Abstract
Hedgehog (Hh) signaling is critical for embryonic development and in differentiation, proliferation, and maintenance of multiple adult tissues. De-regulation of the Hh pathway is associated with birth defects and cancer. In the gastrointestinal tract, Hh ligands Sonic (Shh) and Indian (Ihh), as well as the receptor Patched (Ptch1), and transcription factors of Glioblastoma family (Gli) are all expressed during development. In the adult, Shh expression is restricted to the stomach and colon, while Ihh expression occurs throughout the luminal gastrointestinal tract, its expression being highest in the proximal duodenum. Several studies have demonstrated a requirement for Hh signaling during gastrointestinal tract development. However to date, the specific role of the Hh pathway in the adult stomach and intestine is not completely understood. The current review will place into context the implications of recent published data related to the biochemistry and cell biology of Hh signaling on the luminal gastrointestinal tract during development, normal physiology and subsequently carcinogenesis.
Keywords: Hedgehog signaling, Gastric epithelium, Intestine, Colon cancer, Gastric cancer, Gastrointestinal development
Hedgehog (Hh) signaling is critical during embryonic development. It is involved in the patterning of the neural tube, lung, skin, axial skeleton, and gastrointestinal tract [1–3] (reviewed in [4] and [5]). In multiple adult tissues, Hh signaling remains active and contributes to differentiation, proliferation, and maintenance. By contrast, deregulation of the Hh pathway is associated with birth defects and cancer (reviewed in [6], [7–9]). Specifically, Hh signaling is constitutively active in medulloblastoma, basal cell carcinoma, small cell lung cancer, breast, pancreas, and gastric cancer. As a result, phase 2 clinical trials for Hh signaling inhibitor GDC-0449 are underway for basal cell [10], medulloblastoma [11], ovarian [12], pancreatic, and metastatic colon cancers (Curis & Genentech).
Although a number of reviews on the Hh pathway and cancer have been published, most have focused on extra-gastrointestinal cancers. In addition, new information related to the biochemistry and cell biology of Hh signaling is rapidly accumulating. Therefore the current review will attempt to place into context the implications of this new information for the luminal gastrointestinal tract, during development, normal physiology and subsequently carcinogenesis.
1. Ligands, Receptors, Signal Transduction
There are three members of the mammalian Hh family: Sonic (Shh), Indian (Ihh), and Desert (Dhh) [13,14]. All the ligands bind with similar affinity to a 12-transmembrane receptor called Patched (Ptch1) that restricts Hh signaling by inhibiting a 7-transmembrane receptor called Smoothened (Smo) [15]. The cytoplasmic tail of Smo serves as a nidus for the accumulation of the Glioblastoma transcription factor protein family (Gli) held in a complex with regulatory proteins, e.g. Costal 2 (Cos), Fused (Fu) and Suppressor of Fused (Sufu) [16]. Upon binding of ligand to Ptch1, the inhibition on Smo is removed and processed forms of Gli migrate to the nucleus to bind Hh target genes ([17–19], reviewed in [5,6,20]). Of the three Hh ligands, Shh is most related to its Drosophila homolog and has been studied most intensively [21]. Thus, we will focus on Shh except where noted.
Shh ligand is expressed as a ~45 kilodalton (kDa) precursor that is cleaved autocatalytically, yielding a 19 kDa amino terminal form, and a residual 26 kDa carboxy-terminal form that acts as a cholesterol transferase. In the stomach, Shh processing depends on the acid-activated protease pepsin [22]. However, whether protease-cleaved Shh is lipid modified and occurs in other tissues has not yet been determined. The final Shh protein is cholesterol modified at a C-terminal cysteine and palmitoylated at its amino terminus [23]. These post-translational hydrophobic modifications facilitate long-range signaling despite their intrinsically poor solubility [24,25]. Although poor solubility and long-range signaling appear counterintuitive, Grobe and coworkers, shed light on this concept by demonstrating that Shh forms stable high molecular weight oligomers with high biological activity [26,27]. Additionally, the lipid modified-Hh interacts with cell-secreted proteoglyglans [28,29], heparan [27], and chondroitin sulfates [30], the latter two have been associated with proper Shh and Ihh signalining respectively. Under-sulfated proteoglycans or Shh mutated in the proteoglycan interaction domain impair Hh signaling of various developmental stages during mouse embryogenesis, resulting in decreased proliferation of chondrocytes and overall growth alterations, but not patterning. In Drosophila and mouse embryos, proteoglycans and heparan sulfate facilitate the distribution on tissues to reach target cells, and on the internalization of the Hh-Ptch1 complex by the cell [31,32]. However the role of proteoglycans has only be examined during neural central tube development. There are no functional studies examining the role of these molecules in adult Hh signaling.
ADAM17 and metalloproteases appear to be involved in Hh release from the producing cell, along with Dispatched, a 12-transmembrane protein with a sterol-sensing domain. To test its function, Dispatched null mice were examined and showed a phenotype similar to mice lacking Smo, i.e., embryonic lethality [33–37]. The authors concluded that Dispatched is necessary for the release of cholesterol-modified Hh, and that this modification regulates Hh signaling in the developing nervous system [37], bone [38], and limb bud [39]. To our knowledge, there are no studies addressing the role of Dispatched in adult tissues. We [22,40] and others [41–43] have shown in vitro that recombinant Shh exhibits regulatory activity, albeit at a lower potency compared to tissue generated Shh presumably because it is not lipid-modified as endogenous Shh [22]. Indeed, Robbins and coworkers have found some Shh mutations in holoprosencephaly patients, which are inactive or exhibit low-activity function as dominant-negative ligands [44], suggesting that variable Shh activity contributes to different phenotypes.
Hh ligands bind to Ptch1, which itself contains a cholesterol-binding domain. Vertebrates have Ptch1 [45] and Ptch2 [46] variants. Ptch1 is a transcriptional target of Hh signaling and acts by repressing Smo [15]. The specificity of Ptch1 is somewhat controversial since its function depends on the cell type, and protein domain involved [47,48]. Hematopoietic cell-specific deletion of Ptch1 does not result in constitutively active Hh signaling [49], as Hh signaling has recently been shown to be dispensable for hematopoiesis [50,51], whereas in skin, brain and colon, mutations in Ptch1 result in unregulated Hh signaling causing basal cell carcinoma of the skin [52,53], medulloblastoma of the brain [54,55], and Gorlin syndrome [56,57], an autosomal dominant disorder characterized by the early appearance of basal cell carcinomas and cancers in other organs, e.g., brain, colon and ovary.
In the presence of Hh ligands, the repression of Smo by Ptch1 is relieved [58,59], activated Smo accumulates in primary cilia and Hh signaling is initiated [60]. In addition to Ptch1, other membrane proteins have been identified that can bind to Hh ligands to modulate signaling. Hip (Hh interacting protein) is the best studied [61,62]. Recently Gas1 (growth arrest specific gene 1) [63–66], Cdo (CAM related/down regulated by oncogene) [67] and BOC (brother of Cdo) [68] have been reported to modulate Hh signaling during development [69], including the gastrointestinal tract [70,71]. However their expression and possible function in adult tissues has not been established, though expression of Cdo and BOC messenger RNA has been demonstrated in normal stomach and gastric cancer samples by RT-PCR [72].
Once Smo is released from the inhibiting influence of Ptch1, it translocates to primary cilia and mediates the activation of Gli proteins [73]. In the absence of Hh ligands, Gli proteins are sequestered in the cytoplasm in a complex containing the kinesin-related protein Kif7, the mammalian homolog of Drosophila Cos2 [74,75]; the serine/ threonine kinase Fu, and Sufu [76–78]. As a complex, Kif7 and Fu direct the proteosomal degradation of the full length Gli protein to its repressor form [79,80]. Gli proteins contain a C-terminal zinc finger DNA binding domain and a N-terminal repressor domain (R) [81], which translocates to the nucleus to suppress the expression of Hh targets [18].
The mechanism of Ptch1 repression on Smo is not completely understood, though involvement of 7-dehydrocholesterol (provitamin D3) secretion has been suggested [15]. Also, a recent report has shown that Tow (target of Wingless) protein, is a modulator of Hh signaling that acts downstream of Ptch1 but upstream of Smo. Tow is a Drosophila homolog of a previously uncharacterized protein family that controls the availability of Smo by regulating the degradation of lipophorin particles [82]. Homologs of Tow have only been identified in insects [83] however; proteins with similar functions may exist in vertebrates. Tow is suppressed by wingless, and its over-expression causes planar cell polarity defects [83]. Thus at least in insects, Hh signaling appears to be suppressed by the Wnt pathway.
Ligand-mediated de-repression of Smo stabilizes full length Gli that transactivates some Hh targets, e.g., Ptch1, Gli1, N-myc, and Wnt5a. Other Hh target genes require further cleavage of Gli to produce a N-terminally-derived activator (Gli-A) before their translocation to the nucleus. Gli2 and Gli3, but not Gli1, are further processed from full-length forms via phosphorylation at different residues primarily by PKA, which results in the release of the activator forms [19]. Therefore Gli 2 and 3 proteins are capable of target gene activation or repression.
