Abstract
Purpose e Cross-sectional imaging methods are important for objective evaluationof small intestinal inflammationinCrohn’sdisease(CD).The primary aim was to compare relative parameters of intestinal perfusion between contrast-enhanced ultrasonography (CEUS) and dynamic contrast-enhanced magnetic resonance enterography (DCE-MRE) in CD. Furthermore, we aimed at testing the repeatability of regions of interest (ROIs) for CEUS.
Methods This prospective study included 25 patients: 12 females (age: 37, range: 19–66) with moderate to severe CD and a bowel wall thickness>3mm evaluated with DCE-MRE and CEUS. CEUS bolus injection was performed twice for repeatability and analyzed in VueBox®. Correlations between modalities were described with Spearman’s rho, limits of agreement(LoA) and intraclass correlation coefficient(ICC). ROIrepeatability for CEUS was assessed.
Results s The correlation between modalities was good and very good for bowel wall thickness (ICC=0.71, P<0.001) and length of the inflamed segment (ICC=0.89, P<0.001). Moderate-weak correlations were found for the time-intensity curve parameters: peak intensity (r=0.59, P=0.006), maximum wash-in-rate (r=0.62, P=0.004), and wash-in perfusion index (r=0.47, P=0.036). Best CEUS repeatability for peak enhancement was a mean difference of 0.73 dB (95% CI: 0.17 to 1.28, P=0.01) and 95% LoA from −3.8 to 5.3 dB. Good quality of curve fit improved LoA to −2.3 to 2.8 dB.
Conclusion The relative perfusion of small intestinal CD assessed with DCE-MRE and CEUS shows only a moderate correlation. Applying strict criteria for ROIs is important and allows for good CEUS repeatability
Key words: ultrasonography, magnetic resonance imaging, Crohn’s disease, reproducibility of results, contrast media
Abbreviations
- AIU
Arbitrary intensity units
- CEUS
Contrast-enhanced ultrasound
- CD
Crohn’s disease
- CDAI
Crohn’s Disease Activity Index
- CDI
Color Doppler imaging
- DCE-MRE
Dynamic contrast-enhanced magnetic resonance enterography
- HBI
Harvey Bradshaw Index
- ICC
Intraclass correlation coefficient
- LoA
Limits of agreement
- QoF
Quality of fit
- ROI
Region of interest
- TIC
Time-intensity curve
- US
Ultrasonography
Introduction
In Crohn’s disease (CD) the grading of disease activity has shifted from subjective clinical scoring systems towards more objective measurements, in combination with patient-reported outcomes 1. Endoscopy, although not completely objective, is often considered a gold standard for luminal disease in the colon, rectum, and sometimes the terminal ileum. However, endoscopy is of limited use in stricturing and proximal disease 2 and even well-recognized endoscopic scoring systems are not fully reliable 3. This calls for cross-sectional imaging methods with objective parameters of disease severity 1 4. Currently there is no single imaging modality as the gold standard for transmural disease of the small intestine 5.
The most consistent characteristic of disease activity on imaging is an increased bowel wall thickness of more than 3 mm 4 6 7. Nevertheless, the intestinal wall may be thickened not only by active disease but also by fibrosis 7 8. Other features of inflammatory activity comprise ulcerations, T2-hypersignal, perimural signal, contrast enhancement, comb sign, enlarged lymph nodes, fistulas, abscesses and strictures described in the development of the MR intestinal activity score and MR enterography global assessment 7 9 10. Unfortunately, experts do not agree about the importance of the individual findings 11.
In recent classifications, increased contrast enhancement is considered a relevant marker of disease activity 7 9 12 13. This is in accordance with the characteristics of active inflammation including dilated leaking vessels 14 and neoangiogenesis 15. Additionally, microvascular density has been shown to correlate with intensity on dynamic contrast-enhanced ultrasound (CEUS) 16. Therefore, dynamic imaging techniques could potentially be used for evaluating disease activity and efficacy of treatment 7 17.
The 2 promising modalities to assess relative bowel wall perfusion are CEUS and dynamic contrast-enhanced MR enterography (DCE-MRE). However, there are significant differences in contrast behavior between modalities. MR gadolinium-based contrast agents are relatively small and exhibit extravasation over time, whereas CEUS gas-filled lipid-shell contrast acts as a true intravascular agent. The time-intensity curves (TICs) recorded from the former are therefore a combination of perfusion and permeability, rather than perfusion alone. The relationship between signal intensity and MRI contrast agent concentration is complex and depends on a number of parameters, such as the native tissue relaxation rate, relaxivity of the contrast agent, local field inhomogeneity and the applied flip angle and inversion-recovery time 18. US contrast agent on the other hand has a direct correlation with the signal intensity measured in dB 19. Since perfusion is difficult to measure if the bowel wall is less than 3 mm thick 20, the parameters should only be used for grading disease activity or to follow treatment efficacy 6 21.
In the present study, we hypothesized that intensity and time parameters of the initial time-intensity curves correlate well between modalities as the amount of MR contrast which is extravasated during the initial pass is low.
The main objective of this study was to compare objective parameters of relative perfusion obtained with DCE-MRE and CEUS in patients with moderate to severe CD. Our secondary objectives were to test the repeatability of regions of interest (ROIs) for CEUS and to evaluate the inter-rater reliability of CD characteristics assessed with MRE and US.
Materials and Methods
This GCP monitored prospective double-blind observational study was approved by the Danish national authorities (2011-005886-19) and the local research ethics committee (1-10-72-340-12) for the off-label use of US contrast agents. All participants gave written informed consent before entering the study. Inclusion criteria were known CD with moderate to severe clinical activity based on either the CD Activity Index 22 (CDAI)>220 or Harvey Bradshaw Index 23 (HBI)>7. Furthermore, patients had to be ≥18 years of age and have a US-detectable intestinal segment with bowel wall thickness>3 mm. Patients were excluded if they were pregnant, breastfeeding or had any contraindications for DCE-MRE or CEUS.
25 patients (mean age: 37 years; range: 19–66 years; 12 females) were recruited for the study from September 2012 to March 2014. Due to screening failure, 2 patients were excluded and another 2 were subsequently recruited to reach the desired inclusion of 25 patients, Fig. 1. The Montreal classification 24, CDAI, HBI, gastrointestinal symptoms, smoking status, and medical history were recorded and blood and stool samples were taken during the first visit. For full patient demographics, Table 1.
Fig. 1.
