Abstract
This letter describes the chemical optimization of a novel series of M4 positive allosteric modulators (PAMs) based on a 5-amino-thieno[2,3-c]pyridazine core, developed via iterative parallel synthesis, and culminating in the highly utilized rodent in vivo tool compound, VU0467154 (5). This is the first report of the optimization campaign (SAR and DMPK profiling) that led to the discovery of VU0467154, and details all of the challenges faced in allosteric modulator programs (steep SAR, species differences in PAM pharmacology and subtle structural changes affecting CNS penetration).
Keywords: M4, Muscarinic acetylcholine receptor, Positive allosteric modulator (PAM), Schizophrenia, Structure-Activity Relationship (SAR)
Graphical abstract
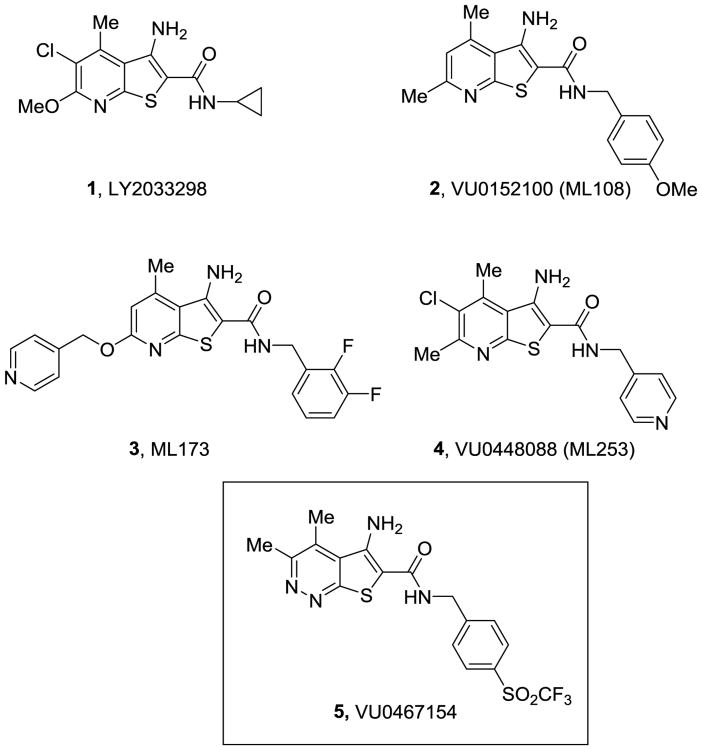
M4 (muscarinic acetylcholine receptor subtype 4) positive allosteric modulators (PAMs) represent an exciting therapeutic strategy to treat multiple domains of schizophrenia,1-18 as well as other CNS disorders,19,20 via a new molecular mechanism.21 However, the great potenital has been hampered by limited chemical diversity centered on a 3-amino-thieno[2,3-b]pyridine core, as in 1-4, (Figure 1), which engenders steep SAR, species differences (rat versus human M4 PAM potency, affinity/cooperativity and subtype selectivity), poor solubility, and/or low CNS penetration.1-18 Early in vivo tool compounds, such as 2,1,2 energized the field but were not optimal. Recently, we disclosed VU0467154 (5) based on a novel 5-amino-thieno[2,3-c]pyridazine core that has proven to be a valuable rodent in vivo M4 PAM tool compound with robust efficacy in a broad range of preclinical mouse and rat models of psychosis and cognition, as well as Huntington's disease.11,17,18 Here, we describe for the first time the optimization campaign (SAR and intriguing DMPK profiles) that advanced PAMs 2-4, into 5 with exceptional rodent M4 PAM potency, selectivity, DMPK profile and CNS penetration; however, a significant species disconnect (35x less potent on human M4) precluded advancement as a clinical candidate.11,17,18
Figure 1.
Structures of representative M4 PAMs 1-4, highlighting the conserved 3-amino-thieno[2,3-b]pyridine chemotype, and the optimized rodent in vivo tool M4 PAM, VU0467154 (5) with a novel pyridazine core.