The genes targeted by Hh signal transduction depend on whether the promoters are occupied by the Gli activator or repressor form. Gli1 and Ptch1 are the classic genes activated by Hh signaling, e.g., Gli2-A [84]. Moreover, Gli2 appears to be the key transcriptional effector of Hh signaling in skin and other organs, including the stomach [85–90]. Studies in epidermal cells have grouped Hh target genes in to 3 classes: Class I are those genes in which expression is controlled by the Gli-R form (i.e. Pax3); Class II promoters are influenced by both activator and repressor forms, and their expression might be regulated in a signal inducible manner (i.e. Ptch1, Cyclin D1, N-myc). Class III genes are only induced by Gli-A forms (i.e. Gli1, Wnt5a) [91,92].
Primary cilia are necessary to process full-length Gli to Gli-R in the absence of Hh ligand [93,94]. By contrast, Sufu is required for maximal Hh signaling, modulating Hh-target genes in mouse embryonic fibroblasts and during neural tube development [95–97]. Recent studies have demonstrated that Gli processing not only depend on Smo activation, but is also actively regulated by the negative Hh regulator Sufu. Kise et al. showed that Sufu functions as a recruiter for GSK3b, the kinase participating in Gli3 processing in the presence of Hh [80]. Furthermore, Chen et al. demonstrated that Sufu antagonizes the activity of Speckle-type POZ/BTB protein (Spop), the mammalian homolog of the Drosophila Hh-induced BTB domain protein Hib, that acts as a Gli-degrading factor [98]. Therefore Sufu prevents the processing of the full-length Gli2 and Gli3 proteins so that they are available for processing in a cilia-independent manner [99,100]. Further studies on the cytoplasmic proteins involved in Hh pathway signaling will provide some understanding of the signal transduction regulations, particularly in adult tissues.
2. Hedgehog signaling in cancer
Hh signaling is not only important during development, but it also has implications in the maintenance of adult tissues. Its deregulation can lead to the development of several cancers, which are now the focus of Phase II clinical trials. Rubin and de Sauvage proposed three basic models for Hh pathway activity in cancer. Type I cancers, e.g., basal cell carcinomas, are ligand-independent because they involve constitutive activation of either the receptor or downstream signaling molecules. Type II cancers (e.g., pancreatic cancers) are ligand-dependent, implying that they involve autocrine or juxtacrine signaling mechanisms. Autocrine or juxtacrine means that the Hh-producing and responding cells are either the same cell (autocrine) or originate from adjacent tumor cells (juxtacrine). Type IIIa cancers are also ligand-dependent, but exhibit paracrine signaling, i.e., tumor cells produce Hh that activates downstream targets within the mesenchyme. Hh-activated mesenchymal cells produce proliferative signals that feedback to the tumor to promote growth and survival [101]. Recently, these authors included a Type IIIb mechanism called “reverse paracrine” signaling, where Hh ligands are generated from mesenchymal cells, and received by responsive tumor cells in the epithelium [9]. Interestingly, none of the models proposed consider the possibility of non-canonical signaling.
Non-canonical Hh signaling has been described in various studies. Some studies have analyzed Ptch1 interaction with cyclin B1. Ptch1 and cyclin B1 have been shown to associate with cyclin dependent kinase-1 (Cdk1) at the cell membrane in vitro (293T cells) [102] and in vivo (human fetal kidney) [103], resulting in decreased proliferation, and reduction of the mitotic index by the sequestration of the cyclin B1/Cdk1 complex. These functions are antagonized by Shh, suggesting that Ptch1 participates in a G2/M phase checkpoint independent of other downstream Hh pathway components. Another report suggests that activation of Ptch1 modulates apoptosis through activation of caspase 3 by cleavage of the C-terminal cytoplasmic tail of Ptch1 [104,105]. Similarly, Shh blocks Ptch-mediated caspase cleavage. Additionally, treatment of mouse fibroblasts with soluble Shh induces reorganization of the actin cytoskeleton, and the formation of lamellipodia. These changes are observed during approximately the first 10 minutes of treatment before changes in Gli1 are observed, suggesting that Shh can induce cell motility separate from the classic canonical signaling through Gli1 [106]. On the other hand, TGFβ signaling activates Gli2 independent of Hh ligands, which subsequently activates other gene targets, such as follistatin, an inhibitor of stromal activins [107,108]. Nevertheless, the existence of non-canonical signaling remains controversial primarily because the evidence for this pathway depends completely upon the absence of Gli1-mediated signaling assessed using a Gli1 reporter assay.
3. Primary Cilia
Primary cilia have been defined as indispensable for Hh signaling in mammalian cells [60,109]. Ptch1 blocks Smo localization to the cilia in the absence of Shh. In the presence of the ligand, Smo migrates to the primary cilia and then activates Gli proteins. In the skin, Shh is expressed in hair follicles [110], while Ptch1 and Glis are expressed in dermal fibroblasts surrounding Shh secreting cells [90]. Primary cilia are present in murine skin and hair follicles throughout morphogenesis, and during hair follicle cycling in postnatal life on both epithelial and mesenchymal cells [111]. Purkinje cells in the brain and granule precursor neurons express primary cilia too [112]. In these tissues, the requirement of primary cilia in Hh signaling has been demonstrated. In the pancreas and prostate not all cells express primary cilia. However, there is synergy between Hh signaling and cilia positive cells in these two organs [113–115].
Interestingly, some cancer types, and non-canonical Hh signals do not require primary cilia. In basal cell carcinoma, the Hh pathway is constitutively active in the absence of ligand due to mutations in Ptch1 or Smo [52,116,117]. Both medulloblastoma, and basal cell carcinoma development can be associated with constitutively active Smo or Gli2. In the case of Smo, primary cilia are necessary for pathway activation. By contrast, cilia ablation was required for these tumors to grow when constitutively active Gli2 was present [118,119].
We have observed the presence of primary cilia expressed in both the gastric epithelium and mesenchyme (unpublished observations). The presence of primary cilia in the gastric epithelium supports the idea that both epithelial-epithelial, and epithelial-mesenchymal Hh signaling might occur. Thus direction of Hh signaling in the gastric mucosa should be viewed with some caution based on the presence of primary cilia. Indeed, non-canonical signaling remains a possible mechanism.
4. Hedgehog in the Gastrointestinal Tract
4.1. The Stomach
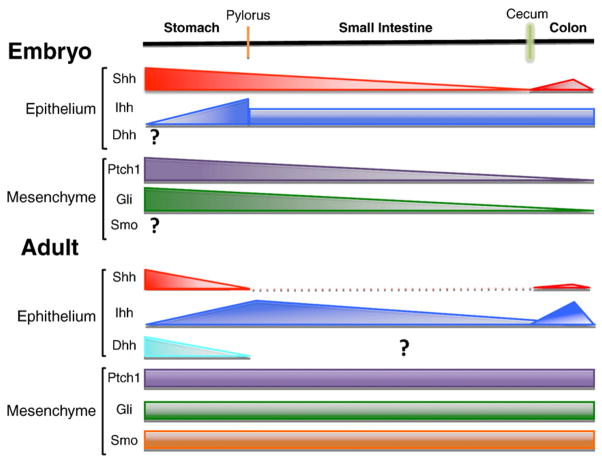
Within the luminal GI tract, Hh signaling in the adult stomach has generated the greatest number of studies due to the original observation by Ramalho-Santos et al. who reported that there are metaplastic changes in day old Shh null mice. Subsequently, a study comparing the phenotype of Shh null to Gli 1, 2 or 3 null mice reported hyperplastic rather than metaplastic changes since immunofluorescent analysis of the P0 stomachs were positive for H+,K+ATPase [120]. Interestingly, the Gli3 null mice mimicked the Shh null phenotype in the stomach [120]. Until E15.5, Shh is expressed at high levels in the squamous forestomach and anterior glandular stomach (Fig. 1). Shh expression tapers down upon reaching the hindstomach (antrum), where Ihh is highly expressed. Gli1 and Ptch1 expression mirrors the expression of Shh [71]. Subsequently at E18.5, Shh and Ihh are expressed in the gastric mucosa and decrease along the intestine [3,121]. By this time, Ptch1 and Gli1 expression is restricted to the mesenchyme while in the embryo; they are also present in the epithelium [122] (Fig. 1). The specific role of Shh during gastric maturation is difficult to define because Shh−/− mice die in utero or within 24 h after birth when the gastric mucosa is not completely differentiated and functional.
Fig. 1.
Expression of hedgehog pathway molecules in the gastrointestinal (GI) tract. During development, Sonic hedgehog (Shh) is highly expressed in the stomach and its expression decreases along the intestine. Shh is expressed in the colon glands. Indian hedgehog (Ihh) expression peaks at the pyloric junction and is constant in the small intestine and colon. There are no studies addressing the pattern of Desert hedgehog (Dhh) expression in the developing GI tract. The Hh receptor Patched (Ptch1) and the Glioblastoma (Gli) transcriptional factor family proteins are expressed in the mesenchyme, mirroring Shh expression. In the adult GI tract, Shh expression is limited to the stomach and colon, with the proximal stomach being the main source of the morphogen. Ihh is expressed in the small intestine, with highest levels in the proximal duodenum. Ihh is also expressed in the colonic glands. Dhh expression has been reported in the gastric mucosa, but has not been localized in the small intestine. Ptch1, Gli proteins, and Smo are expressed primarily in the mesenchyme of the adult GI tract.