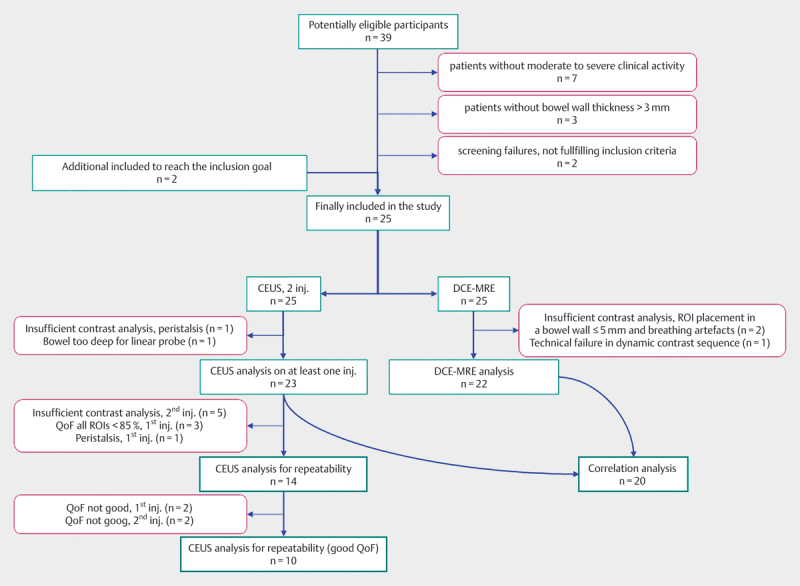
Flowchart of inclusion and analysis. Purple-colored boxes show the reason for no inclusion, exclusion or no analysis. The large number of patients with insufficient contrast analysis of 2nd contrast injection is due to in-and-out-of-plane motion artifacts in the non-optimal scan plane. CEUS=contrastenhanced ultrasound, DCE-MRE=dynamic contrast-enhanced magnetic resonance enterography, QoF=quality of fit, inj.=injection(s)
Table 1 Patient demographics.
| Parameter | No. of Patients |
|---|---|
| Included patients | 25 |
| Female | 13 (52) |
| Age, years | 37 [19–66] |
| Body mass index (kg/m2) | 24.5±4.4 |
| Disease duration | |
| <2 years | 10 (40) |
| 2–10 years | 6 (24) |
| >10 years | 7 (28) |
| Unknown | 2 (8) |
| Location of disease | |
| Terminal Ileum | 16 (64) |
| Colon | 1 (4) |
| Ileocolon | 6 (24) |
| Upper disease | 0 (0) |
| Unknown | 2 (8) |
| Medical therapy, n (%) | |
| None | 11 (44) |
| Corticosteroids | 5 (20) |
| Immunomodulators | 6 (24) |
| Biological therapy | 2 (8) |
| Combo treatment | 1 (4) |
| Crohn’s Disease Activity Index | 298±85 |
| Harvey Bradshaw Index | 9.9±3.5 |
| Fecal calprotectin (μg/g)* | 356 [63–3 600] |
| C-reactive protein (mg/l)* | 5.9 [0.7–34.4] |
| Hemoglobin (mmol/l) | 8.6±0.8 |
| Albumin (g/l) | 36.7±4.5 |
| Vitamin D (nmol/l) | 65±20.5 |
| Hematocrit | 0.40±0.035 |
| Time between examinations, days* | 0 [0–4] |
| Symptoms within last flair, n (%), days* | |
| Pain | 23 (92), 157 days [11–2 906] |
| Nausea | 17 (68), 70 days [3–2 495] |
| Vomit | 11 (44), 35 days [3–265] |
| Diarrhea | 19 (76), 303 days [3–5 751] |
| Bloody stools | 6 (24), 29.5 days [5–105] |
| Bloating | 17 (68), 166 days [26–4 093] |
| Weight loss | 16 (64), 108 days [3–2 468] |
| Fatigue | 5 (20), 189 days [22–1 764] |
Note – Numbers in parenthesis are percentages. Numbers in brackets are ranges
Unless otherwise indicated, data are means±standard deviations
*Median values and ranges
Ultrasonography
Participants were investigated after a 4-h fast. Ultrasonography (US) was performed by one physician (RW) with 2 years of experience with the procedure. The investigator was blinded to the MRE scan and biochemical results. However, the patients’ symptoms were known. An Acuson S3000 ultrasound machine with a 4–9 MHz linear matrix transducer and a 1–6 MHz curvilinear transducer was used (Siemens Medical Solutions, Malvern, PA). Color Doppler imaging (CDI) was set with a transmit frequency of 6.75 MHz, gain 1 dB, pulsed repetition frequency 1 099, low wall filter of 2, and a scale of 6 cm/s. The most severely inflamed bowel segment was identified based on wall thickness and the highest CDI signal score according to the Limberg classification 25. The total length of each affected segment, bowel wall pattern, presence of ulcers, stenosis and prestenotic dilatation were registered.
All CEUS scans were performed on the Acuson machine using the 9L4 probe. The settings were: fixed mechanical index of 0.06–0.08, dynamic range 80, frame rate of 10 per second, frequency 4 MHz, and the focal zone beneath the bowel wall. Sulfur hexafluoride microbubbles (SonoVue®; Bracco Imaging, Milan, Italy) 2.4 ml×2 were injected by trained nurses followed by a 5 ml saline flush over 2 s. Scans were recorded for 90 s. The scan plane was kept constant and patients were instructed regarding gentle breathing. More than 5 min after the first injection, the scan was repeated at the same spot, but in a different scan plane, to cover the segment in both the transverse and longitudinal axes. The chosen bowel segments were terminal ileum, neo-terminal ileum or proximal ileum. The location for CEUS was determined as the most inflamed area of the segment according to a prior classification 12.
Analysis of contrast-enhanced ultrasonography
Cine loop files were exported in DICOM format, re-linearized, and quantified on VueBox® 5.1 (Bracco Suisse SA, Geneva, Switzerland) as described earlier 17.
If possible, four ROIs were drawn using the following criteria: all ROIs had to be larger than 0.1 cm2 and within the bowel wall at all times. Shapes and placement of ROIs were optimized to obtain a quality of fit (QoF) of the fitted curve larger than 90% or as high as possible. Built-in motion compensation was applied whenever beneficial. The first ROI was drawn as large as possible and typically covering the full bowel wall thickness of the anterior and posterior bowel wall avoiding the lumen. VueBox includes the possibility to apply a heat map for the parameters of interest. 3 additional ROIs were placed in areas with the highest peak enhancement according to the heat map and without overlapping, Fig. 2 and video (Online Resource). Analyses were then compared for repeatability between the largest ROI, the maximum peak ROI and the mean of the 3 latter ROIs, exhibiting a QOF>85%, entitled mean ROI. The average (log-converted) values of the best reproducible method were subsequently chosen for comparison with DCE-MRE results. Data post-processing was badge-analyzed by the same investigator more than 6 months after US and clinical scoring of the last patient to ensure effective blinding of data.
Fig. 2.
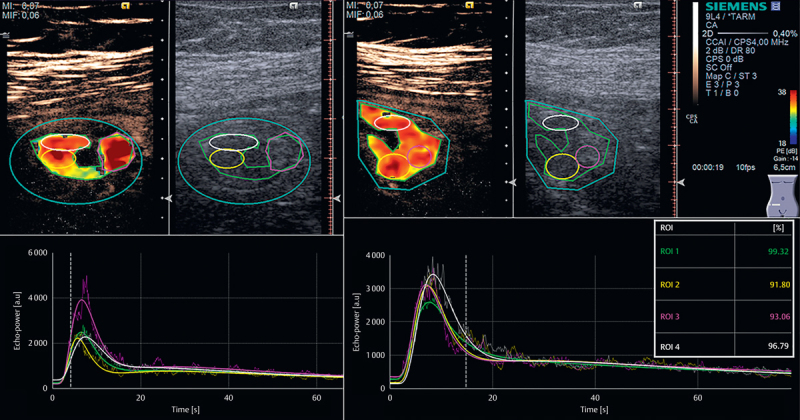
Contrast-enhanced ultrasonography with SonoVue in a 66-year-old woman. Quantification using VueBox. Upper left: Axial view of first bolus injection. The contrast image is seen on the left, while the corresponding B-mode image is shown on the right. The outer turquoise oval-shaped ROI is the area of investigation and motion compensation. The green region of interest (ROI) is ROI1 and the largest possible ROI. The yellow ROI is ROI2, the purple ROI is ROI3, and the fourth ROI is white. Lower left: Corresponding time intensity curves. Upper right: bowel in longitudinal scan after second bolus injection with 4 new ROIs. Lower right: TICs for injection 2. NB. Y-axis is slightly different from injection 1. Quality of fit is shown in the box on the right, indicating the largest ROI (ROI1) has the best curve fit. ROI2 and ROI3 are almost identical. ROI=region of interest.