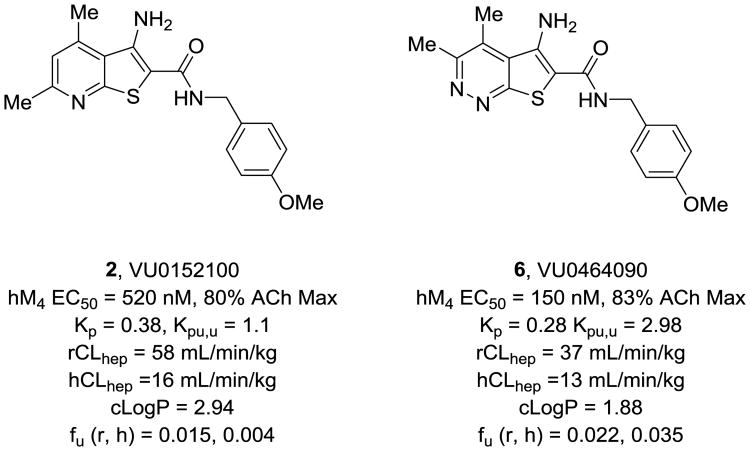
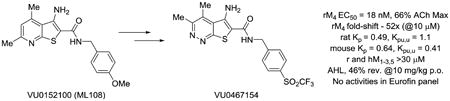
Within the 3-amino-thieno[2,3-b]pyridine series, balancing M4 PAM potency and solubility was a distinct challenge;1-18 therefore, initial efforts focused on replacements for the pyridine ring, as very few substituents were tolerated. Regioisomeric pyridines were evaluated, along with isomeric pyrimidines, but all lost considerable M4 PAM potency at both human and rat M4. Based on the attractive physiochemical properties and high dielectric constant of the pyridazine ring, we prepared a 5-amino-thieno[2,3-c]pyridazine congener 6 of 2 (Figure 2). This modification proved favorable (M4 PAM potency enhanced 4-fold, free fraction increased up to 9-fold, CLhep improved as well as Kp,uu and cLogP with no change in molecular weight), but the PMB amide proved to be a metabolic ‘hot spot’ (CYP-mediated oxidative demethylation) and would need to be replaced with an alternate amide.22 At this point, multiple avenues of inquiry were pursued, and here we will focus on amide moieties containing sulfur (thioethers, sulfoxides, sulfones and SF5) moieties.
Figure 2.
Structures of the first in vivo M4 PAM tool compound 2, and the new 5-amino-thieno[2,3-c]pyridazine congener 6, which displayed improved physiochemical, pharmacological and DMPK properties.
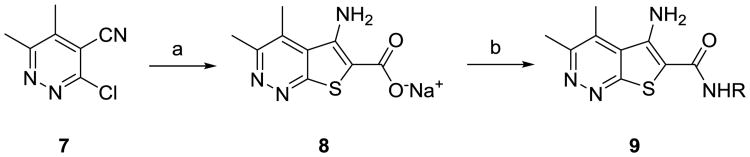
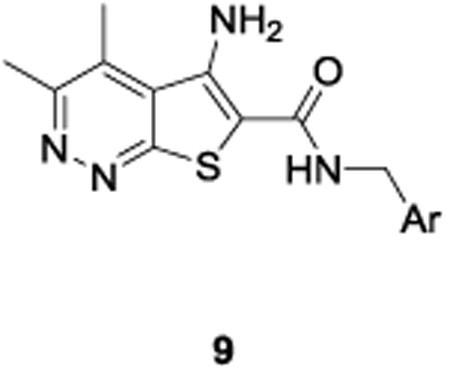
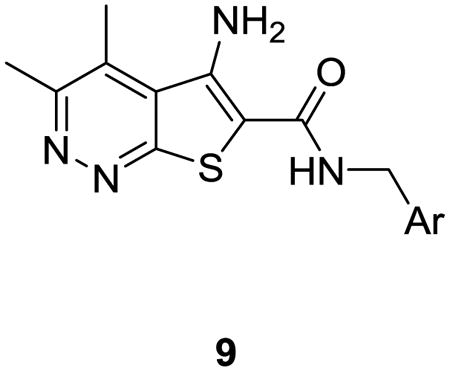
The synthesis of analogs 9 proved to be straightforward. Condensation of commercially available 3-chloro-5,6-dimethylpyridazine-4-carbonitrile 7 with methyl thioglycolateunder basic conditions smoothly affords the sodiumcarboxylate 8 in 78% yield.11 A HATU-mediated amide coupling with various benzyl amines then delivered analogs 9in yields ranging from 45-92%. In all cases, either the desired benzylamine or the corresponding nitrile, which was easily reduced to the requisite benzyl amine, was commercially available.
