Abstract
The spindle assembly checkpoint (SAC) monitors mistakes in kinetochore-microtubule interaction and its activation prevents anaphase entry. The SAC remains active until all chromosomes have achieved bipolar attachment which applies tension on kinetochores. Our previous data in budding yeast Saccharomyces cerevisiae show that Ipl1/Aurora B kinase and a centromere-associated protein, Sgo1, are required to prevent SAC silencing prior to tension generation, but we believe that this regulatory network is incomplete. Bub1 kinase is one of the SAC components, and Bub1-dependent H2A phosphorylation triggers centromere recruitment of Sgo1 by H2A in yeast and human cells. Although yeast cells lacking the kinase domain of Bub1 show competent SAC activation, we found that the mutant cells fail to maintain a prolonged checkpoint arrest in the presence of tensionless attachment. Mutation of the Bub1 phosphorylation site in H2A also results in premature SAC silencing in yeast cells. Previous data indicate that Sgo1 protein binds to PP2ARts1, and we found that rts1Δ mutants exhibited premature SAC silencing as well. We further revealed that sgo1 mutants with abolished binding to H2A or PP2ARts1 displayed premature SAC silencing. Together, our results suggest that, in budding yeast S. cerevisiae, the Bub1-H2A-Sgo1-PP2ARts1 axis prevents SAC silencing and helps prolonged checkpoint arrest prior to tension establishment at kinetochores.
Keywords: Bub1 kinase, PP2A, SAC silencing, Sgo1, spindle assembly checkpoint
THE spindle assembly checkpoint (SAC) monitors defects in kinetochore-microtubule interaction and an active SAC blocks anaphase onset, thus this checkpoint is crucial for faithful chromosome segregation. The SAC components include Bub1, Bub3, Mad1, Mad2, Mad3/BubR1, and Mps1 (Stukenberg and Burke 2015). Recent evidence from budding yeast Saccharomyces cerevisiae and fission yeast S. pombe indicates that SAC proteins Bub1 and Bub3 bind to a kinetochore protein Spc105/Knl1, and protein kinase Mps1-mediated phosphorylation of Spc105 promotes the interaction of its MELT domains with Bub3 (Shepperd et al. 2012; Primorac et al. 2013). Kinetochore-associated Bub3-Bub1 complexes further recruit Mad1 and Mad2, where Mad2 is converted to a closed form that prevents the activation of anaphase-promoting complex/cyclosome and blocks the degradation of anaphase inhibitor Pds1 (Luo et al. 2000; London and Biggins 2014).
The SAC has to be silenced to allow anaphase onset, but the regulation of SAC silencing is poorly understood. One model is that kinetochore-microtubule attachment removes the SAC kinase Mps1 from kinetochores to silence the SAC, which is supported by recent observations in mammalian cells (Hiruma et al. 2015; Ji et al. 2015). Research work in budding yeast supports the model that the interaction of microtubule-associated Dam1 complex with the Ndc80 kinetochore complex separates Ndc80-associated Mps1 from its substrates to trigger SAC silencing (Aravamudhan et al. 2015). On the other hand, protein phosphatase PP1 has been shown to be essential for SAC silencing likely through its dephosphorylation of Mps1 kinase substrates at the kinetochore (London et al. 2012). The yeast kinetochore protein Spc105 binds to PP1 through a conserved motif and mutation of this motif blocks anaphase entry, resulting in lethality. Interestingly, this cell cycle arrest and lethality are rescued by deletion of a SAC gene, indicating that Spc105-PP1 interaction is essential for SAC silencing (Rosenberg et al. 2011). Furthermore, PP1 overexpression causes premature SAC silencing in the presence of detached kinetochores (Pinsky et al. 2009; London et al. 2012). In addition to Spc105, PP1 also binds to another yeast kinetochore protein, Fin1 (Akiyoshi et al. 2009). Our recent data demonstrate that the Fin1-PP1 complex promotes the removal of Bub1 from the kinetochore. Because Fin1 localizes to the kinetochore after anaphase entry, this regulation is not essential for SAC silencing, but untimely Fin1 kinetochore localization leads to premature SAC silencing (Bokros and Wang 2016; Bokros et al. 2016), indicating that PP1 negatively regulates SAC at multiple levels.
