radiat. Phys. Chem. 1992;39:527. [Google Scholar]
- 45.Medel FJ, Kurtz SM, Hozack WJ, Parvizi J, Purtill JJ, Sharkey PF, MacDonald D, Kraay MJ, Goldberg V, Rimnac CM. J. Bone Jt. Surg. Am. 2009;91:839. doi: 10.2106/JBJS.H.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorati R, Colonna C, Tomasi C, Genta I, Modena T, Faucitano A, Buttafava A, Conti B. AAPS PharmSciTech. 2008;9:1110. doi: 10.1208/s12249-008-9150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.