In the normal adult gastric corpus, Shh protein expression is greatest in parietal cells (Table 1, Fig. 1). Recently using Shh-LacZ reporter mice, we have shown that Shh mRNA is also expressed in zymogenic, surface pit, and mucous neck cells, supporting previous reports by other groups [40,72,123] (Table 1). In addition Shh mRNA and protein expression and Hh signal transduction is observed in the antrum [40]. This finding was surprising, as prior studies indicated no Shh expression in the antrum [121].
Table 1.
Expression of Hedgehog related molecules in the stomach and gastric cancer.
| Molecule | Normal Stomach | Intestinal Metaplasia | Cancer | Methods | Reference | |
|---|---|---|---|---|---|---|
|
| ||||||
| Intestinal | Diffuse | |||||
| Shh | Neck / Gland Epithelium | Low or absent | High | Low or undetectable | a, b | [72] |
| a, b | [193] | |||||
| b | [154] | |||||
| a, b, c | [123] | |||||
| a | [125] | |||||
| Ihh | Pit Epithelium | ND | High | Low | a, b | [72] |
| a, b, c | [123] | |||||
| Dhh | Gland Epithelium | Low or absent | Low | High | a, b | [72] |
| a, b, c | [123] | |||||
| a | [125] | |||||
| Ptch1 | Pit Mesenchyme | D | High | High | a, b | [72] |
| a, b, c | [123] | |||||
| a, d | [125] | |||||
| Smo | Pit / Gland Mesenchyme | D | High | High | a, b | [72] |
| a, b, c | [123] | |||||
| Gli1 | Pit / Gland Mesenchyme | Low or absent | High | High | a, b | [72] |
| a, b, c | [123] | |||||
| Gli2 | Pit Mesenchyme | Low or absent | High | High | a, b | [72] |
| a, b, c | [123] | |||||
| Hip | D | NR | Low | NR | a, c | [193] |
| BOC | Pit | ND | High | High | a, b | [72] |
D: Detected, ND: Not detected, NR: Not reported, a: RT-PCR, b: Immunohistochemistry, c: Immunofluorescence, d: LacZ reporter.
There is controversy regarding how Hh signaling occurs in the stomach. A major source of the controversy resides in the lack of immunohistochemistry for Ptch1 and Gli to demonstrate that the mRNA levels reflect protein expression. Most studies indicate that the gastric epithelium secretes Shh and activates Hh receptors in the mesenchyme. Both in situ hybridization, and Lac-Z reporter mice for Ptch1 and Gli proteins show robust expression in the gastric mesenchyme supporting this argument [71,124]. Berman et al. showed that there is Ptch-LacZ expression in gastric parietal cells in the gastric epithelium [125]. However other groups have not reproduced their results. They also showed RNA expression of Ptch1, Gli, Shh and Ihh in resected human tumor samples [125]. In addition, Fukaya et al. demonstrated the expression of the three Hh ligands and downstream signaling molecules by both immunohistochemistry and RT-PCR analysis of tissues from human and mouse gastric epithelium [123] (Table 1). Since this study was performed using laser micro dissection, contamination of the epithelial sample by mesenchymal cells cannot be excluded.
Since Hh signaling is essential for the development of viable embryos, defining its role in adult tissues including the stomach requires the use of cell specific knockouts. Unfortunately, there are very few reliable tissue-specific promoters that will drive Cre recombinase in the stomach. Zavros et al. recently used the H+,K+ATPase-β subunit promoter, which faithfully drives Cre in gastric parietal cells to generate a conditional Shh knockout [126]. These mice develop hypergastrinemia, an expanded mucous pit cell compartment and parietal cells unresponsive to histamine stimulation. Interestingly, D cells (somatostatin secreting cells), zymogenic and mucous neck cells populations decreased.
Prior studies by Todisco and coworkers revealed that Shh stimulates H+,K+ATPase gene expression [127]. Presumably, chemoreceptors on D cells detect the increase in pH (hypochlorhydria), triggering a regulatory feedback loop that results in a decrease in somatostatin and hypergastrinemia [128]. In point of fact, we previously showed that the loss somatostatin in null mice is the most potent inducer of gastrin gene expression [129]. Since bona fide chemoreceptors have never been identified, the D cell regulation by Shh functioning as a proton-sensing molecule might also explain the acid-mediated feedback regulation. Confirmation of this model awaits demonstration that D cells directly respond to Hh signaling. Another important observation is that loss of Shh did not cause parietal cell atrophy. Thus one might conclude that Shh regulates target genes that modulate parietal cell function, but not survival. The unresponsiveness of parietal cells to histamine, and the changes observed during tubulovesicle formation in the Shh-depleted model [126] may be associated with ezrin, a membrane cytoskeleton linker protein that mobilizes H+,K+ATPase in the gastric parietal cells by a mechanism dependent on PKA activation [130,131]. Although it has been shown that gastrin regulates ezrin expression by parietal cells in gastrin deficient mice [132], the effects of hypergastrinemia on ezrin expression and localization have not been yet studied.
We have demonstrated that the Shh 45 kDa form processing to the 19 kDa form in the stomach requires cleavage by pepsin A, which itself requires acidic conditions to become active [22]. Acid is released into the gastric lumen by parietal cells, making it available for pepsin A activation. Thus in the stomach, we assume that Shh is released into the lumen to be fully processed and active. However, Shh processing within an acidic endocytic vesicle cannot be excluded. A recent study by Robbins et al. has indicated that the unprocessed form of Shh has biological activity [133]. Indeed, we found that the predominant form of Shh in the transformed, hypochlorhydric stomach is the unprocessed 45 kDa protein [22]. In addition, some human gastric cell lines are responsive to Shh in vitro (unpublished data), raising the possibility that Hh signaling can occur in through autocrine or non-canonical signaling.
Shh activation is acid-dependent in the stomach, therefore it has been suggested that Shh is released at the apical surface of parietal cells where acid is secreted [134]. Zymogenic, surface pit and mucous neck cells also express Shh, however there is no data available on the mechanism of Shh release from these cells. Recently, Etheridge et al. have shown that Disp1 is required to produce and release Shh basolaterally. Ptch1 in the adjacent cells binds to Shh, indicating that both Ptch1 and Disp1 control the trafficking of Shh away from its site of synthesis [135]. If Hh signaling is paracrine with responsive cells located exclusively in the mesenchyme, then the mechanism by which secreted Shh is received by target cells might involve Disp1 and will require further clarification. Megalin has been proposed as the intracellular transporter for Shh in epithelial cells during development and in the efferent duct epithelial cells of adult rat [136]. Megalin is involved in forming vesicles that deliver Shh to the basal membrane of the producing cell [137]. However there is no evidence that Shh-producing cells in the GI tract express megalin, raising the possibility that ezrin could fill that role in the stomach.
The eventual loss of zymogenic and mucous neck cell populations in the Shh conditional null mouse suggests that Shh also affects the trans-differentiation of mucous neck to zymogenic cells. Since the helix-loop-helix transcription factor Mist1 mediates the transition of this cell lineage in the gastric mucosa [138,139], Shh might modulate the function of this transcription factor and subsequently the appearance of these gastric cell types. Additional evidence supporting a role for Shh in cell differentiation has emerged from studies of aspirin-induced gastric ulceration in rats, which showed that Shh was not required for cell proliferation, but rather for differentiation of parietal, and mucous neck cells. By contrast, blocking Hh signaling with cyclopamine, a Smo inhibitor increased the proliferation of gastric progenitor cells without expansion of the parietal and mucous neck cell compartments [59,140]. Other studies have shown that cyclopamine treatment reduces the expression of H+,K+ATPase in canine parietal cells [127] and mice hypergastrinemic stomach [140]. Collectively, these results suggest that Shh is necessary for progenitor cell differentiation into various gastric cell lineages rather than proliferation and for parietal cell function. Specifically, loss of Shh expands the progenitor cell compartment at the expense of terminally differentiated lineages [124,126].
Depleting Shh in the mouse parietal cells also induced expression of Ihh, Gli1, TGF, Wnt, SNAIL, and cyclin-D1, while E-cadherin was decreased [126]. Modulations in SNAIL and E-cadherin expression have been associated with carcinogenesis, particularly in the intestine and skin, where Hh activation induces SNAIL expression increasing its metastatic potential [141,142]. Therefore one might consider that Shh regulation of these genes, along with the associated changes in proliferation and apoptosis, might explain mucosal transformation during gastric carcinogenesis. Nevertheless, induction of Ihh did not compensate for the loss of Shh in parietal cells. This result raises the possibility that the three Hh proteins possess distinct functions in the gastric mucosa, similar to their distinct roles in the hair follicle and the sebaceous epithelial cell precursors of the skin [143,144].
H. pylori infection is the main cause of gastric inflammation, and has a strong association with intestinal type gastric cancer development in humans. Helicobacter-infection models in rodents have provided valuable information on the early mechanisms involved in gastric carcinogenesis and the participation of Shh in them. During H. pylori infection, acid secretion by parietal cells is compromised and Shh levels are reduced [145,146]. We have recently shown that IL-1 also reduces Shh gene expression in parietal cells, H+,K+ATPase expression and that gastric acid secretion subsequently blocks the release of intracellular calcium [40]. Therefore, changes in gastric acidity can result in the development of gastric atrophy, which in turn creates a permissive environment for the expression of intestine-specific transcription factors, e.g., CDX2, which correlates with the development metaplasia [147,148].