Ipl1/Aurora B kinase regulates the stability of kinetochore-microtubule interaction as well as the SAC silencing process (Cheeseman et al. 2002). In budding yeast, Ipl1 phosphorylates Dam1, a protein of a 10-subunit kinetochore complex which mediates Ndc80-microtubule interaction (Janke et al. 2002; Li et al. 2002). We found that phospho-deficient dam1 mutants showed premature SAC silencing, while the phospho-mimetic dam1 mutants exhibited delayed anaphase entry. This delay is mainly attributed to the failure of SAC silencing, whereas defective kinetochore attachment only plays a minor role (Jin and Wang 2013). Because Dam1 dephosphorylation is triggered by tension at kinetochores (Keating et al. 2009), Ipl1-dependent Dam1 phosphorylation likely prevents SAC silencing until chromosome bipolar attachment generates tension at kinetochores. Therefore, this mechanism links bipolar attachment and SAC silencing (Wang et al. 2014). SGO1 encodes a centromere-binding protein (Indjeian et al. 2005), and SGO1 deletion also leads to premature SAC silencing (Jin et al. 2012; Jin and Wang 2013). However, it is largely unknown how Sgo1 regulates SAC silencing at the molecular level.
In yeast and human cells, kinetochore-localized checkpoint protein Bub1 phosphorylates histone H2A to promote its association with Sgo1, resulting in the recruitment of Sgo1 to centromeres and pericentromeres (Kawashima et al. 2010). Interestingly, results from budding yeast indicate that the kinase domain of Bub1 is dispensable for SAC activation (Fernius and Hardwick 2007). In this report, we determine the role of the Bub1 kinase domain in the regulation of SAC silencing in budding yeast S. cerevisiae. We found that deletion of the Bub1 kinase domain or mutation of the Bub1 phosphorylation site in H2A leads to premature SAC silencing, without compromising SAC activation. Sgo1 interacts with protein phosphatase PP2ARts1 (Riedel et al. 2006; Eshleman and Morgan 2014), and we found that deletion of RTS1 also led to premature SAC silencing. In addition, mutation of the H2A or PP2ARts1 binding motif in Sgo1 causes anaphase entry in the presence of tensionless chromosome attachment. Therefore, our results suggest that the Bub1-H2A-Sgo1-PP2ARts1 axis prevents SAC silencing until cells have achieved chromosome bipolar attachment.
Materials and Methods
Plasmids construction
The SGO1 gene was cloned by PCR using the forward primer 5′-GATTCCCCGCGGGGACTACTTCGATTGGGTTATTGA-3′ and the reverse primer 5′-GATACCATCGATGGTAGGGACGTTAAAGACATTGA-3′. The SGO1 DNA fragment from PCR was inserted into the pRS403 vector after digestion with ClaI and SacII restriction enzymes. Using this constructed SGO1 plasmid, site-directed mutagenesis was performed to generate the plasmid harboring the sgo1-N51I mutant gene, in which the PP2A binding motif was mutated. To generate the sgo1-4A plasmid, the DNA that encodes the H2A binding motif (KMRR) in SGO1 gene was mutated to the sequence encoding four alanine residues.
Yeast strain and growth conditions
The relevant genotypes and the sources of the strains used in this study are listed in Supplemental Material, Table S1. All of the strains listed are isogenic to Y300, a derivative of W303. The Bub1 kinase deletion allele (bub1-∆K) was constructed by inserting a 13myc-Sphis5+ or 3HA-Sphis5+ cassette after the 608 residue of Bub1 by a PCR-based method (Longtine et al. 1998). The resulting strains express truncated Bub1 lacking the fragment that encodes the amino acid residues from 609 to 1021. The bub1-∆K mutants were verified by determining the protein size of the tagged protein fragment using Western blotting. The rts1Δ strain was also created using a PCR-based method. The h2a-121A and the H2A-wild type (WT) strains are a gift from the Watanabe Laboratory. The endogenous H2A gene was deleted in these strains, but the strains express WT H2A or mutated h2A-S121A from plasmids. The constructed plasmids containing SGO1, sgo1-N51I, and sgo1-4A genes were inserted into the genome of sgo1Δ strains at the endogenous locus to generate SGO1, sgo1-N51I, and sgo1-4A yeast strains.
The yeast cell growth, synchronization, and CIK1-CC overexpression were performed as described previously (Jin et al. 2012). Briefly, yeast cells with a control vector or PGALCIK1-CC plasmid were grown in synthetic medium containing raffinose to midlog phase and then arrested in G1 phase with α factor. The arrested cells were released into medium containing galactose to induce CIK1-CC overexpression. For nocodazole treatment, G1-arrested cells were released into YPD containing 20 µg/ml nocodazole and 1% of DMSO. We added 10 µg/ml of nocodazole every hour.