Dynamic contrast-enhanced magnetic resonance enterography
Patients were instructed to fast for 4 h and drink 1 l of oral contrast 1 h before the scan. Oral contrast comprised a suspension of 125 ml mannitol 15% (Fresenius Kabi, Bad Homburg, Germany) in 875 ml of tap water, 30 ml psyllium HUSK® Fibre, and ice cubes. Peristalsis was suppressed by intravenous injection of 20 mg hyoscine butylbromide (Buscopan®; Boehringer Ingelheim, Ingelheim, Germany) prior to non-dynamic sequences and repeated before contrast injection. Images were acquired using a 1.5T MR unit (Avanto; Siemens, Erlangen, Germany) with patients in the prone position. The intravenous contrast agent used was gadoterate meglumine (Dotarem®; Guerbet, Villepinte, France) with 0.2 mg/kg bodyweight at 5 ml/s followed by a 24 ml saline flush. Patients were instructed to hyperventilate prior to a long breath hold followed by gentle breathing. The MR scanning protocols can be seen in Table 2.
Table 2 Magnetic resonance enterography parameters.
| Sequences Parameter | True FISP | T2w single-shot turbo spin-echo | T2w single-shot turbo spin-echo fat sat | T1w Turboflash fat sat | T2w TRUEFISP | T1w spoiled 3D flash | T1w spoiled 3d flash | Turboflash fat sat | T1w VIBE |
|---|---|---|---|---|---|---|---|---|---|
| Image plane(s) | Coronal | Coronal | Coronal | Coronal | Axial | Coronal | Coronal | Coronal+Axial | Coronal |
| Field of view (mm) | 450×366 | 450×338 | 450×338 | 450×366 | 400×300 | 360×240 | 360×240 | 450×366/400×300 | 400×400 |
| No. of sections | 5 | 20 | 20 | 24 | 30 | 20 | 20 | 24/22 | 96 |
| No. of stacks | 2–3 | 1 | 1 | 1 | 3–4 | 1 | 1 | 1/3–4 | 1 |
| Repetition time (msec) | 36.9 | 2 000 | 2 000 | 212 | 3.42 | 3.00 | 3.00 | 212/203 | 9:33 |
| Echo time (msec) | 1.04 | 81 | 81 | 4.76 | 1.45 | 0.82 | 0.82 | 4.76/4.76 | 4:44 |
| Acquisition time per stack (min) | 0:19 | 1:20 | 1:20 | 0:40 | 0:12 | 0:05:4 | 3:36 | 0:40/0:36 | 0:20 |
| No. of sequential acquisitions | 10 | 1 | 1 | 1 | 1 | 1 | 120 | 1 | 1 |
| Matrix | 192×192 | 320×260 | 320×260 | 256×205 | 256×256 | 256×128 | 256×128 | 256×205 | 256×154 |
| Section thickness (mm) | 10 | 6 | 6 | 5 | 4 | 5 | 5 | 5/5 | 2.5 |
| Section gap (mm) | 2 | 2 | 2 | 2.5 | 0 | 1 | 1 | 2.5/0.5 | 0.5 |
| Turbo factor | NA | 194 | 194 | NA | NA | NA | NA | NA | NA |
| Parallel imaging* | GRAPPA | GRAPPA | GRAPPA | GRAPPA | GRAPPA | GRAPPA | GRAPPA | GRAPPA | GRAPPA |
| Flip angle(s) (degrees) | 77 | 150§ | 150§ | 70 | 60 | 5, 10, 15, 20, 25 | 24 | 70/70 | 20 |
*GRAPPA=Generalized autocalibrating partially parallel acquisitions, applied left to right with a factor of 2 in conjunction with a body matrix coil
§ Refocusing flip angles
NA=Not applicable
Analysis of magnetic resonance enterography
Interpretation of MRE-based pathoanatomical data was performed individually by 2 radiologists with 9 (AHN) and 4 (VPH) years of experience, respectively. Both were blinded to the findings on US. The maximum wall thickness and total length of disease were described in continuous measurements for the most pathological bowel segment. Average values between readers were used for comparison with bowel wall thickness and length of involvement measured on US. The presence of mural edema, ulcers, wall enhancement pattern, perimural involvement, and presence of complications like stenosis and penetrating disease were also registered for the segment, based on the MRE global score 9 10.
The ROI for DCE-MRE analysis was placed in the bowel wall at the site of the largest wall thickness and highest enhancement within the same bowel segment examined by CEUS, using a custom-made program in MATLAB® (MathWorks®, Natick, MA). The ROI was manually moved in order to stay within the bowel wall during the dynamic series, Fig. 3. TICs were interpolated using a cubic spline. This interpolated curve was used to derive the parameters described in Table 3.
Fig. 3.
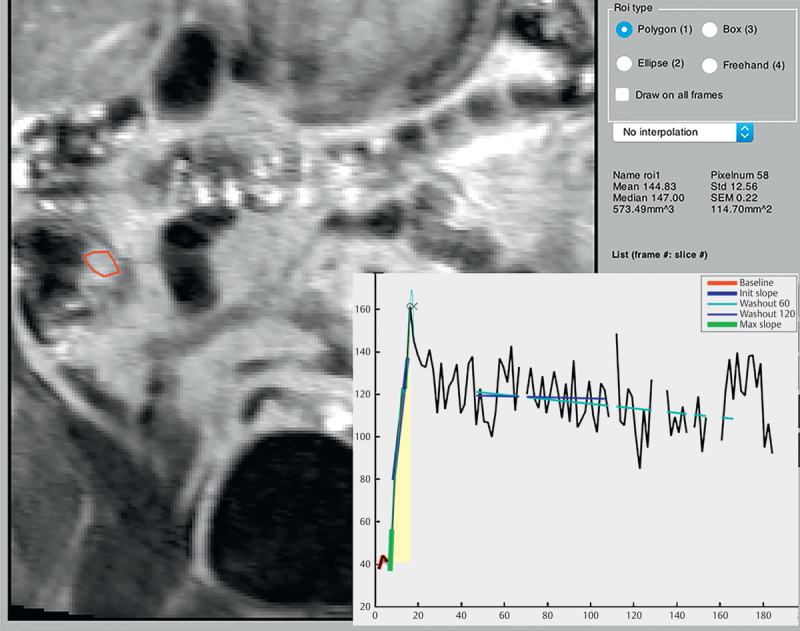
RoiTool. Dynamic contrast-enhanced MR enterography quantification, using RoiTool. Coronal T1- weighted spoiled 3D flash sequence of a 35-year-old woman. A region of interest is drawn within the thickened bowel at the terminal ileum. Corresponding graphs are produced in MatLab. The red line indicates the baseline. The bold blue line indicates the initial slope. The bold green line indicates the maximum slope. The yellow area shows the wash-in area under the curve. The two thin lines can calculate the plateau over time (not utilized in our study). ROI=region of interest.