4.2. Shh in Gastric Cancer
Gastric cancers are classified histologically into two major types: intestinal and diffuse [149]. The intestinal type is thought to develop from a series of mucosal changes, triggered by chronic inflammation (gastritis), then followed by parietal cell atrophy (atrophic gastritis), gastric mucous cell hyperplasia, intestinal metaplasia, dysplasia, and cancer [150]. On the other hand, diffuse gastric cancer is thought to arise from deregulation of stem or precursor cells [151,152]. In addition, the familial form of diffuse gastric cancer is due to mutations in E-cadherin [153].
The two types of gastric cancer show different Hh ligand expression profiles, perhaps due to their cell origin (See Table 1). The analysis of human metaplastic and gastric cancer samples showed that the three Hh ligands, Ptch1, Smo, and the three Gli transcription factors were only weakly expressed or absent. In intestinal type gastric cancer, Ihh and Shh expression were detected. However, the expression of Smo, Gli 1, and 2 was infrequent but low when detectable [72].
Ihh, Shh, and Dhh expression was variable in diffuse gastric cancers, but in contrast to the intestinal-type cancer, diffuse cancers expressed high levels of Ptch1, Smo, and Gli proteins (Table 1). Some diffuse cancer cells showed histological expression of Ihh, while Shh expression was not detected in epithelial cells of the cancer, but instead were expressed in the adjacent fibroblast cells [123,154]. The latter observation is suggestive of the Type IIIb signaling described by de Sauvage and colleagues, in which the ligand is produced in the supporting stromal cells [9,101]. Thus, some diffuse gastric cancers belong to the Type IIIb group [125], while others might express receptor mutations, or non-canonical pathway activation, as suggested by the lack of response to cyclopamine-mediated inhibition of Hh signaling [140,155], although these tumor types are rare. Gastric metaplasia and intestinal cancer cannot be classified by these criteria because they appear to involve the initial loss, then subsequent gain in Hh signaling once the epithelium is irreversibly transformed (Table 1). Therefore, we suggest adding a Type IV signaling pathway to the classification for cancers in which the normal adult epithelium requires Hh signaling. With neoplastic transformation, Hh signaling is lost, with or without gain of function mutations in the Hh signaling molecules, e.g., Ptch1, Smo or the SuFu-Gli complex [140,155].
In summary, the normal gastric mucosa expresses Shh, Ihh and Dhh (Table 1, Fig. 1). Shh regulates parietal, zymogenic, and mucous neck cell differentiation, and proliferation, while Ihh appears to modulate the surface pit cells without recapitulating the function of Shh. The role of Dhh in the stomach is not yet known. During H. pylori infection, Shh expression reduced by IL-1β results in the lack of acid secretion and hypergastrinemia [40]. Even when gastrin stimulates Shh expression, acid-dependent processing of the Shh precursor is compromised, and parietal cells fail to become properly functional [22], possibly due to changes in ezrin. Hypochlorhydria, hypergastrinemia, and low levels of Shh result in parietal cell atrophy, and mucous cell expansion, with impaired zymogenic cell differentiation, along with the expression of intestinal markers, such as Cdx2 and Muc2, along with the expansion of cells with low Hh responsiveness (low levels of Smo and Gli). Diffuse tumor carcinogenesis is associated with hyperplasia of stem or precursor cells, but it is not yet clear whether Hh signaling contributes to its pathogenesis. Although in some of these tumors, the signaling shifts from its normal gastric epithelial to a mesenchymal origin (Table 1). In addition, mutations in the TGFβ growth factor receptor, activins, or mutations in Ptch1, Smo, and other Hh pathway mediators such as Sufu or hedgehog interacting protein (Hip), can result in constitutively active growth signals.
4.3. The Small Intestine and Colon
During gut development (reviewed in [156]), Hh signaling plays an important role in the left-right axis specification, radial patterning by regulating villus formation, smooth muscle genesis, and development of the enteric nervous system. At E11.5 and E12.5, Shh and Ihh are expressed throughout the endoderm of the developing intestine. Shh expression is down regulated in the small intestine at E14.5, with the highest expression remaining in the duodenum. Ihh expression remains unchanged in the developing intestine and colon. At E18.5 their expression is restricted to the intervillus epithelium in the small intestine. In the colon, Shh is expressed at the base of the crypts, whereas Ihh is expressed in surface colonocytes. Ptch1 and Gli1 are exclusively expressed in the intestinal mesenchyme [71,157,158]. Shh but not Ihh signaling is necessary for normal anorectal development [3] (Fig. 1).
The adult small intestinal epithelium is established after the crypts are completely formed by the third postnatal week. The level of Shh mRNA in the adult small intestine and colon are very low compared to those in the stomach, whereas Ihh expression appears to be more robust, and present mainly at the crypt-villus junction [159]. Ptch1 is expressed at low levels and only in the mesenchyme. The distal colon expresses Ihh, but not Shh in epithelial colonocytes, and Ptch1 expression is also restricted to the mesenchyme underlying the Ihh positive epithelium [157,158].
Both in vivo and in vitro studies show that Hh signaling is necessary for enterocyte differentiation, such as brush border staining for villin, and carbonic anhydrase IV [160]. Since Ptch1 expression is exclusively mesenchymal, enterocyte differentiation must be either under paracrine or non-canonical Hh regulation. In vitro, Hh signaling is necessary and sufficient for induction of differentiation markers in the human colon adenocarcinoma HT-29 cell line [161], although these cell lines express Ptch1 and Gli1 [162], suggesting autocrine regulation, which differs from what is currently understood regarding Hh-mediated enterocyte differentiation.
Ulcerative colitis (UC), and Crohn’s disease are the prevalent inflammatory bowel disorders (IBD) with high morbidity. Lees et al. analyzed samples of human colonic inflammation and found that the levels of Gli1, Ptch1, Hip, and Ihh were lower in UC patients. They also identified a germ line variation in Gli1 that resulted in reduced Hh signaling. In vivo analysis of mice heterozygous for Gli1 demonstrated increased acute inflammation when the mice were treated with dextran sulfate (DSS). These authors found that Hh signaling also regulates myeloid cells, including dendritic cells in the colon, during both homeostasis and under inflammatory conditions [163].
The association of Hh signaling with cancers of the intestine or colon is controversial. Mouse models with deleted or silenced Hh signaling molecules that have been analyzed thus far do not develop gastrointestinal cancers, except in the pancreas (reviewed in [122], and [164]). Some reports show that expression of Hh signaling components is increased in hyperplastic polyps, adenomas and adenocarcinomas of the human colon. These Hh components are also expressed in many colon adenoma or adenocarcinoma cell lines [165,166]. In contrast, it has been reported that Ihh antagonizes Wnt signaling in a colon cancer cell line and that loss of Ihh contributes to intestinal tumor formation [160]. Transgenic expression of the Hh ligand-inhibitor Hip in the small intestine also enhances activation of Wnt signaling. In addition, treatment with cyclopamine does not alter the expression of Ptch1 and Gli1 in some colon cell lines [158]. Therefore, Hh participation in colon cancer development might occur by enhancing or inhibiting the Wnt pathway [167].
The distal colon looses Ihh expression early in the process of the adenoma to carcinoma sequence of colorectal cancer. Ihh expression loss is caused by mutations in adenomatous polyposis coli (APC) or other genes that result in uncontrolled Wnt pathway activity [160,167,168]. Recently, Smo was found to contribute to intestinal tumorigenesis by increasing Wnt signaling, in the APC mutant mice treated with cyclopamine, and this role was confirmed by further specific deletion of Smo in the intestine of these mice [167]. Indeed, Smo expression is high in adenomatous polyps with APC mutations compared to normal intestine where it is hardly detectable. Smo can stimulate intestinal tumor cell proliferation by increasing-catenin in the intestinal tumor cells, altering the β-catenin/Tcf dependent Wnt signaling, resulting in the expression of Wnt targets, Gli independent or non-canonical Hh signaling [169]. Collectively, these data suggest that Hh signaling is important for normal enterocyte differentiation, and its role in intestinal or colon cancer development is not direct, involving both mesenchymal paracrine signaling [170] and Hh pathway molecules acting in Wnt signaling [171,172].
4.4. Esophagus, pancreas and liver
Hh signaling in the esophagus during development mirrors the observed expression in the stomach. The esophagus consists of three to four layers of squamous epithelium. During development at E17, some ciliated cells are also observed, however the ciliated cells disappear 1 week after birth [173]. Human and mice esophageal epithelia differ in that the mouse esophagus becomes keratinized one month after birth, while the human esophagus is not keratinized [174]. In the human adult esophagus, Shh expression is restricted to the basal cell layer, while Dhh is expressed in differentiated cells and Ihh is absent. Gli1 expression was localized in the nuclei of the basal and parabasal cells, and keratinocytes differentiation is blocked by cyclopamine, suggesting Hh signaling is necessary for epithelium differentiation but absent in differentiated cells [175]. Hh signaling is activated in most esophageal squamous cells carcinomas, along with several markers of epithelial-mesenchymal transformation (EMT) that are regulated by Gli1 [175], suggesting that esophageal carcinomas arise from aberrant Hh signaling cross-talk between the mesenchyme and epithelium. However the information available on these carcinomas and Hh signaling is scarce.