Western blot analysis
We collected 1.5 ml yeast cell culture and the cell pellets were resuspended in 100 µl H2O and then 100 µl 0.2 N NaOH was added. The mixture was left at room temperature for 5 min. The pellet was resuspended in the loading buffer. For Pds1-18myc protein detection, we used 10% acrylamide gels for SDS-PAGE. For the detection of Mad1 modification, we used and 8% acrylamide gels. The anti-myc antibody (9E10) and anti-HA (16B12) (Covance Research Products) were used at a 1:1000 dilution. Phosphoglycerate kinase 1 (Pgk1) antibody (Molecular Probes, Eugene, OR) was used at a 1:5000 dilution. Proteins were detected with ECL (Perkin-Elmar-Cetus, Norwalk, CT).
Chromosome segregation assays
Strains containing GFP-labeled chromosome IV (CEN4-GFP) and Tub1-mCherry were collected and fixed with 3.7% formaldehyde for 5 min at room temperature. After centrifugation, the pellet was resuspended in 1× PBS buffer. The fluorescence signals were analyzed in cells with an elongated spindle using a fluorescence microscope (EVOS from Life Technologies).
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Bub1 kinase activity is required for the cell cycle delay induced by syntelic chromosome attachment
Cik1 and Kar3 form a hetero-motor complex through their coiled-coil domains (Barrett et al. 2000). We demonstrate that overexpression of the coiled-coil domain of CIK1 (CIK1-CC) disrupts Cik1-Kar3 interaction and causes chromosome syntelic attachment, i.e., both sister kinetochores are attached by microtubules from the same spindle pole. We showed that Ipl1 kinase and Sgo1 are required to prevent SAC silencing in response to syntelic attachment induced by CIK1-CC overexpression (Jin et al. 2012; Jin and Wang 2013). Because the centromere localization of Sgo1 depends on H2A phosphorylation by Bub1 kinase (Fernius and Hardwick 2007; Kawashima et al. 2010; Nerusheva et al. 2014), Bub1 kinase activity might be required to prevent SAC silencing in response to syntelic attachment, although this kinase activity is dispensable for SAC activation (Fernius and Hardwick 2007).
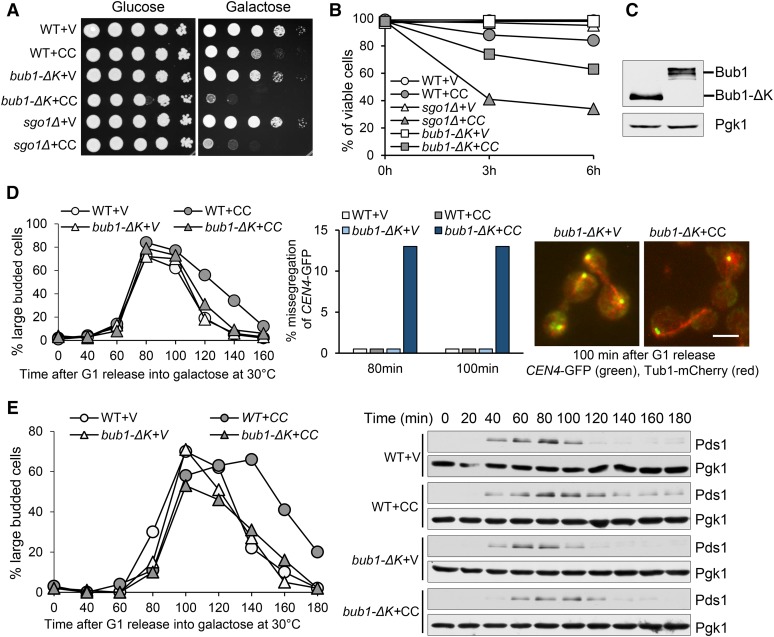
To investigate the role of the Bub1 kinase domain in preventing SAC silencing, we first examined the sensitivity of yeast cells lacking this domain (bub1-ΔK) to CIK1-CC overexpression. In bub1-ΔK mutant, the C-terminal fraction of the BUB1 gene that encodes 609–1021 amino acid residues was deleted to eliminate the kinase domain. We introduced PGALCIK1-CC plasmids, which contain CIK1-CC under the control of a galactose promotor (GAL), into bub1-ΔK cells. Similarly to sgo1Δ, bub1-ΔK cells grew a little slower than WT cells, but the mutant cells with PGALCIK1-CC plasmids failed to grow on galactose plates (Figure 1A). After CIK1-CC induction for 6 hr, 37% bub1-ΔK cells lost viability; compared to 15% viability loss in WT cells (Figure 1B). The expression of truncated Bub1 (Bub1-ΔK-13myc) was similar to that of Bub1-13myc (Figure 1C). Thus, the observed phenotype of bub1-ΔK mutant is unlikely to be attributed to decreased Bub1 expression.