Table 3 Time intensity curve parameters, dynamic contrast-enhanced magnetic resonance enterography.
| Value | Description |
|---|---|
| Baseline | Mean of initial frames before rapid rise in enhancement. First frame was discarded. |
| Peak | Highest enhancement within first 7 frames (15 s). In the upslope, all preceding values should present in an increasing manner. Only a single dip was allowed. |
| Rise time | Time between end of baseline and peak |
| Peak enhancement | Absolute value between peak and baseline |
| Slope | Peak enhancement divided by rise time |
| Robust slope | Best line fitted between values from 25 to 75% of peak enhancement |
| Max slope | Steepest slope over an average of 1 s |
| Wash-in AUC | Area under curve from baseline to peak – subtracted by baseline |
| AUC70 s | Area under the curve within the first 70 s |
| Time to peak | Calculated time to peak enhancement value based on extrapolation of the robust slope |
AUC=area under curve
Statistical analysis
Statistical analysis was performed using Stata 13.1 for MAC (Stata Corp LP, College Station, TX). If no disease was observed on MRE, the bowel wall thickness was set to 3 mm and the length to 0 cm. Existing data in the literature were too scarce to allow for a power calculation. However, we estimated 25 patients to be sufficient. None of the linearized CEUS intensity data, expressed as arbitrary intensity units (AIU), followed a Gaussian distribution. Hence, they were log-converted as by default in US systems using 10×log10 (AIU) and expressed in dB for further analysis 12. Time parameters for both CEUS and DCE-MRE, C-reactive protein, and fecal-calprotectin were analyzed log-converted. Correlations between DCE-MRE and CEUS TIC parameters were described with Spearman’s correlation, since DCE-MRE data were slightly skewed 26 even with log-conversion. Correlation coefficients were interpreted as suggested earlier 26. CEUS repeatability was assessed with 95% limits of agreement (LoA), using a mixed effect model with independent residuals per ROI 27. Data for length of disease and MRE global score did not follow a Gaussian distribution regardless of log conversion. Hence only intraclass correlation coefficients (ICC) are reported for these data. P-values<0.05 were considered statistically significant. Data were not corrected for multiple testing. However, final conclusions were drawn having multiple testing in mind.
Results
All but 2 patients had CEUS and DCE-MRE performed within the same day. The remaining 2 patients were scanned 4 days apart. All patients completed both examinations without adverse events or serious discomfort.
Pathoanatomical data
The thickest bowel wall segments had a mean of 7.9 mm (range: 4–12 mm) when assessed with US and 8.1 mm (range: 4–14.5 mm) when assessed with MRE. The mean difference was 0.22 mm (LoA −4.3 to 3.9) and the corresponding ICC was 0.71 (0.44–0.86, P<0.001) (Fig. 4). The median length of the inflamed segment was 15 cm (range 3–57 cm) on US and 12 cm (range 1–70 cm) on MRE. The corresponding ICC was 0.89 (0.76–0.95, P<0.001).
Fig. 4.
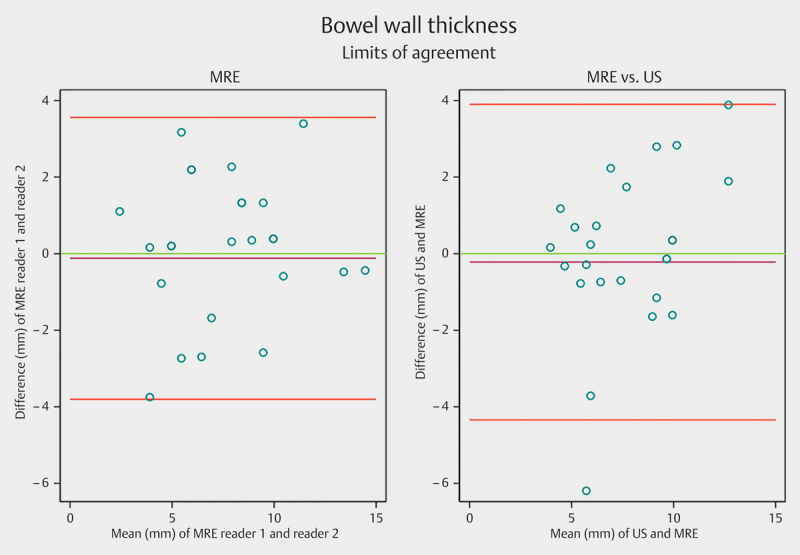
Limits of agreement for bowel wall thickness measured by ultrasound (US) and magnetic resonance enterography (MRE). The purple line shows the observed average agreement. The red lines indicate 95% limits of agreements and the green line is the perfect average agreement. MRE=magnetic resonance enterography, US=ultrasonography
Associations between perfusion data from contrast-enhanced ultrasonography and dynamic contrast-enhanced magnetic resonance enterography
Data from 3 MRE and 2 CEUS scans were excluded from further analysis, Fig. 1. All compared segments were either from the terminal ileum (n=19) or the ileum (n=1).
The total area under curve, including wash-in and wash-out for CEUS and wash-in and plateau-phase at 70 s for DCE-MRE, had a low and insignificant correlation between the 2 methods (r=0.16, P=0.494). The wash-in area under curve also showed poor correlation (r=0.18, P=0.443). Likewise, the rise time and time to peak showed no correlation between modalities (r=0.11, P=0.659 and r=0.02, P=0.930, respectively). The slope and maximum slope for DCE-MRE and wash-in rate for CEUS correlated moderately well (r=0.60, P=0.005, and r=0.62, P=0.004), Fig. 5. The peak intensity and wash-in perfusion index determined by each of the 2 methods were moderately and moderately to weakly correlated (r=0.59, P=0.006 and r=0.47, P=0.036 respectively). No significant correlation was found between peak enhancement of CEUS and of DCE-MRE (r=0.41, P=0.076).
Fig. 5.
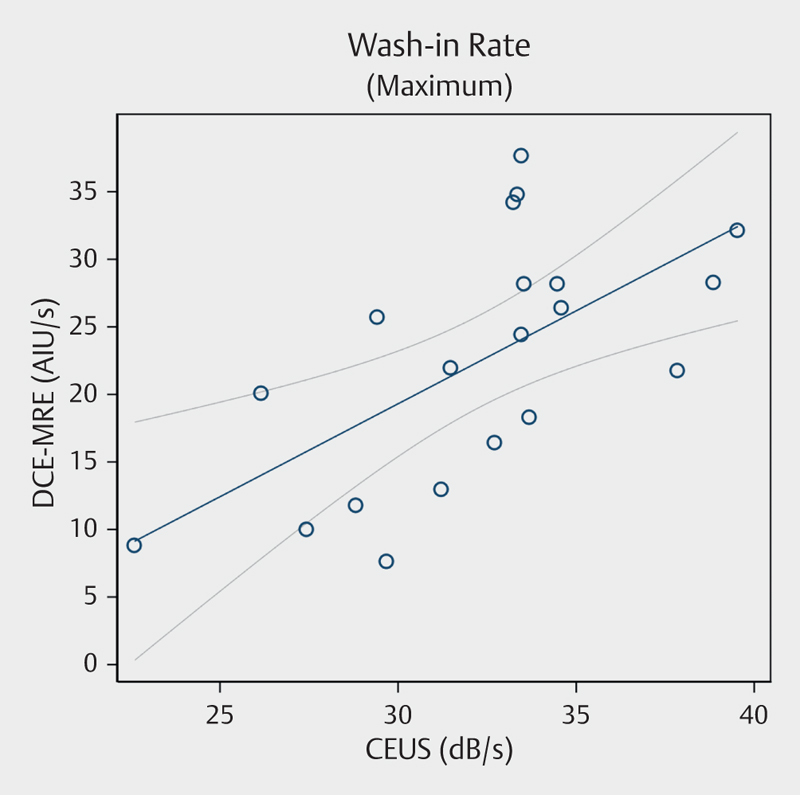
Scatter plot showing correlation between dynamic contrast-enhanced magnetic resonance enterography and contrast-enhanced ultrasound for maximum wash-in rate. Spearman’s rho=0.618, P=0.004. MRE=magnetic resonance enterography. CEUS=contrast-enhanced ultrasound. AIU=arbitrary intensity units.