The pancreas represents an interesting and controversial organ in terms of Hh signaling. In contrast to the other organs mentioned in this review, Shh expression is absent in the embryonic pancreatic bud [176,177]. Ihh, Dhh and Ptch1 are expressed in the pancreas from embryonic day 13.5 onward, and their expression remains constant in adult islet and duct cells [178,179]. Activated Hh signaling in the developing pancreas results in loss of endocrine cells [177] while models where Hh signaling is lost, result in an expansion of the pancreas [178,180]. Recently, Hebrok and colleagues demonstrated that Hh signaling is active in the pancreatic epithelium during development, and Smo mutant mice are glucose intolerant and produce less insulin [181]. Therefore Hh signaling in the developing pancreas is required for endocrine function. Hh signaling in pancreatic cancer has been widely studied. Teglund et al. recently published a comprehensive review of Hh signaling and pancreatic cancer, among other forms of cancer [182]. In summary, most of the studies report the expression of both Shh and Ihh in pancreatic cancer. Apparently, Shh ligand is expressed transiently in the injured pancreas, but remains “on” in the transforming pancreas (intraepithelial neoplasia PanIN) suggesting aberrant Hh pathway activation in the pancreas contributing to cancer development. However Hh signaling alterations are not sufficient cause cancer and mutation of oncogenes, i.e. Kras, are necessary for pancreatic cancer development.
There is little information about Hh signaling in the liver. Hepatic stellate cells and liver epithelial progenitor cells express and respond to Hh ligands [183–185]. Activation of Hh signaling has been reported in hepatocellular carcinoma [186,187] and Hh signaling in the liver appears to be related to EMT involving the transformation of quiescent stellate cells to myofibroblastic stellate cells, via the reduction of Hip expression, as evidenced in a model of induced cirrhosis [188]. Thus the development of hepatocellular carcinoma involves cross-talk between the Wnt and TGFβ pathways and has not been linked exclusively to alterations in Hh signaling.
4.5. Future directions
Hh signaling is extremely complex. Recent studies have highlighted the importance of different pathway members, namely Smo, Sufu, Kif7, Disp1, and other participants, such as primary cilia, proteoglycans, and heparan sulfate, in Hh signaling regulation. Nevertheless, we still lack a complete understanding of the mechanisms involving Hh signaling.
The roles of Ihh and Dhh in the gastrointestinal mucosa, and mesenchyme have yet to be resolved. There are reports of Ihh and Dhh expression in the gastrointestinal epithelium, but one should exercise caution when analyzing these often-contradictory studies. Most of the antibodies commercially available for Hh ligands cross-react with the other family members, and reports using antibodies against Ptch1 and Gli proteins are not consistent, nor are those antibodies commercially available. Arguably, the best tool available to analyze Hh mRNA expression in vivo is in-situ hybridization. This technology has proven very powerful, in particular to establish the localization of Hh ligand and signaling molecules, e.g., Shh in the stomach, in contrast to Ihh expression in the distal intestine and colon. Nevertheless following the corresponding proteins has been problematic using antibodies. Transgenic reporter mice have also provided important insight; however, complete null mice are not suitable for adult tissue analysis due to the critical role of Hh signaling in development. Conditional and inducible deletion models are available for analysis in the intestine due to the existence of well-characterized tissue specific promoters to express Cre recombinase. However, stomach-specific promoters are limited, making development of deletion models a challenge for the field.
Finally, the duration of Hh signaling might be tissue specific. In the skin, Shh is transiently expressed in periods of hair active growth when Hh signaling is intermittent [189]. During development, Hh signaling is active in the intestinal tract, but in the adult, intestinal Hh signaling is due to Ihh rather than Shh. Shh expression is constant in the stomach, as are Ihh and Dhh. Depletion of Shh expression results in an increase in Ihh expression without apparent functional overlap [126]. The turnover time for each cell type in the stomach is different, ranging from 4 days for mucous cells to close to 60 days for parietal cells [190–192]. Shh is important in proliferation and the differentiation of gastric cell types but, to our knowledge, there are no studies on the expression of different Hh ligands over time in the adult gastric mucosa. Hh proteins may exert differential roles related to specific cell turnover cycles, but that concept has yet to be explored.
References
- 1.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 2.Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- 3.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 4.Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- 5.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 6.Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12:5924–5928. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- 7.Fan H, Oro AE, Scott MP, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med. 1997;3:788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- 8.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 9.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Mackey HM, Lum BL, Darbonne WC, Marsters JC, Jr, de Sauvage FJ, Low JA. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 11.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, He J, Huang S, Zhang X, Bian Y, He N, Zhang H, Xie J. Activation of hedgehog signaling is not a frequent event in ovarian cancers. Mol Cancer. 2009;8:112. doi: 10.1186/1476-4598-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 14.Marigo V, Roberts DJ, Lee SM, Tsukurov O, Levi T, Gastier JM, Epstein DJ, Gilbert DJ, Copeland NG, Seidman CE, et al. Cloning, expression and chromosomal location of SHH and IHH: two human homologues of the Drosophila segment polarity gene hedgehog. Genomics. 1995;28:44–51. doi: 10.1006/geno.1995.1104. [DOI] [PubMed] [Google Scholar]
- 15.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-) vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, Beachy PA. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–1274. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim SC, Kim YS, Jetten AM. Kruppel-like zinc finger protein Gli-similar 2 (Glis2) represses transcription through interaction with C-terminal binding protein 1 (CtBP1) Nucleic Acids Res. 2005;33:6805–6815. doi: 10.1093/nar/gki985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murone M, Rosenthal A, de Sauvage FJ. Hedgehog signal transduction: from flies to vertebrates. Exp Cell Res. 1999;253:25–33. doi: 10.1006/excr.1999.4676. [DOI] [PubMed] [Google Scholar]
- 21.Oldak M, Grzela T, Lazarczyk M, Malejczyk J, Skopinski P. Clinical aspects of disrupted Hedgehog signaling (Review) Int J Mol Med. 2001;8:445–452. [PubMed] [Google Scholar]
- 22.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, LGD, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem. 2007;282:33265–33274. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]
- 23.Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of sonic hedgehog. Mol Cell Biol. 1995;15:2294–2303. doi: 10.1128/mcb.15.4.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121:2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- 25.Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, Beachy PA. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374:363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- 26.Dierker T, Dreier R, Migone M, Hamer S, Grobe K. Heparan sulfate and transglutaminase activity are required for the formation of covalently cross-linked hedgehog oligomers. J Biol Chem. 2009;284:32562–32571. doi: 10.1074/jbc.M109.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dierker T, Dreier R, Petersen A, Bordych C, Grobe K. Heparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cells. J Biol Chem. 2009;284:8013–8022. doi: 10.1074/jbc.M806838200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin JB, Choi Y, Segal RA. Cerebellar proteoglycans regulate sonic hedgehog responses during development. Development. 2002;129:2223–2232. doi: 10.1242/dev.129.9.2223. [DOI] [PubMed] [Google Scholar]
- 29.Chan JA, Balasubramanian S, Witt RM, Nazemi KJ, Choi Y, Pazyra-Murphy MF, Walsh CO, Thompson M, Segal RA. Proteoglycan interactions with Sonic Hedgehog specify mitogenic responses. Nat Neurosci. 2009;12:409–417. doi: 10.1038/nn.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortes M, Baria AT, Schwartz NB. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development. 2009;136:1697–1706. doi: 10.1242/dev.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Incardona JP, Lee JH, Robertson CP, Enga K, Kapur RP, Roelink H. Receptor-mediated endocytosis of soluble and membrane-tethered Sonic hedgehog by Patched-1. Proc Natl Acad Sci USA. 2000;97:12044–12049. doi: 10.1073/pnas.220251997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Incardona JP, Eaton S. Cholesterol in signal transduction. Curr Opin Cell Biol. 2000;12:193–203. doi: 10.1016/s0955-0674(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y, Erkner A, Gong R, Yao S, Taipale J, Basler K, Beachy PA. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111:63–75. doi: 10.1016/s0092-8674(02)00977-7. [DOI] [PubMed] [Google Scholar]
- 34.Caspary T, Garcia-Garcia MJ, Huangfu D, Eggenschwiler JT, Wyler MR, Rakeman AS, Alcorn HL, Anderson KV. Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr Biol. 2002;12:1628–1632. doi: 10.1016/s0960-9822(02)01147-8. [DOI] [PubMed] [Google Scholar]