Figure 1.
bub1-∆K mutants exhibit premature SAC silencing in response to CIK1-CC-induced syntelic attachment. (A) Overexpression of CIK1-CC is toxic to bub1-∆K cells. Saturated WT and mutant cells with a vector (V) or a PGALCIK1-CC plasmid (CC) were 10-fold serial diluted, spotted onto glucose and galactose plates, and incubated at 30° for 2 days. (B) The viability loss of bub1-∆K cells after CIK1-CC overexpression. WT and bub1-∆K cells with a vector or a PGALCIK1-CC plasmid were grown to log phase in raffinose medium and then released into galactose medium at 30°. Cells were collected at 0, 3, and 6 hr and spread onto YPD plates to determine the plating efficiency after overnight growth at 25° (n ≥ 200). (C) The expression of truncated Bub1. BUB1-13-myc and bub1-∆K-13myc cells in midlog phase were treated with 20 µg/ml of nocodazole for 120 min at 30°. The cells lysates were prepared for the examination of myc-tagged Bub1 and Bub1-∆K. (D) CIK1-CC overexpression causes chromosome missegregation in bub1-∆K mutant cells. A vector or a PGALCIK1-CC plasmid was introduced into WT and bub1-∆K cells with CEN4-GFP TUB1-mCherry. The transformants were first arrested in G1 phase in raffinose medium and then released into galactose medium at 30°. α-factor was restored after budding to block the second round of cell cycle. Cells were collected at the indicated time points for the examination of fluorescence signals. The percentage of CEN4-GFP missegregation is shown in the center panel; the distribution of CEN4-GFP and spindle morphology in some representative cells is shown on the right. The budding index is shown in the left panel. Bar, 5 µm. (E) bub1-∆K mutation alleviates the delay of Pds1 degradation induced by CIK1-CC overexpression. G1-arrested PDS1-18myc and bub1-∆K PDS1-18myc cells with a vector or a PGALCIK1-CC plasmid were released into galactose medium and incubated at 30°. α-factor was restored after budding. Cells were collected at the indicated time points and protein samples were prepared for Western blotting. The budding index and Pds1 levels are shown. Pgk1 protein levels are used as a loading control.
The sensitivity of bub1-ΔK cells to CIK1-CC overexpression might be a result of chromosome missegregation. To test this idea, a control vector or PGALCIK1-CC plasmid was introduced into WT and bub1-ΔK mutants with the GFP-marked centromere of chromosome IV (CEN4-GFP) and mCherry-labeled spindle (Tub1-mCherry). The cells were arrested in G1 phase in noninducible raffinose medium and then released into galactose medium to induce CIK1-CC overexpression. CIK1-CC overexpression caused a cell cycle delay in WT cells as indicated by the higher proportion of large-budded cells in later time points, but this delay was largely suppressed by bub1-ΔK. In anaphase cells, CIK1-CC overexpression resulted in 13% missegregation of chromosome IV in bub1-ΔK cells, as indicated by two CEN4-GFP dots in one daughter cell (Figure 1D). The cosegregation of CEN4-GFP in bub1-ΔK cells indicates checkpoint arrest failure. Our previous study showed that overexpression of CIK1-CC in sgo1Δ cells caused >30% CEN4-GFP missegregation using the same analysis (Jin et al. 2012), which is higher than that of bub1-ΔK cells. One explanation is that Sgo1 is still functional enough in bub1-ΔK cells to prevent SAC silencing, but less efficient due to the failure of centromere localization.
To further demonstrate the premature SAC silencing in bub1-ΔK cells, we analyzed the degradation kinetics of the anaphase inhibitor Pds1 in cells overexpressing CIK1-CC, which marks anaphase entry. G1-arrested PDS1-18myc and bub1-ΔK PDS1-18myc cells carrying either a vector or a PGALCIK1-CC plasmid were released into galactose medium. Overexpression of CIK1-CC induced moderate cell cycle delay in WT cells, as indicated by the budding index as well as by delayed Pds1 degradation. However, this delay largely disappeared in bub1-∆K cells, indicating that this anaphase entry delay depends on the kinase domain of Bub1 (Figure 1E). Because previous research indicates that the kinase activity of Bub1 is dispensable for SAC activation, it is likely that this kinase domain is specifically required to prevent premature SAC silencing and maintain prolonged checkpoint arrest when tensionless attachment occurs.