SAR was driven on rat M4, as the objective was an in vivo POC tool compound, but key compounds were assessed on human M4 as well. As shown in Table 1, many potent rat M4 PAMs were discovered, and several displayed excellent CNS pentration (Kp and Kp,uu). The direct thioether analog 9a of 6 displayed an ∼10-fold increase in rat M4 PAM potency (EC50= 11.2 nM), but diminished CNS exposure (Kp = 0.13). Oxidation to the corresponding sulfoxide 9b lost potency(EC50 = 139 nM), which was restored by the methyl sulfone9c (EC50 = 51.3 nM; however, CNS penetration was poor, Kp= 0.04). Moving the sulfone from the 4-position to the 3-position, as in 9d, was poorly tolerated (EC50 = 417 nM), while steric bulk, as in 9e-g, retained good rat M4 PAM potency (EC50s 22-48 nM), but with low, variable Kps (0.05 to 0.11). Thus, steric bulk alone was not sufficient to balance PAM potency and Kp, therefore increased lipophilicity, in the form of fluorine atoms, was then evaluated. Addition of a single fluorine atom alpha to the methyl sulfone (9h) maintained PAM potency (EC50 = 58 nM), and CNS penetration improved (Kp = 0.51, Kp,uu = 2.78). Consecutive addition of fluorines to the methyl moiety of 9c afforded various fluoromethyl sulfones 9i-j with potent rat M4 PAM activity (EC50s 17 to 23 nM), but unexpected and highly variable CNS exposure. The mono-fluoromethyl analog 9i was not detected in the CNS, while brain distribution of the difluoromethyl congener 9j was modest (Kp = 0.11, Kp,uu = 0.10) and that of the trifluoromethyl derivative 5 was exceptional (Kp = 0.49, Kp,uu = 1.1). Finally, an unusual pentaflurosulfur (SF5) analog, 9l, was a potent rat M4 PAM (EC50 = 30 nM) with excellent CNS distribution (Kp = 1.4, Kp,uu = 1.0). Of these, 5 and 9l stood out as potential candidates as rodent in vivo tool compounds, but we wanted to assess their activity at human M4 (as species differences are common amongst M4 PAM ligands) to determine if these could translate into clinical candidates.8-11,13,14 Unfortunately, as shown in Table 2, there was a 6-to 35-fold rightward shift in human PAM potency, precluding these analogs from consideration as clinical candidates.
Table 1.
Structures and activities for rat M4 PAM 5 and analogs 9.
| ||||
|---|---|---|---|---|
| Cpd | Ar | rM4EC50 (nM)a[% ACh Max ±SEM] | rM4 pEC50 (±SEM) | Rat Kp(Kp,uu)b |
| 9a |
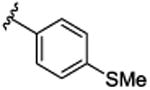
|
11.2 [75.6±1.7] | 7.95±0.09 | 0.13 (0.48) |
| 9b |
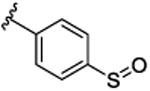
|
139 [74.2±1.9] | 6.86±0.04 | ND |
| 9c |
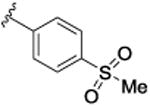
|
51.3 [72.6±2.2] | 7.29±0.08 | 0.04 (0.14) |
| 9d |
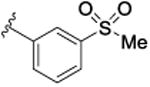
|
417 [74.6±2.1] | 6.38±0.17 | ND |
| 9e |
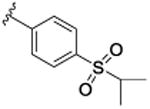
|
47.9 [76.8±2.7] | 7.32±0.06 | 0.07 (0.67) |
| 9f |
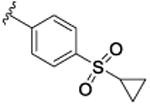
|
22.4 [67.1±3.1] | 7.65±0.05 | 0.05 (0.29) |
| 9g |
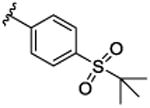
|
44.7 [72.1±2.2] | 7.35±0.06 | 0.11 (0.34) |
| 9h |
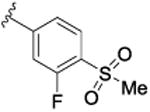
|
57.7 [69.2±3.5] | 7.24±0.10 | 0.51 (2.8) |
| 9i |
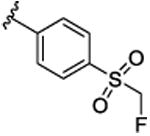
|
21.9 [62.5±5.5] | 7.66±0.04 | BLQ |
| 9j |
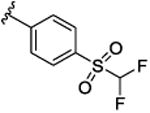
|
17.8 [65.2±2.4] | 7.75±0.14 | 0.11 (0.10) |
| 5 |
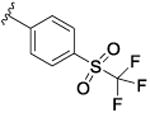
|
17.8 [68.1±1.6] | 7.75±0.06 | 0.49 (1.1)c |
| 9k |
|
23.4 [76.7±2.6] | 7.63±0.05 | BLQ |
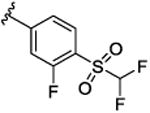
| 9l |
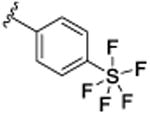
|
30.2 [75.7±1.6] | 7.52±0.03 | 1.4 (1.0) |
Calcium mobilization assays with rM4/Gqi5-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine; values represent means from three (n=3) independent experiments performed in triplicate.