- 35.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami T, Kawcak T, Li YJ, Zhang W, Hu Y, Chuang PT. Mouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signaling. Development. 2002;129:5753–5765. doi: 10.1242/dev.00178. [DOI] [PubMed] [Google Scholar]
- 37.Tian H, Jeong J, Harfe BD, Tabin CJ, McMahon AP. Mouse Disp1 is required in sonic hedgehog-expressing cells for paracrine activity of the cholesterol-modified ligand. Development. 2005;132:133–142. doi: 10.1242/dev.01563. [DOI] [PubMed] [Google Scholar]
- 38.Tsiairis CD, McMahon AP. Disp1 regulates growth of mammalian long bones through the control of Ihh distribution. Dev Biol. 2008;317:480–485. doi: 10.1016/j.ydbio.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Zhang H, Litingtung Y, Chiang C. Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc Natl Acad Sci USA. 2006;103:6548–6553. doi: 10.1073/pnas.0600124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1beta Promotes Gastric Atrophy Through Suppression of Sonic Hedgehog. Gastroenterology. 2010;138:562–572. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng W, Yeung CK, Ng YK, Zhang JR, Hui CC, Kim PC. Sonic Hedgehog mediator Gli2 regulates bladder mesenchymal patterning. J Urol. 2008;180:1543–1550. doi: 10.1016/j.juro.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Stewart GA, Lowrey JA, Wakelin SJ, Fitch PM, Lindey S, Dallman MJ, Lamb JR, Howie SE. Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4+ T cells. J Immunol. 2002;169:5451–5457. doi: 10.4049/jimmunol.169.10.5451. [DOI] [PubMed] [Google Scholar]
- 43.Abe Y, Oda-Sato E, Tobiume K, Kawauchi K, Taya Y, Okamoto K, Oren M, Tanaka N. Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc Natl Acad Sci USA. 2008;105:4838–4843. doi: 10.1073/pnas.0712216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh S, Tokhunts R, Baubet V, Goetz JA, Huang ZJ, Schilling NS, Black KE, MacKenzie TA, Dahmane N, Robbins DJ. Sonic hedgehog mutations identified in holoprosencephaly patients can act in a dominant negative manner. Hum Genet. 2009;125:95–103. doi: 10.1007/s00439-008-0599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 46.Motoyama J, Takabatake T, Takeshima K, Hui C. Ptch2, a second mouse Patched gene is co-expressed with Sonic hedgehog. Nat Genet. 1998;18:104–106. doi: 10.1038/ng0298-104. [DOI] [PubMed] [Google Scholar]
- 47.Mullor JL, Guerrero I. A gain-of-function mutant of patched dissects different responses to the hedgehog gradient. Dev Biol. 2000;228:211–224. doi: 10.1006/dbio.2000.9862. [DOI] [PubMed] [Google Scholar]
- 48.Thomas C, Ingham PW. Hedgehog signaling in the Drosophila eye and head: an analysis of the effects of different patched trans-heterozygotes. Genetics. 2003;165:1915–1928. doi: 10.1093/genetics/165.4.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siggins SL, Nguyen NY, McCormack MP, Vasudevan S, Villani R, Jane SM, Wainwright BJ, Curtis DJ. The Hedgehog receptor Patched1 regulates myeloid and lymphoid progenitors by distinct cell-extrinsic mechanisms. Blood. 2009;114:995–1004. doi: 10.1182/blood-2009-03-208330. [DOI] [PubMed] [Google Scholar]
- 50.Hofmann I, Stover EH, Cullen DE, Mao J, Morgan KJ, Lee BH, Kharas MG, Miller PG, Cornejo MG, Okabe R, Armstrong SA, Ghilardi N, Gould S, de Sauvage FJ, McMahon AP, Gilliland DG. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell. 2009;4:559–567. doi: 10.1016/j.stem.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao J, Graves S, Koch U, Liu S, Jankovic V, Buonamici S, El Andaloussi A, Nimer SD, Kee BL, Taichman R, Radtke F, Aifantis I. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell. 2009;4:548–558. doi: 10.1016/j.stem.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr, Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 53.Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Jr, Scott MP. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 54.Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schuller U, Machold R, Fishell G, Rowitch DH, Wainwright BJ, Wechsler-Reya RJ. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 56.Valin A, Barnay-Verdier S, Robert T, Ripoche H, Brellier F, Chevallier-Lagente O, Avril MF, Magnaldo T. PTCH1 +/− dermal fibroblasts isolated from healthy skin of Gorlin syndrome patients exhibit features of carcinoma associated fibroblasts. PLoS ONE. 2009;4:e4818. doi: 10.1371/journal.pone.0004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crawford JR, Rood BR, Rossi CT, Vezina G. Medulloblastoma associated with novel PTCH mutation as primary manifestation of Gorlin syndrome. Neurology. 2009;72:1618. doi: 10.1212/WNL.0b013e3181a413d6. [DOI] [PubMed] [Google Scholar]
- 58.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 59.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 61.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 62.Zeng X, Goetz JA, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- 63.Marques G, Fan CM. Growth arrest specific gene 1: a fuel for driving growth in the cerebellum. Cerebellum. 2002;1:259–263. doi: 10.1080/147342202320883560. [DOI] [PubMed] [Google Scholar]
- 64.Martinelli DC, Fan CM. A sonic hedgehog missense mutation associated with holoprosencephaly causes defective binding to GAS1. J Biol Chem. 2009;284:19169–19172. doi: 10.1074/jbc.C109.011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dominguez-Monzon G, Benitez JA, Vergara P, Lorenzana R, Segovia J. Gas1 inhibits cell proliferation and induces apoptosis of human primary gliomas in the absence of Shh. Int J Dev Neurosci. 2009;27:305–313. doi: 10.1016/j.ijdevneu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J Clin Invest. 2007;117:1575–1584. doi: 10.1172/JCI32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell. 2006;10:657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- 69.Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21:1244–1257. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Udager AM, Hu C, Qiao XT, Richards N, Gumucio DL. Dynamic patterning at the pylorus: Formation of an epithelial intestine-stomach boundary in late fetal life. Dev Dyn. 2009;238:3205–3217. doi: 10.1002/dvdy.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, Madison BB, Waghray M, Ferris JE, Hu C, Merchant JL, Dlugosz AA, Kottmann AH, Gumucio DL. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–628. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohta H, Aoyagi K, Fukaya M, Danjoh I, Ohta A, Isohata N, Saeki N, Taniguchi H, Sakamoto H, Shimoda T, Tani T, Yoshida T, Sasaki H. Cross talk between hedgehog and epithelial-mesenchymal transition pathways in gastric pit cells and in diffuse-type gastric cancers. Br J Cancer. 2009;100:389–398. doi: 10.1038/sj.bjc.6604846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci USA. 2009;106:3196–3201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheung HO, Zhang X, Ribeiro A, Mo R, Makino S, Puviindran V, Law KK, Briscoe J, Hui CC. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- 75.Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, Phamluong K, Davis M, Scales SJ, Solloway MJ, de Sauvage FJ, Peterson AS. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr Biol. 2009;19:1320–1326. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 76.Methot N, Basler K. Suppressor of fused opposes hedgehog signal transduction by impeding nuclear accumulation of the activator form of Cubitus interruptus. Development. 2000;127:4001–4010. doi: 10.1242/dev.127.18.4001. [DOI] [PubMed] [Google Scholar]
- 77.Wang G, Amanai K, Wang B, Jiang J. Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes Dev. 2000;14:2893–2905. doi: 10.1101/gad.843900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ho KS, Suyama K, Fish M, Scott MP. Differential regulation of Hedgehog target gene transcription by Costal2 and Suppressor of Fused. Development. 2005;132:1401–1412. doi: 10.1242/dev.01689. [DOI] [PubMed] [Google Scholar]
- 79.Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, Phillips H, Brush J, Goddard A, de Sauvage FJ, Rosenthal A. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci. 1999;112(Pt 23):4437–4448. doi: 10.1242/jcs.112.23.4437. [DOI] [PubMed] [Google Scholar]
- 80.Kise Y, Morinaka A, Teglund S, Miki H. Sufu recruits GSK3beta for efficient processing of Gli3. Biochem Biophys Res Commun. 2009;387:569–574. doi: 10.1016/j.bbrc.2009.07.087. [DOI] [PubMed] [Google Scholar]
- 81.Kim YS, Lewandoski M, Perantoni AO, Kurebayashi S, Nakanishi G, Jetten AM. Identification of Glis1, a novel Gli-related, Kruppel-like zinc finger protein containing transactivation and repressor functions. J Biol Chem. 2002;277:30901–30913. doi: 10.1074/jbc.M203563200. [DOI] [PubMed] [Google Scholar]
- 82.Ayers KL, Rodriguez R, Gallet A, Ruel L, Therond P. Tow (Target of Wingless), a novel repressor of the Hedgehog pathway in Drosophila. Dev Biol. 2009;329:280–293. doi: 10.1016/j.ydbio.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 83.Chung S, Kim S, Yoon J, Adler PN, Yim J. The balance between the novel protein target of wingless and the Drosophila Rho-associated kinase pathway regulates planar cell polarity in the Drosophila wing. Genetics. 2007;176:891–903. doi: 10.1534/genetics.106.069021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 85.Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- 86.Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–2811. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- 87.Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, Rosenblum ND. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006;133:569–578. doi: 10.1242/dev.02220. [DOI] [PubMed] [Google Scholar]