Repeatability of contrast-enhanced ultrasonography and reproducibility of magnetic resonance enterography
For CEUS, the smallest mean difference between 2 contrast injections was found for the maximum peak ROI. However, the narrowest limit of agreement was consistently found for the mean ROIs, Table 4. In a post hoc analysis restricted to ROIs with QoF>90%, or if 2 ROIs could not qualify for this, at least one ROI with QoF>85% and the other>90%, LoA could be further reduced, Fig. 6 and Table 4 for all LoA, Table 5 for QoF.
Table 4 Repeatability of time intensity curve parameters, dynamic contrast-enhanced ultrasonography (CEUS).
| CEUS parameter region of interest (ROI) | Mean difference between inj. 1 and inj. 2 | P-value | Limits of agreement | Difference from large ROI | P-value |
|---|---|---|---|---|---|
| Peak Enhancement | |||||
| Large ROI | 1.36 dB (0.77–1.96) | P<0.001 | [−4.0 to 6.8] dB | Reference | NA |
| Good QoF | −0.14 dB (−0.66 to 0.38) | P=0.588 | [−4.2 to 3.9] dB | Reference | NA |
| Maximum Peak ROI | 0.63 dB (0.05–1.20) | P=0.032 | [−4.4 to 5.7] dB | 1.34 dB (0.93–1.75) | P<0.0001 |
| Good QoF | −0.49 dB (−0.89 to−0.08) | P=0.018 | [−3.7 to 2.7] dB | 1.78 dB (1.45–2.10) | P<0.0001 |
| Mean ROI | 0.73 dB (0.17–1.28) | P=0.010 | [−3.8 to 5.3] dB | 0.90 dB (0.50–1.30) | P<0.0001 |
| Good QoF | 0.24 dB (−0.13 to 0.61) | P=0.198 | [−2.3 to 2.8] dB | 1.18 dB (0.87–1.50) | P<0.0001 |
| Area under curve | |||||
| Large ROI | 1.46 dB (0.78–2.13) | P<0.0001 | [−4.6 to 7.5] dB | Reference | NA |
| Good QoF | 0.46 dB (−0.03 to 0.95) | P=0.068 | [−3.4 to 4.3] dB | Reference | NA |
| Maximum Peak ROI | 0.18 dB (−0.34 to 0.71) | P=0.489 | [−4.3 to 4.7] dB | 0.88 dB (0.46–1.30) | P<0.0001 |
| Good QoF | 0.16 dB (−0.32 to 0.63) | P=0.515 | [−3.5 to 3.8] dB | 1.31 dB (0.98–1.64) | P<0.0001 |
| Mean ROI | 0.64 dB (0.15–1.13) | P=0.010 | [−3.3 to 4.6] dB | 0.32 dB (−0.09 to 0.73) | P=0.122 |
| Good QoF | 0.75 dB (0.32–1.17) | P<0.001 | [−2.2 to 3.7] dB | 0.79 dB (0.48–1.11) | P<0.0001 |
| Wash−in rate | |||||
| Large ROI | 1.41 dB/s (0.74–2.09) | P<0.0001 | [−4.7 to 7.6] dB/s | Reference | NA |
| Good QoF | −0.59 dB/s (−1.09 to −0.08) | P=0.023 | [−4.6 to 3.4] dB/s | Reference | NA |
| Maximum Peak ROI | 0.90 dB/s (0.20–1.59) | P=0.011 | [−5.2 to 7.0] dB/s | 1.54 dB/s (1.06–2.02) | P<0.0001 |
| Good QoF | −0.68 dB/s (−1.13 to −0.24) | P=0.003 | [−4.2 to 2.8] dB/s | 1.94 dB/s (1.61–2.27) | P<0.0001 |
| Mean ROI | 0.61 dB/s (−0.00 to 1.23) | P=0.051 | [−4.4 to 5.6] dB/s | 1.17 dB/s (0.72–1.62) | P<0.0001 |
| Good QoF | −0.16 dB/s (−0.53 to 0.21) | P=0.393 | [−2.8 to 2.4] dB/s | 1.35 dB/S (1.04–1.66) | P<0.0001 |
| Wash−in perfusion index | |||||
| Large ROI | 1.34 dB/s (0.75–1.93) | P<0.0001 | [−4.0 to 6.7] dB/s | Reference | NA |
| Good QoF | −0.13 dB/s (−0.64 to 0.38) | P=0.616 | [−4.2 to 3.9] dB/s | Reference | NA |
| Maximum Peak ROI | 0.57 dB/s (0.01–1.14) | P=0.045 | [−4.4 to 5.5] dB/s | 1.31 dB/s (0.91–1.72) | P<0.0001 |
| Good QoF | −0.50 dB/s (−0.90 to −0.10) | P=0.016 | [−3.7 to 2.7] dB/s | 1.74 dB/s (1.42–2.06) | P<0.0001 |
| Mean ROI | 0.71 dB/s (0.17–1.26) | P=0.011 | [−3.8 to 5.2] dB/s | 0.87 dB/s (0.48–1.27) | P<0.0001 |
| Good QoF | 0.25 dB/s (−0.12 to 0.61) | P=0.191 | [−2.3 to 2.8] dB/s | 1.16 dB/s (0.85–1.47) | P<0.0001 |
Note – Numbers in parenthesis are 95% confidence intervals. Numbers in brackets are 95% limits of agreement
ROI=region of interest, QoF=quality of fit, CEUS=contrast-enhanced ultrasonography, inj.=injection
Fig. 6.
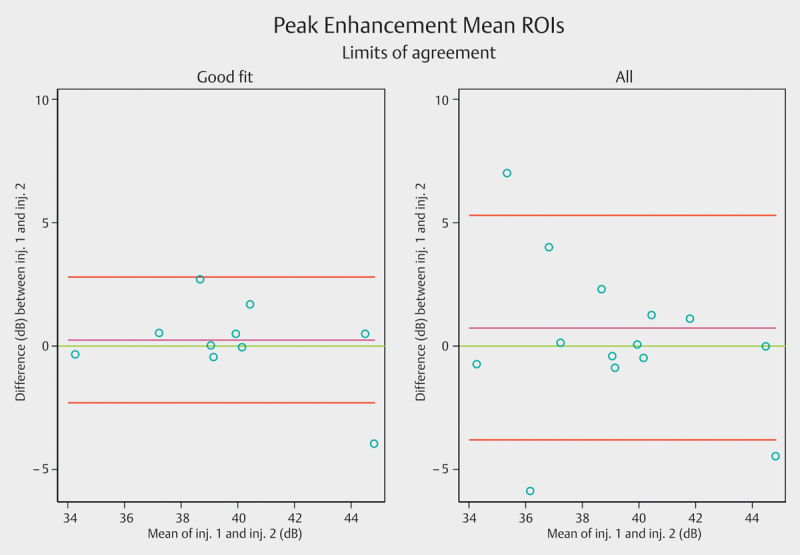
Limits of agreement (LoA) for peak enhancement mean regions of interest. The purple line shows the observed average agreement. The red lines indicate 95% limits of agreement and the green line is the perfect average agreement. ROI=region of interest. Inj.=injection.