Total and calculated unbound brain:plasma partition coefficients determined at 0.25 hr post-administration of an IV cassette dose (0.20-0.25 mg/kg) to male, SD rat (n=1); in conjunction with in vitro rat plasma protein and brain homogenate binding assay data. ND = not determined. BLQ = below limit of quantitation.
Table 2.
Structures and human activities for M4 PAM analogs 9.
| |||
|---|---|---|---|
| Cpd | Ar | hM4EC50 (nM)a [% ACh Max ±SEM] | hM4 pEC50 (±SEM) |
| 9a |
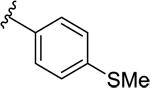
|
60.2 [71.9±1.7] | 7.22±0.09 |
| 9c |
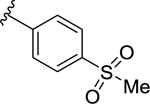
|
363 [73.8±2.2] | 6.44 ±0.08 |
| 9i |
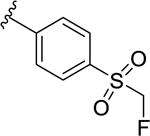
|
209 [62.5±5.5] | 6.68±0.04 |
| 9j |
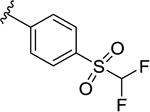
|
251 [72.8±2.4] | 6.60±0.14 |
| 5 |
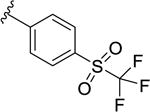
|
631 [56.4±2.4] | 6.20±0.06 |
| 9l |
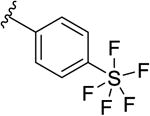
|
550 [66.1±1.6] | 6.26±0.03 |
Calcium mobilization assays with hM4/Gqi5-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine; values represent means from three (n=3) independent experiments performed in triplicate.
Next, we assessed physiochemical properties and DMPK profiles of 9j, 5 and 9l in battery of in vitro and in vivo assays (Table 2). Molecular weights were all similar and both the difluoromethyl sulfone 9j and the trifluoromethyl sulfone 5 had similar TPSAs, but the SF5 analog, 9l, had greatly reduced TPSA. In vitro clearance (predicted CLhep for both human and rat) was similar across the three PAMs, but plasma protein binding did differentiate the PAMs. 9j, with a cLogP of 1.98, had the most favorable fraction unbound (fu (rat, human) of 0.066 and 0.053) as well as fraction unbound in brain (fu rat brain, 0.062). In contrast, the highly lipophilic SF5 (cLogP = 5.13) congener 9l, displayed poor fraction unbound (fu (rat, human) of 0.004 and 0.010). 5 represented the middle ground with moderate fractionun bound in plasma (fu (rat, human) of 0.031 and 0.019) and in brain (fu rat brain, 0.067) with a n optimal cLogP (2.49) predictive of good CNS exposure. The CYP450 profiles were very clean, except for weak inhibition of 2D6 by 9j and 9l. In vivo rat PK (1 mg/kg i.v.) again distinguished 5, with low CLp (7.8 mL/min/kg), a long half-life (t1/2 = 5.7 hours), excellent oral bioavailability (61%F, from 3 mg/kg suspension dose) and with excellent brain distribution (Kp = 0.49 and Kp,uu = 1.1. Interestingly, and despite varying in vivo DMPK profiles, all three afforded robust reversal of amphetamine-induced hyperlocomotion (AHL) when evaluated in single-point oral dosing at either 10 mg/kg (9j, 49.3% and 5, 55.3%) or at 30 mg/kg (9l, 66.3%) – our standard pharmacodynamic assay for M4 PAM optimization. While we have recently reported results from dose-response AHL (as well as MK-801-induced hyperlocomotion) studies with 5 in rat and mouse (Kp for mouse of 0.64), here we show full dose response AHL for 9j and 9l (Fig. 3), and, while not ideal tools, both 9j and 9l are efficacious in vivo. Data for 5 was previously reported, with 46% reversal at 10 mg/kg p.o, 53% reversal at 30 mg/kg p.o. and a minimum effective dose (MED) at 3 mg/kg p.o. (33% reversal).11
Figure 3.