- 88.Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate, adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- 89.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2, Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 90.Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, Hui CC. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17:282–294. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muller B, Basler K. The repressor and activator forms of Cubitus interruptus control Hedgehog target genes through common generic gli-binding sites. Development. 2000;127:2999–3007. doi: 10.1242/dev.127.14.2999. [DOI] [PubMed] [Google Scholar]
- 92.Mill P, Hui CC. Splitting Hairs: Dissecting the Roles of Gli Activator and Repressor Functions during Epidermal Development and Disease. In: Ruiz IAA, editor. Hedgehog-Gli Signaling in Human Disease. Landes Bioscience/Springer; 2006. electronic. [Google Scholar]
- 93.Ashique AM, Choe Y, Karlen M, May SR, Phamluong K, Solloway MJ, Ericson J, Peterson AS. The Rfx4 transcription factor modulates Shh signaling by regional control of ciliogenesis. Sci Signal. 2009;2:ra70. doi: 10.1126/scisignal.2000602. [DOI] [PubMed] [Google Scholar]
- 94.May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 95.Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 96.Svard J, Rozell B, Toftgard R, Teglund S. Tumor suppressor gene co-operativity in compound Patched1 and suppressor of fused heterozygous mutant mice. Mol Carcinog. 2009;48:408–419. doi: 10.1002/mc.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merchant M, Vajdos FF, Ultsch M, Maun HR, Wendt U, Cannon J, Desmarais W, Lazarus RA, de Vos AM, de Sauvage FJ. Suppressor of fused regulates Gli activity through a dual binding mechanism. Mol Cell Biol. 2004;24:8627–8641. doi: 10.1128/MCB.24.19.8627-8641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Q, Shi Q, Chen Y, Yue T, Li S, Wang B, Jiang J. Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proc Natl Acad Sci USA. 2009;106:21191–21196. doi: 10.1073/pnas.0912008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23:1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jia J, Kolterud A, Zeng H, Hoover A, Teglund S, Toftgard R, Liu A. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Dev Biol. 2009;330:452–460. doi: 10.1016/j.ydbio.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 102.Barnes EA, Kong M, Ollendorff V, Donoghue DJ. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 2001;20:2214–2223. doi: 10.1093/emboj/20.9.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jenkins D, Winyard PJ, Woolf AS. Immunohistochemical analysis of Sonic hedgehog signalling in normal human urinary tract development. J Anat. 2007;211:620–629. doi: 10.1111/j.1469-7580.2007.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 105.Mille F, Thibert C, Fombonne J, Rama N, Guix C, Hayashi H, Corset V, Reed JC, Mehlen P. The Patched dependence receptor triggers apoptosis through a DRAL-caspase-9 complex. Nat Cell Biol. 2009;11:739–746. doi: 10.1038/ncb1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bijlsma MF, Borensztajn KS, Roelink H, Peppelenbosch MP, Spek CA. Sonic hedgehog induces transcription-independent cytoskeletal rearrangement and migration regulated by arachidonate metabolites. Cell Signal. 2007;19:2596–2604. doi: 10.1016/j.cellsig.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 107.Kang W, Saqui-Salces M, Zavros Y, Merchant JL. Induction of follistatin precedes gastric transformation in gastrin deficient mice. Biochem Biophys Res Commun. 2008;376:573–577. doi: 10.1016/j.bbrc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eichberger T, Kaser A, Pixner C, Schmid C, Klingler S, Winklmayr M, Hauser-Kronberger C, Aberger F, Frischauf AM. GLI2-specific transcriptional activation of the bone morphogenetic protein/activin antagonist follistatin in human epidermal cells. J Biol Chem. 2008;283:12426–12437. doi: 10.1074/jbc.M707117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 110.Gallego MI, Beachy PA, Hennighausen L, Robinson GW. Differential requirements for Shh in mammary tissue and hair follicle morphogenesis. Dev Biol. 2002;249:131–139. doi: 10.1006/dbio.2002.0761. [DOI] [PubMed] [Google Scholar]
- 111.Lehman JM, Laag E, Michaud EJ, Yoder BK. An essential role for dermal primary cilia in hair follicle morphogenesis. J Investig Dermatol. 2009;129:438–448. doi: 10.1038/jid.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- 114.Cano DA, Sekine S, Hebrok M. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology. 2006;131:1856–1869. doi: 10.1053/j.gastro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 115.Zhang J, Lipinski RJ, Gipp JJ, Shaw AK, Bushman W. Hedgehog pathway responsiveness correlates with the presence of primary cilia on prostate stromal cells. BMC Dev Biol. 2009;9:50. doi: 10.1186/1471-213X-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 117.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 118.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim JH, Huang Z, Mo R. Gli3 null mice display glandular overgrowth of the developing stomach. Dev Dyn. 2005;234:984–991. doi: 10.1002/dvdy.20542. [DOI] [PubMed] [Google Scholar]
- 121.van den Brink GR, Hardwick JC, Nielsen C, Xu C, ten Kate FJ, Glickman J, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51:628–633. doi: 10.1136/gut.51.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–328. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 123.Fukaya M, Isohata N, Ohta H, Aoyagi K, Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H, Sakamoto H, Shimoda T, Nimura Y, Yoshida T, Sasaki H. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131:14–29. doi: 10.1053/j.gastro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 124.Kang DH, Han ME, Song MH, Lee YS, Kim EH, Kim HJ, Kim GH, Kim DH, Yoon S, Baek SY, Kim BS, Kim JB, Oh SO. The role of hedgehog signaling during gastric regeneration. J Gastroenterol. 2009;44:372–379. doi: 10.1007/s00535-009-0006-1. [DOI] [PubMed] [Google Scholar]
- 125.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 126.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of Parietal Cell Expression of Sonic Hedgehog Induces Hypergastrinemia and Hyperproliferation of Surface Mucous Cells. Gastroenterology. 2010;138:550–561. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–15708. doi: 10.1074/jbc.M413037200. [DOI] [PubMed] [Google Scholar]
- 128.Hollinshead JW, Debas HT, Yamada T, Elashoff J, Osadchey B, Walsh JH. Hypergastrinemia develops within 24 hours of truncal vagotomy in dogs. Gastroenterology. 1985;88:35–40. doi: 10.1016/s0016-5085(85)80129-3. [DOI] [PubMed] [Google Scholar]
- 129.Zavros Y, Rathinavelu S, Kao JY, Todisco A, Del Valle J, Weinstock JV, Low MJ, Merchant JL. Treatment of Helicobacter gastritis with IL-4 requires somato-statin. Proc Natl Acad Sci USA. 2003;100:12944–12949. doi: 10.1073/pnas.2135193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mangeat P, Gusdinar T, Sahuquet A, Hanzel DK, Forte JG, Magous R. Acid secretion and membrane reorganization in single gastric parietal cell in primary culture. Biol Cell. 1990;69:223–231. doi: 10.1016/0248-4900(90)90349-8. [DOI] [PubMed] [Google Scholar]
- 131.Zhou R, Cao X, Watson C, Miao Y, Guo Z, Forte JG, Yao X. Characterization of protein kinase A-mediated phosphorylation of ezrin in gastric parietal cell activation. J Biol Chem. 2003;278:35651–35659. doi: 10.1074/jbc.M303416200. [DOI] [PubMed] [Google Scholar]
- 132.Pagliocca A, Hegyi P, Venglovecz V, Rackstraw SA, Khan Z, Burdyga G, Wang TC, Dimaline R, Varro A, Dockray GJ. Identification of ezrin as a target of gastrin in immature mouse gastric parietal cells. Exp Physiol. 2008;93:1174–1189. doi: 10.1113/expphysiol.2008.042648. [DOI] [PubMed] [Google Scholar]
- 133.Tokhunts R, Singh S, Chu T, D’Angelo G, Baubet V, Goetz JA, Huang Z, Yuan Z, Ascano M, Zavros Y, Therond PP, Kunes S, Dahmane N, Robbins DJ. The full-length unprocessed hedgehog protein is an active signaling molecule. J Biol Chem. 2010;285:2562–2568. doi: 10.1074/jbc.M109.078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G99–G111. doi: 10.1152/ajpgi.00389.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Etheridge LA, Crawford TQ, Zhang S, Roelink H. Evidence for a role of vertebrate Disp1 in long-range Shh signaling. Development. 2010;137:133–140. doi: 10.1242/dev.043547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morales CR, Zeng J, El Alfy M, Barth JL, Chintalapudi MR, McCarthy RA, Incardona JP, Argraves WS. Epithelial trafficking of Sonic hedgehog by megalin. J Histochem Cytochem. 2006;54:1115–1127. doi: 10.1369/jhc.5A6899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCarthy RA, Barth JL, Chintalapudi MR, Knaak C, Argraves WS. Megalin functions as an endocytic sonic hedgehog receptor. J Biol Chem. 2002;277:25660–25667. doi: 10.1074/jbc.M201933200. [DOI] [PubMed] [Google Scholar]
- 138.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 140.El-Zaatari M, Grabowska AM, McKenzie AJ, Powe DG, Scotting PJ, Watson SA. Cyclopamine inhibition of the sonic hedgehog pathway in the stomach requires concomitant acid inhibition. Regul Pept. 2008;146:131–139. doi: 10.1016/j.regpep.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 141.Li X, Deng W, Lobo-Ruppert SM, Ruppert JM. Gli1 acts through Snail and E-cadherin to promote nuclear signaling by beta-catenin. Oncogene. 2007;26:4489–4498. doi: 10.1038/sj.onc.1210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fendrich V, Waldmann J, Esni F, Ramaswamy A, Mullendore M, Buchholz M, Maitra A, Feldmann G. Snail and Sonic Hedgehog activation in neuroendocrine tumors of the ileum. Endocr Relat Cancer. 2007;14:865–874. doi: 10.1677/ERC-07-0108. [DOI] [PubMed] [Google Scholar]