Table 5 CEUS region of interest quality of fit.
| Quality of fit | Injection 1 | Injection 2 |
|---|---|---|
| Large ROI | 93.4 (67–99) | 97.1 (80–99) |
| Good QoF | 97.4 (82–99) | 97.4 (90–99) |
| Maximum peak ROI | 91.9 (69–96) | 94.3 (69–98) |
| Good QoF | 92.6 (86–96) | 94.2 (86–96) |
| Mean ROI | 93.7 (82–98) | 93.1 (75–97) |
| Good QoF | 95.0 (90–98) | 94.4 (86–97) |
Note – Numbers are percentages, parentheses are ranges in percentage
MRE interrater variability for bowel wall thickness showed an ICC=0.83 (0.66–0.92 P<0.001) and ICC=0.76 (0.51–0.89 P<0.001) for length of involvement. The mean difference was 1.2 mm with 95% LoA from −3.8 to 3.6 mm for wall thickness. For reproducibility on MR enterography global score, ▶Table 6.
Table 6 MR enterography reproducibility.
| MR enterography global score (MEGS) | Kappa value | P-value |
|---|---|---|
| Total score | ICC=0.79 (0.59–0.90) | P<0.0001 |
| Bowel wall thickness | κ=0.41±0.14 | P=0.0016 |
| Length of involvement | κ=0.42±0.12 | P=0.0004 |
| Lymph nodes | κ=0.51±0.19 | P=0.0046 |
| Enhancement pattern | κ=0.16±0.22 | P=0.2313 |
| Mural T2 signal | κ=0.51±0.14 | P=0.1816 |
| Perimural T2 signal | κ=0.30±0.12 | P=0.0056 |
| Comb sign | κ=0.39±0.18 | P=0.0148 |
| Fistulas | κ=0.65±0.19 | P=0.0003 |
Note – Numbers in parenthesis are 95% confidence intervals
Unless otherwise indicated, data are means±standard error
ICC=intraclass correlation coefficient, κ=kappa
Discussion
The present study compares CEUS and MRE for the description of the severity of ongoing small intestinal inflammation in CD. Even though correlations between basic pathoanatomical findings were good between the 2 modalities, our main finding was only a moderate to weak correlation when assessing relative changes in perfusion.
Since clinical activity scores for CD are poorly associated with the presence of active inflammation and equally poorly predict long-term outcome, their use should be supplemented by objective markers 1. Therefore, cross-sectional imaging is of paramount importance as an adjunct to endoscopy 4. Active inflammation is potentially treatable with effective medication but needs objective description and repeated follow-up to determine treatment response. Stenoses caused by fibrosis do not respond to medical treatment and need surgery 28. In contrast to fibrosis 29, active inflammation causes hyperemia and hyperperfusion 30 which may be quantified by CEUS and MRE.
A few previous studies have shown a significant correlation between dynamic contrast-enhanced cross-sectional imaging and clinical disease activity, biochemistry 31, or a combined score for response 32, the need for surgery 20, and change in medication 17 21 33. Other authors aimed at more objective endpoints like micro-vessel density 16 or mucosal healing or inactive disease defined by endoscopy 21. However, the studies do not agree about which TIC parameters are important. Romanini et al. [16, Saevik et al.17 and Horje et al.34 found a statistically significant difference for almost all TIC parameters and disease activity, whereas others only showed significance for time to peak 31, area under curve 32, or peak enhancement 33. In this present study, we found a significant correlation between the 2 modalities when describing peak and slope-related parameters but, surprisingly, not for area under curve, peak enhancement or rise time.
There is no consensus on how to perform or quantify intestinal perfusion measurement. Consequently, the heterogeneity between studies makes them difficult to compare or reproduce. For example, only a few authors have described the placement and analysis of ROIs for CEUS in detail 21 and only one group did log transformation of data before statistical analysis 35.
Several MRE studies use change in contrast enhancement as an indicator for disease activity 13 29 30. However, most studies have not applied a dynamic protocol and only use a few image acquisitions or the relative change over a predefined timespan after injection. Taylor et al. found an inverse correlation with slope of enhancement on MRE and micro-vessel density 36, which is the opposite of the finding by Romanini et al. using CEUS 16. These studies and our findings, showing a lack of good correlation, suggest that the 2 modalities measure somewhat dissimilar components of “perfusion”, with DCE-MRE TIC measurements being a mixture of perfusion and extravasation. Taylor et al. also found a direct correlation with slope of enhancement and disease duration and speculated that increased enhancement could be caused by ischemia and arteriolar stenosis 36.
In the present study, the interrater variability for structural MRE findings was comparable to those reported in previous studies 37. We only found a moderate correlation in wash-in rate and peak intensity could be established between DCE-MRE and CEUS. Lack of a strong correlation between modalities may likely be due to the dissimilar types and distribution nature of contrast agents, relatively poor MR time resolution, different field of view and scan planes and perhaps also the administration technique between modalities. In the optimal setting, absolute perfusion measurements of tissue blood flow, blood volume and mean transit time should be compared. However, this is complicated, even when using MR contrast agents which act as true intravascular agents, e. g., in cerebral perfusion 38.
The present study demonstrates the consequence of ROI selection in the quantification of perfusion in CD. Our data emphasize the importance of TIC QoF for reliability and reproducibility. Poor QoF 34, e. g., by fitting a burst-replenishment curve on a bolus injection examination 39, will obviously give unreliable results. We therefore recommend that curve fitting quality should be reported alongside test results in future publications. Also, using low perfused tissue as a reference will cause high uncertainty of the final results 40.
This study has some limitations. We did not apply Tofts (extended) model or any other model to reflect pharmacokinetic parameters, like absolute blood flow or permeability measures for pathological conditions 41, as our T1 measurements employing the variable flip angle technique gave unreliable results 42. As an alternative, we used the absolute signal difference technique instead, which has recently been shown to have a linear relationship to contrast agent concentration at low contrast concentrations 43 44.
Furthermore, CEUS was performed without deconvolution 45, thereby only providing semiquantitative measurements. Deconvolution is complex and relies on several assumptions 45 46 that are difficult to fulfil and thus rarely used in daily practice nor in scientific work. A method called bolus tracking and burst replenishment is described by Jirik et al. 47 but the repeatability is not yet established in humans.
Based on existing guidelines, bolus injection techniques were used for CEUS and DCE-MRE. A fixed dose and manual injection of SonoVue was chosen for CEUS quantification 48. For DCE-MRE, gadolinium dose was bodyweight-dependent and administered with an automatic pump. We chose the bodyweight-dependent dose over the fixed dose based on the general recommendation for MR contrast administration 13. We did not measure the exact length defined from an anatomical landmark, like the ileocecal valve, to ensure identical ROI location between modalities. Also, the CEUS scan planes were subjectively chosen and did not necessarily follow the standardized scan planes of MRI. The morphology of CD may vary even within short distances of the bowel and we cannot state that the exact same location was analyzed with the 2 methods 44 49. However, we attempted to do so by analyzing the same bowel segment and the thickest part of it in each patient. As a result of the disease complexity, grading disease activity should ideally involve all changes in segmental inflammation instead of narrow sampling as used in this study. However, complex scores limit use in everyday practice 50.