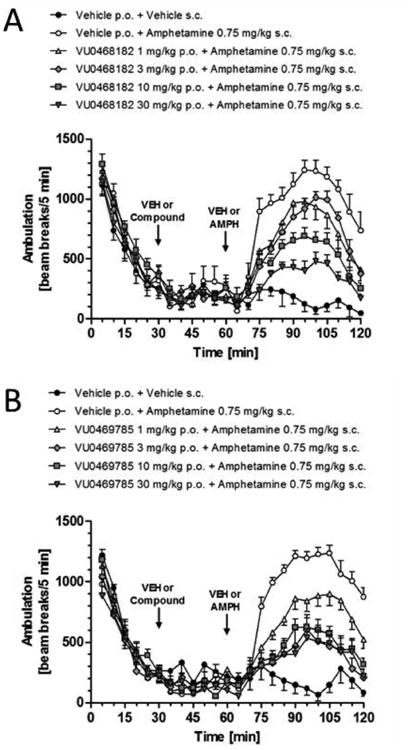
Reversal of amphetamine-induced hyperlocomotion with A) 9j (VU0468182), B) 9l (VU0469785). The M4 PAMs were administered 30 minutes after habituation in the chamber, and either vehicle or 0.75 mg/kg amphetamine administered s.c. at t = 60 min. For each dose group, n = 7-8 rats.
To vet these analogs as potential rodent in vivo tool compounds, we next evaluated selectivity across the other muscarinic receptors (M1-3,5) and broader ancillary pharmacology in Eurofin 's Lead Profiling panel (68 GPCRs, ion channels and transporter radioligand binding assay panel).23 All three M4 PAMs, 9j, 5 and 9l, had no activity (EC50s >30 μM) at both rat and human M1-3,5 and 5 displayed no ancillary pharmacology at any of the 68 targets in the Eurofin panel (no % inhibition >50%@10 μM). Both 9j and 9l showed a less clean profile, showing significant off-target activities at multiple targets (>50% inhibition@10 μM which were then confirmed in full CRC). 9j proved to be a ligand for hCav1 2 (5.7 μM), DAT (175 nM), SERT (600 nM) and 5-HT3 (1.6 μM). Similarly, 9l proved to be a ligand for DAT (150 nM), SERT (520 nM), Ghrelin receptor (6.5 μM), BDZ (1.5 μM), GABA (2.1 μM) and 5-HT3 (1.5 μM) Therefore, 5 emerged as the ideal rodent in vivo M4 PAM tool compound with clean ancillary pharmacology and excellent rodent PK. A subsequent, broader ancillary pharmacology panel identified single off-target activity for 5, the human adenosine transporter, with an IC50 of 240 nM. The full in vitro and in vivo pharmacological profile of 5 (VU0467154) has been described in detail.11
In summary, we have detailed for the first time the progression from the prototypical 3-amino-thieno[2,3-b]pyridine core M4 PAM core to the 5-amino-thieno[2,3-c]pyridazine core, which imparts compounds with improved potency, physiochemical properties and DMPK profiles. However, the sulfone series detailed here still suffers from significant species differences in M4 PAM potency, in favor of rat, and unpredictable variations in CNS penetration based on fluorine content and/or lipophilicity. This effort led to the study of an unusual SF5 congener with potent PAM activity and in vivo efficacy, but the high cLogP proved problematic. Importantly, this gave rise to the highly valuable rodent M4 PAM in vivo tool compound 5 (VU0467154), with a balanced profile and demonstrated utility in target validation studies. Further optimization efforts en route to M4 PAM clinical candidates, with equivalent human and rat M4 PAM potencies within this series, will be reported in due course.