- 143.Allen M, Grachtchouk M, Sheng H, Grachtchouk V, Wang A, Wei L, Liu J, Ramirez A, Metzger D, Chambon P, Jorcano J, Dlugosz AA. Hedgehog signaling regulates sebaceous gland development. Am J Pathol. 2003;163:2173–2178. doi: 10.1016/S0002-9440(10)63574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Niemann C, Unden AB, Lyle S, Zouboulis Ch C, Toftgard R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11873–11880. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Minegishi Y, Suzuki H, Arakawa M, Fukushima Y, Masaoka T, Ishikawa T, Wright NA, Hibi T. Reduced Shh expression in TFF2-overexpressing lesions of the gastric fundus under hypochlorhydric conditions. J Pathol. 2007;213:161–169. doi: 10.1002/path.2221. [DOI] [PubMed] [Google Scholar]
- 146.Nishizawa T, Suzuki H, Masaoka T, Minegishi Y, Iwasahi E, Hibi T. Helicobacter pylori eradication restored sonic hedgehog expression in the stomach. Hepatogastroenterology. 2007;54:697–700. [PubMed] [Google Scholar]
- 147.Tsukamoto T, Mizoshita T, Tatematsu M. Gastric-and-intestinal mixed-type intestinal metaplasia: aberrant expression of transcription factors and stem cell intestinalization. Gastric Cancer. 2006;9:156–166. doi: 10.1007/s10120-006-0375-6. [DOI] [PubMed] [Google Scholar]
- 148.Shiotani A, Kamada T, Yamanaka Y, Manabe N, Kusunoki H, Hata J, Haruma K. Sonic hedgehog and CDX2 expression in the stomach. J Gastroenterol Hepatol. 2008;23(Suppl 2):S161–S166. doi: 10.1111/j.1440-1746.2008.05406.x. [DOI] [PubMed] [Google Scholar]
- 149.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 150.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 151.Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004:327–349. [PubMed] [Google Scholar]
- 152.Milne AN, Carneiro F, O’Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126:615–628. doi: 10.1007/s00439-009-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, Butterfield YS, Jeyes J, Schinas J, Bacani J, Kelsey M, Ferreira P, MacGillivray B, MacLeod P, Micek M, Ford J, Foulkes W, Australie K, Greenberg C, LaPointe M, Gilpin C, Nikkel S, Gilchrist D, Hughes R, Jackson CE, Monaghan KG, Oliveira MJ, Seruca R, Gallinger S, Caldas C, Huntsman D. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41:508–517. doi: 10.1136/jmg.2004.018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee SY, Han HS, Lee KY, Hwang TS, Kim JH, Sung IK, Park HS, Jin CJ, Choi KW. Sonic hedgehog expression in gastric cancer and gastric adenoma. Oncol Rep. 2007;17:1051–1055. [PubMed] [Google Scholar]
- 155.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 156.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 157.Li X, Madison BB, Zacharias W, Kolterud A, States D, Gumucio DL. Deconvoluting the intestine: molecular evidence for a major role of the mesenchyme in the modulation of signaling cross talk. Physiol Genomics. 2007;29:290–301. doi: 10.1152/physiolgenomics.00269.2006. [DOI] [PubMed] [Google Scholar]
- 158.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 159.Batts LE, Polk DB, Dubois RN, Kulessa H. Bmp signaling is required for intestinal growth and morphogenesis. Dev Dyn. 2006;235:1563–1570. doi: 10.1002/dvdy.20741. [DOI] [PubMed] [Google Scholar]
- 160.van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, Nielsen C, Gaffield W, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36:277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 161.Varnat F, Heggeler BB, Grisel P, Boucard N, Corthesy-Theulaz I, Wahli W, Desvergne B. PPARbeta/delta regulates paneth cell differentiation via controlling the hedgehog signaling pathway. Gastroenterology. 2006;131:538–553. doi: 10.1053/j.gastro.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 162.Qualtrough D, Buda A, Gaffield W, Williams AC, Paraskeva C. Hedgehog signalling in colorectal tumour cells: induction of apoptosis with cyclopamine treatment. Int J Cancer. 2004;110:831–837. doi: 10.1002/ijc.20227. [DOI] [PubMed] [Google Scholar]
- 163.Lees CW, Zacharias WJ, Tremelling M, Noble CL, Nimmo ER, Tenesa A, Cornelius J, Torkvist L, Kao J, Farrington S, Drummond HE, Ho GT, Arnott ID, Appelman HD, Diehl L, Campbell H, Dunlop MG, Parkes M, Howie SE, Gumucio DL, Satsangi J. Analysis of germline GLI1 variation implicates hedgehog signalling in the regulation of intestinal inflammatory pathways. PLoS Med. 2008;5:e239. doi: 10.1371/journal.pmed.0050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van den Brink GR, Hardwick JC. Hedgehog Wnteraction in colorectal cancer. Gut. 2006;55:912–914. doi: 10.1136/gut.2005.085902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oniscu A, James RM, Morris RG, Bader S, Malcomson RD, Harrison DJ. Expression of Sonic hedgehog pathway genes is altered in colonic neoplasia. J Pathol. 2004;203:909–917. doi: 10.1002/path.1591. [DOI] [PubMed] [Google Scholar]
- 166.Yoshikawa K, Shimada M, Miyamoto H, Higashijima J, Miyatani T, Nishioka M, Kurita N, Iwata T, Uehara H. Sonic hedgehog relates to colorectal carcinogenesis. J Gastroenterol. 2009;44:1113–1117. doi: 10.1007/s00535-009-0110-2. [DOI] [PubMed] [Google Scholar]
- 167.Varnat F, Zacchetti G, Ruiz IAA. Hedgehog pathway activity is required for the lethality and intestinal phenotypes of mice with hyperactive Wnt signaling. Mech Dev. 2010;127:73–81. doi: 10.1016/j.mod.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 168.Akiyoshi T, Nakamura M, Koga K, Nakashima H, Yao T, Tsuneyoshi M, Tanaka M, Katano M. Gli1, downregulated in colorectal cancers, inhibits proliferation of colon cancer cells involving Wnt signalling activation. Gut. 2006;55:991–999. doi: 10.1136/gut.2005.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arimura S, Matsunaga A, Kitamura T, Aoki K, Aoki M, Taketo MM. Reduced level of smoothened suppresses intestinal tumorigenesis by down-regulation of Wnt signaling. Gastroenterology. 2009;137:629–638. doi: 10.1053/j.gastro.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 170.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 171.Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Varnat F, Zacchetti G, Ruiz i Altaba A. Hedgehog pathway activity is required for the lethality and intestinal phenotypes of mice with hyperactive Wnt signaling. Mech Dev. 2010;127:73–81. doi: 10.1016/j.mod.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 173.Raymond C, Anne V, Millane G. Development of esophageal epithelium in the fetal and neonatal mouse. Anat Rec. 1991;230:225–234. doi: 10.1002/ar.1092300210. [DOI] [PubMed] [Google Scholar]
- 174.Duan H, Gao F, Li S, Nagata T. Postnatal development and aging of esophageal epithelium in mouse: a light and electron microscopic radioautographic study. Cell Mol Biol (Noisy-le-grand) 1993;39:309–316. [PubMed] [Google Scholar]
- 175.Isohata N, Aoyagi K, Mabuchi T, Daiko H, Fukaya M, Ohta H, Ogawa K, Yoshida T, Sasaki H. Hedgehog and epithelial-mesenchymal transition signaling in normal and malignant epithelial cells of the esophagus. Int J Cancer. 2009;125:1212–1221. doi: 10.1002/ijc.24400. [DOI] [PubMed] [Google Scholar]
- 176.Kawahira H, Ma NH, Tzanakakis ES, McMahon AP, Chuang PT, Hebrok M. Combined activities of hedgehog signaling inhibitors regulate pancreas development. Development. 2003;130:4871–4879. doi: 10.1242/dev.00653. [DOI] [PubMed] [Google Scholar]
- 177.Kawahira H, Scheel DW, Smith SB, German MS, Hebrok M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol. 2005;280:111–121. doi: 10.1016/j.ydbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 178.Hebrok M, Kim SK, St Jacques B, McMahon AP, Melton DA. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–4913. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- 179.Thomas MK, Rastalsky N, Lee JH, Habener JF. Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes. 2000;49:2039–2047. doi: 10.2337/diabetes.49.12.2039. [DOI] [PubMed] [Google Scholar]
- 180.Kim SK, Melton DA. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor. Proc Natl Acad Sci USA. 1998;95:13036–13041. doi: 10.1073/pnas.95.22.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lau J, Hebrok M. Hedgehog signaling in pancreas epithelium regulates embryonic organ formation and adult {beta}-cell function. Diabetes. 2010;59:1211–1221. doi: 10.2337/db09-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 183.Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang J, Zdanowicz M, Camp T, Torbenson MS, Rojkind M, Diehl AM. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85:1368–1380. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- 184.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, Fair JH, Ludlow JW, McClelland RE, Reid LM, Diehl AM. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–G870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 185.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang S, He J, Zhang X, Bian Y, Yang L, Xie G, Zhang K, Tang W, Stelter AA, Wang Q, Zhang H, Xie J. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006;27:1334–1340. doi: 10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- 187.Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS, Diehl AM. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–757. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- 188.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA, Rojkind M, Diehl AM. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–G1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 190.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–296. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 191.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 192.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 193.Taniguchi H, Yamamoto H, Akutsu N, Nosho K, Adachi Y, Imai K, Shinomura Y. Transcriptional silencing of hedgehog-interacting protein by CpG hypermethylation and chromatic structure in human gastrointestinal cancer. J Pathol. 2007;213:131–139. doi: 10.1002/path.2216. [DOI] [PubMed] [Google Scholar]