Since there are no guidelines on the optimal scan plane, 2 different scan planes were employed for the assessment of the repeatability of CEUS ROIs. Full repeatability of findings from the same segment in an identical scan plane along with reproducibility between investigators is still warranted. However, based on the present findings within patient repeatability seems acceptable for the clinical use of CEUS in CD, especially when applying strict criteria for size and QoF. Lack of strict criteria or the use of a low perfused tissue as the reference tissue will lead to poor reproducibility 35 40.
We chose to restrict the inclusion of patients to those with moderate to severe disease activity based on clinical symptoms. Investigating perfusion in a normal bowel wall is difficult because of peristalsis and a small ROI size results in poor QoF. However, clinical symptoms are often poorly correlated to objective signs of active disease. Based on wall thickness and biochemical findings, we covered the full disease spectrum of active small bowel disease.
In summary, there is only a moderate to weak correlation between CEUS and DCE-MRE slope-related and peak intensity parameters in CD. This is likely to be caused by the inherently different nature of the contrast agents and scanning modalities. Additionally, we have elucidated the importance of quality of fit for ROI selection in CEUS. The value of perfusion measurements as activity assessment in CD still remains to be clarified and validated against more objective endpoints.
References
- 1.Peyrin-Biroulet L, Sandborn W, Sands B E, Reinisch W, Bemelman W, Bryant R V. et al. Selecting therapeutic targets in inflammatory bowel disease (stride): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 2.Wilkens R, Novak K L, Lebeuf-Taylor E, Wilson S R. Impact of intestinal ultrasound on classification and management of Crohn disease patients with inconclusive colonoscopy. Can J Gastroenterol Hepatol. 2015;29:1–7. [PubMed] [Google Scholar]
- 3.Dubcenco E, Zou G, Stitt L, Baker J P, Jeejeebhoy K N, Kandel G. et al. Effect of standardised scoring conventions on inter-rater reliability in the endoscopic evaluation of crohn’s disease. J Crohns Colitis. 2016;22:S17. doi: 10.1093/ecco-jcc/jjw120. [DOI] [PubMed] [Google Scholar]
- 4.Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor S A, Baumgart D C. et al. Imaging techniques for assessment of inflammatory bowel disease: Joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Taylor S, Mallett S, Bhatnagar G, Bloom S, Gupta A, Halligan S. et al. METRIC (MREnterography or ulTRasound in Crohn’s disease): a study protocol for a multicentre, non-randomised, single-arm, prospective comparison study of magnetic resonance enterography and small bowel ultrasound compared to a reference standard in those. BMC Gastroenterol. 2014;14:142. doi: 10.1186/1471-230X-14-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horsthuis K, Bipat S, Bennink R J, Stoker J. Inflammatory bowel disease diagnosed with us, mr, scintigraphy, and ct: meta-analysis of prospective studies. Radiology. 2008;247:64–79. doi: 10.1148/radiol.2471070611. [DOI] [PubMed] [Google Scholar]
- 7.Murphy D J, Smyth A E, McEvoy S H, Gibson D J, Doherty G A, Malone D E. Subclassification of small bowel Crohn’s disease using magnetic resonance enterography: A review using evidence-based medicine methodology. Clin Radiol. 2015;70:1336–1343. doi: 10.1016/j.crad.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Zappa M, Stefanescu C, Cazals-Hatem D, Bretagnol F, Deschamps L, Attar A. et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011;17:984–993. doi: 10.1002/ibd.21414. [DOI] [PubMed] [Google Scholar]
- 9.Steward M J, Punwani S, Proctor I, Adjei-Gyamfi Y, Chatterjee F, Bloom S. et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81:2080–2088. doi: 10.1016/j.ejrad.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Makanyanga J C, Pendsé D, Dikaios N, Bloom S, McCartney S, Helbren E. et al. Evaluation of Crohn’s disease activity: Initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014;24:277–287. doi: 10.1007/s00330-013-3010-z. [DOI] [PubMed] [Google Scholar]
- 11.Ziech ML W, Bossuyt PM M, Laghi A, Lauenstein T C, Taylor S A, Stoker J. Grading luminal Crohn’s disease: which MRI features are considered as important? Eur J Radiol. 2012;81:e467–e472. doi: 10.1016/j.ejrad.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Medellin-Kowalewski A, Wilkens R, Wilson A, Ruan J, Wilson S R. Quantitative contrast-enhanced ultrasound parameters in crohn disease: their role in disease activity determination with ultrasound. AJR Am J Roentgenol. 2016;206:64–73. doi: 10.2214/AJR.15.14506. [DOI] [PubMed] [Google Scholar]
- 13.Rimola J, Rodriguez S, García-Bosch O, Ordás I, Ayala E, Aceituno M. et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–1120. doi: 10.1136/gut.2008.167957. [DOI] [PubMed] [Google Scholar]
- 14.Darwin F. On the primary vascular dilatation in acute inflammation. J Anat Physiol. 1875;10:1–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips M H. et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 16.Romanini L, Passamonti M, Navarria M, Lanzarotto F, Villanacci V, Grazioli L. et al. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in inflammatory bowel disease. Eur J Radiol. 2014;83:1317–1323. doi: 10.1016/j.ejrad.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Saevik F, Nylund K, Hausken T, Odegaard S, Gilja O H. Bowel perfusion measured with dynamic contrast-enhanced ultrasound predicts treatment outcome in patients with crohn’s disease. Inflamm Bowel Dis. 2014;20:2029–2037. doi: 10.1097/MIB.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckley D L. Uncertainty in the analysis of tracer kinetics using dynamic contrast-enhancedT1-weighted MRI. Magn Reson Med. 2002;47:601–606. doi: 10.1002/mrm.10080. [DOI] [PubMed] [Google Scholar]
- 19.Payen T, Coron A, Lamuraglia M, Le Guillou-Buffello D, Gaud E, Arditi M. et al. Echo-power estimation from log-compressed video data in dynamic contrast-enhanced ultrasound imaging. Ultrasound Med Biol. 2013;39:1826–1837. doi: 10.1016/j.ultrasmedbio.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Nylund K, Jirik R, Mezl M, Leh S, Hausken T, Pfeffer F. et al. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with crohn’s disease. Ultrasound Med Biol. 2013;39:1197–1206. doi: 10.1016/j.ultrasmedbio.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Quaia E, Sozzi M, Angileri R, Gennari A G, Cova M A. time-intensity curves obtained after microbubble injection can be used to differentiate responders from nonresponders among patients with clinically active crohn disease after 6 weeks of pharmacologic treatment. Radiology 2016. 0:152461. doi: 10.1148/radiol.2016152461. [DOI] [PubMed] [Google Scholar]
- 22.Best W R, Becktel J M, Singleton J W, Kern F Jr. Development of a Crohn’s disease activity index. National cooperative crohn’s disease study. Gastroenterology. 1976;70:439. [PubMed] [Google Scholar]