Scheme 1.
Synthesis of M4 PAM analogs 9.a
aReagents and conditions: (a) Methyl thioglycolate, MeOH, 1M aq. NaOH, 150 °C, microwave, 30 min, 78%; (b) NH2CH2Ar, HATU, DMF, DIEPA, 2 h, 45-92%.
Table 3.
In vitro and in vivo DMPK properties of 9j, 5 and 9l.
| Property | 9j | 5 | 9l |
|---|---|---|---|
| MW | 426.4 | 444.4 | 438.4 |
| cLogP | 1.98 | 2.49 | 5.13 |
| TPSA | 114 | 114 | 79.8 |
| In vitro PK parameters | |||
| CLINT (mL/min/kg), rat | 48.1 | 47.7 | 95.9 |
| CLHEP (mL/min/kg), rat | 28.1 | 28.4 | 40.5 |
| CLINT (mL/min/kg), human | 15.6 | 19.8 | 52.8 |
| CLHEP (mL/min/kg), human | 9.34 | 10.2 | 15.0 |
| Rat fuplasma | 0.066 | 0.031 | 0.004 |
| Human fuplasma | 0.053 | 0.019 | 0.010 |
| Rat fubrain | 0.062 | 0.067 | 0.004 |
| Cytochrome P450 (IC50, μM) | |||
| 1A2 | >30 | >30 | >30 |
| 2C9 | >30 | >30 | >30 |
| 2D6 | 20.8 | >30 | 23.9 |
| 3A4 | >30 | >30 | >30 |
| In vivo PK (Sprague-Dawley rats) | |||
| IV (1 mg/kg) | |||
| CLp (mL/min/kg) | 16 | 7.8 | 12 |
| t ½ (h) | 2.4 | 5.7 | 3.6 |
| Vss (L/kg) | 2.9 | 3.1 | 3.1 |
| Tissue Distribution (1 mg/kg, IP) Sample time (0.25 h) | |||
| Kp, brain | 0.11 | 0.49 | 1.0 |
| Kpuu, brain | 0.10 | 1.1 | 0.75 |
| % reversal in AHL (10 mg/kg, p.o.) | 49.3 | 55.3 | 66.3 |
Acknowledgments
We thank the NIH for funding via the NIH Roadmap Initiative 1X01 MH077607 (C.M.N.), the Molecular Libraries Probe Center Network (U54MH084659 to C.W.L.) and U01MH087965 (Vanderbilt NCDDG). We also thank William K. Warren, Jr. and the William K. Warren Foundation who funded the William K. Warren, Jr. Chair in Medicine (to C.W.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan WY, McKinize DL, Bose S, Mitchell SN, Witkins JM, Thompson RC, Christopoulos A, Birdsall NJ, Bymaster FP, Felder CC. Proc Natl Acad Sci USA. 2008;105:10978–10983. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach K, Loiancono RE, Felder CC, McKinize DL, Mogg A, Shaw DB, Sexton PM, Christopoulos A. Neuropsychopharmacology. 2010;35:855–869. doi: 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady A, Jones CK, Bridges TM, Kennedy PJ, Thompson AD, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey J, Conn PJ, Lindsley CW. J Pharm & Exp Ther. 2008;327:941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishnakar R, Kelm ND, Damon S, Bridges TM, Melancon BJ, Tarr JC, Brogan JT, Avison MJ, Deutch AY, Wess J, Wood MR, Lindsley CW, Gore JC, Conn PJ, Jones CK. Neuropsychopharmacology. 2014;39:1578–1593. doi: 10.1038/npp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell M, Roth BL. Neuropsychopharmacology. 2010;35:851–852. doi: 10.1038/npp.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones CK, Byun N, Bubser M. Neuropsychopharmacology. 2012;37:16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirey JK, Xiang Z, Orton D, Brady AE, Johnson KA, Williams R, Ayala JE, Rodriguez AL, Wess J, Weaver D, Niswender CM, Conn PJ. Nat Chem Bio. 2008;4:42–50. doi: 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- 8.Le U, Melancon BJ, Bridges TM, Utley TJ, Lamsal A, Vinson PN, Sheffler DJ, Jones CK, Morrison R, Wood MR, Daniels JS, Conn PJ, Niswender CM, Lindsley CW, Hopkins CR. Bioorg Med Chem Lett. 2013;23:346–350. doi: 10.1016/j.bmcl.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy JP, Bridges TM, Gentry PR, Brogan JT, Brady AE, Shirey JK, Jones CK, Conn PJ, Lindsley CW. ChemMedChem. 2009;4:1600–1607. doi: 10.1002/cmdc.200900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salovich JM, Sheffler DJ, Vinson PN, Lamsal A, Utley TJ, Blobaum AL, Bridges TM, Le U, Jones CK, Wood MR, Daniels JS, Conn PJ, Niswender CM, Lindsley CW, Hopkins CR. Bioorg Med Chem Lett. 2012;22:5084–5088. doi: 10.1016/j.bmcl.2012.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubser M, Bridges TM, Thorbeck DD, Gould RW, Grannan M, Noetzel MJ, Niswender CM, Daniels JS, Melancon BJ, Tarr JC, Wess J, Duggan ME, Brandon NJ, Dunlop J, Wood MW, Wood MR, Lindsley CW, Conn PJ, Jones CK. ACS Chem Neurosci. 2014;5:920–942. doi: 10.1021/cn500128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith E, Chase P, Niswender CM, Conn PJ, Lindsley CW, Madoux F, Acosta M, Scampavia L, Spicer T, Hodder P. J Biomol Screening. 2015;20:858–868. doi: 10.1177/1087057115581770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood MR, Noetzel MJ, Tarr JC, Rodriguez AL, Lamsal A, Chang S, Foster JJ, Smith E, Hodder PS, Engers DW, Niswender CM, Brandon NJ, Wood MW, Duggan ME, Conn PJ, Bridges TM, Lindsley CW. Bioorg Med Chem Lett. 2016;26:4282–4286. doi: 10.1016/j.bmcl.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood MR, Noetzel MJ, Engers JL, Bollinger KA, Melancon BJ, Tarr JC, Han C, West M, Gregro AR, Lamsal A, Chang S, Ajmera S, Smith E, Chase P, Hodder PS, Bubser M, Jones CK, Hopkins CR, Emmitte KA, Niswender CM, Wood MW, Duggan ME, Conn PJ, Bridges TM, Lindsley CW. Bioorg Med Chem Lett. 2016;26:3029–3033. doi: 10.1016/j.bmcl.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabo M, Huynh T, Valant C, Lane JR, Sexton PM, Christopoulos A, Capuano B. MedChemComm. 2015;6:1998–2003. [Google Scholar]
- 16.Huynh T, Valant C, Crosby IT, Sexton PM, Christopoulos A, Capuano B. ACS Chem Neurosci. 2015;6:1592–1599. doi: 10.1021/acschemneuro.5b00035. [DOI] [PubMed] [Google Scholar]
- 17.Croy CH, Schober DA, Xiao H, Quets A, Christopoulos A, Felder CC. Mol Pharmacol. 2014;86:106–115. doi: 10.1124/mol.114.091751. [DOI] [PubMed] [Google Scholar]
- 18.Huynh T, Valant C, Crosby IT, Sexton PM, Christopoulos A, Capuano B. J Med Chem. 2013;56:1592–1599. doi: 10.1021/jm401032k. [DOI] [PubMed] [Google Scholar]
- 19.Pancani T, Foster DJ, Bichell T, Bradley E, Bridges TM, Klar R, Daniels JS, Jones CK, Bowman AB, Lindsley CW, Xiang Z, Conn PJ. Proc Natl Acad Sci USA. 2015;112:14078–14083. doi: 10.1073/pnas.1512812112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen W, Plotkin JL, Francardo V, Ko WKD, Xie Z, Li Q, Fieblinger T, Wess J, Neubig RR, Lindsley CW, Conn PJ, Greengrad P, Bezard E, Cenci MA, Surmeier DJ. Neuron. 2015;88:762–773. doi: 10.1016/j.neuron.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ. Neuron. 2016;91:1224–1252. doi: 10.1016/j.neuron.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuscript in preparation.
- 23.For information on the Eurofin LeadProfiling Screen see: www.eurofin.com