- 23.Harvey R F, Bradshaw J M. A simple index of Crohn’s-disease activity. Lancet (London, England) 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg M S, Satsangi J, Ahmad T, Arnott ID R, Bernstein C N, Brant S R. et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 25.Limberg B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Zeitschrift Für Gastroenterol. 1999;37:495–508. [PubMed] [Google Scholar]
- 26.Zou K H, Tuncali K, Silverman S G. Correlation and simple linear regression. Radiology. 2003;227:617–622. doi: 10.1148/radiol.2273011499. [DOI] [PubMed] [Google Scholar]
- 27.Raunig D L, McShane L M, Pennello G, Gatsonis C, Carson P L, Voyvodic J T. et al. Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Stat Methods Med Res. 2015;24:27–67. doi: 10.1177/0962280214537344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spinelli A, Correale C, Szabo H, Montorsi M. Intestinal fibrosis in Crohn’s disease: medical treatment or surgery? Curr Drug Targets. 2010;11:242–248. doi: 10.2174/138945010790309984. [DOI] [PubMed] [Google Scholar]
- 29.Rimola J, Planell N, Rodríguez S, Delgado S, Ordás I, Ramírez-Morros A. et al. Characterization of Inflammation and Fibrosis in Crohn’s Disease Lesions by Magnetic Resonance Imaging. Am J Gastroenterol. 2015;110:432–441. doi: 10.1038/ajg.2014.424. [DOI] [PubMed] [Google Scholar]
- 30.Röttgen R, Grandke T, Grieser C, Lehmkuhl L, Hamm B, Lüdemann L. Measurement of MRI enhancement kinetics for evaluation of inflammatory activity in Crohn’s disease. Clin Imaging. 2010;34:29–35. doi: 10.1016/j.clinimag.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Girlich C, Schacherer D, Jung E M, Schreyer A, Büttner R. Comparison between a clinical activity index (Harvey-Bradshaw-Index), laboratory inflammation markers and quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound in Crohn’s disease. Eur J Radiol. 2012;81:1105–1109. doi: 10.1016/j.ejrad.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 32.Quaia E, Cabibbo B, De Paoli L, Toscano W, Poillucci G, Cova M A. The value of time-intensity curves obtained after microbubble contrast agent injection to discriminate responders from non-responders to anti-inflammatory medication among patients with Crohn’s disease. Eur Radiol. 2013;23:1650–1659. doi: 10.1007/s00330-012-2754-1. [DOI] [PubMed] [Google Scholar]
- 33.Moreno N, Ripollés T, Paredes J M, Ortiz I, Martínez M J, López A. et al. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis. 2014;8:1079–1087. doi: 10.1016/j.crohns.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Horjus Talabur Horje C S, Bruijnen R, Roovers L, Groenen MJ M, Joosten FB M, Wahab P J. Contrast enhanced abdominal ultrasound in the assessment of ileal inflammation in crohn’s disease: a comparison with mr enterography. PLoS One. 2015;10:e0136105. doi: 10.1371/journal.pone.0136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Socaciu M, Ciobanu L, Diaconu B, Hagiu C, Seicean A, Badea R. Non-Invasive assessment of inflammation and treatment response in patients with crohn’s disease and ulcerative colitis using contrast-enhanced ultrasonography quantification. J Gastrointestin Liver Dis. 2015;24:457–465. doi: 10.15403/jgld.2014.1121.244.chr. [DOI] [PubMed] [Google Scholar]
- 36.Taylor S A, Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E. et al. Radiology. 2009;251:369–379. doi: 10.1148/radiol.2512081292. [DOI] [PubMed] [Google Scholar]
- 37.Makanyanga J C, Pendsé D, Dikaios N, Bloom S, McCartney S, Helbren E. et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014;24:277–287. doi: 10.1007/s00330-013-3010-z. [DOI] [PubMed] [Google Scholar]
- 38.Calamante F. Arterial input function in perfusion MRI: a comprehensive review. Prog Nucl Magn Reson Spectrosc. 2013;74:1–32. doi: 10.1016/j.pnmrs.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Wong D D, Forbes G M, Zelesco M, Mason R, Pawlik J, Mendelson R M. Crohn’s disease activity: quantitative contrast-enhanced ultrasound assessment. Abdom Imaging. 2012;37:369–376. doi: 10.1007/s00261-011-9792-z. [DOI] [PubMed] [Google Scholar]
- 40.Zink F, Kratzer W, Schmidt S, Oeztuerk S, Mason R A, Porzner M. et al. Comparison of two high-end ultrasound systems for contrast-enhanced ultrasound quantification of mural microvascularity in crohn’s disease. Ultraschall Med. 2016;37:74–81. doi: 10.1055/s-0034-1398746. [DOI] [PubMed] [Google Scholar]
- 41.Tofts P S, Brix G, Buckley D L, Evelhoch J L, Henderson E, Knopp M V. et al. Estimating kinetic parameters from dynamic contrast-enhanced t1-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(SICI)1522-2586(199909)10:3<223::AID-JMRI2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 42.Sourbron S P, Buckley D L. On the scope and interpretation of the Tofts models for DCE-MRI. Magn Reson Med. 2011;66:735–745. doi: 10.1002/mrm.22861. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Xue Y, Zhao X, Yu J, Rosen M, Song H K. Effects of flip angle uncertainty and noise on the accuracy of DCE-MRI metrics: comparison between standard concentration-based and signal difference methods. Magn Reson Imaging. 2015;33:166–173. doi: 10.1016/j.mri.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharman A, Zealley I A, Greenhalgh R, Bassett P, Taylor S A. MRI of small bowel Crohn’s disease: determining the reproducibility of bowel wall gadolinium enhancement measurements. Eur Radiol. 2009;19:1960–1967. doi: 10.1007/s00330-009-1371-0. [DOI] [PubMed] [Google Scholar]
- 45.Mezl M, Jirik R, Harabis V, Kolar R, Standara M, Nylund K. et al. Absolute ultrasound perfusion parameter quantification of a tissue-mimicking phantom using bolus tracking [Correspondence] IEEE Trans Ultrason Ferroelectr Freq Control. 2015;62:983–987. doi: 10.1109/TUFFC.2014.006896. [DOI] [PubMed] [Google Scholar]
- 46.Gauthier M, Tabarout F, Leguerney I, Polrot M, Pitre S, Peronneau P. et al. Assessment of quantitative perfusion parameters by dynamic contrast-enhanced sonography using a deconvolution method: an in vitro and in vivo study. J Ultrasound Med. 2012;31:595–608. doi: 10.7863/jum.2012.31.4.595. [DOI] [PubMed] [Google Scholar]
- 47.Jirik R, Nylund K, Gilja O H, Mezl M, Harabis V, Kolar R. et al. Ultrasound perfusion analysis combining bolus-tracking and burst-replenishment. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:310–319. doi: 10.1109/TUFFC.2013.2567. [DOI] [PubMed] [Google Scholar]
- 48.Piscaglia F, Nolsøe C, Dietrich C F, Cosgrove D O, Gilja O H, Bachmann Nielsen M. et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (ceus): update 2011 on non-hepatic applications. Ultraschall Med 2011. :33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 49.Borley N R, Mortensen N J, Jewell D P, Warren B F. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn’s disease: evidence for a possible causative link. J Pathol. 2000;190:196–202. doi: 10.1002/(SICI)1096-9896(200002)190:2<196::AID-PATH513>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Calabrese E, Zorzi F, Zuzzi S, Ooka S, Onali S, Petruzziello C. et al. Development of a numerical index quantitating small bowel damage as detected by ultrasonography in Crohn’s disease. J Crohns Colitis. 2012;6:852–860. doi: 10.1016/j.crohns.2012.01.015. [DOI] [PubMed] [Google Scholar]