The anaphase entry delay in dam1-3D mutants is alleviated by bub1-∆K
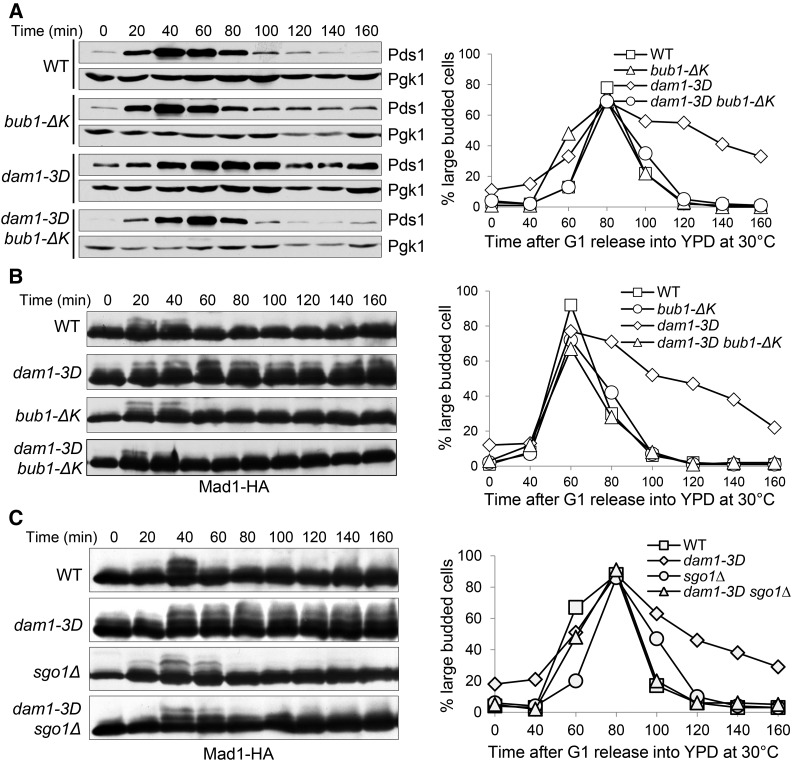
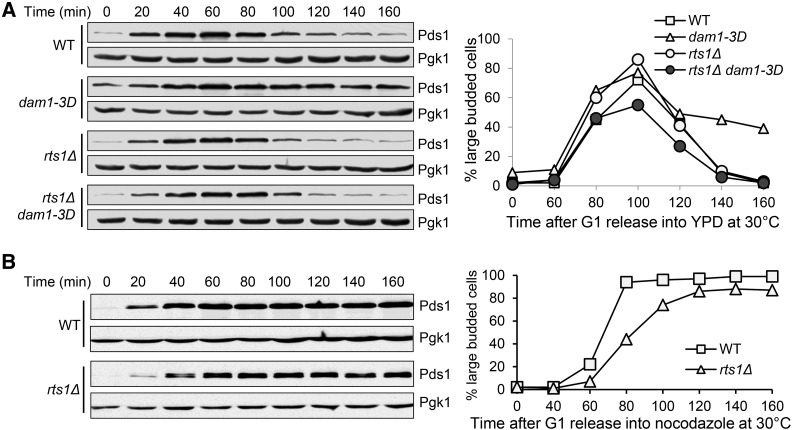
The phosphorylation of kinetochore protein Dam1 by Ipl1 kinase destabilizes kinetochore-microtubule attachment to facilitate correction (Pinsky et al. 2006; Tien et al. 2010), but the delayed anaphase entry in phospho-mimetic mutant dam1-3D is primarily attributed to its inability to silence the SAC (Jin and Wang 2013). Thus, we tested if the anaphase entry delay in dam1-3D requires the kinase activity of Bub1. First, we compared the anaphase entry process in synchronized dam1-3D and dam1-3D bub1-ΔK cells by examining Pds1 protein levels. Consistent with our previous report, dam1-3D exhibited delayed Pds1 degradation and persistent appearance of large-budded cells, but these phenotypes were abolished in dam1-3D bub1-ΔK cells (Figure 2A). Therefore, the anaphase entry delay in dam1-3D cells depends on the kinase activity of Bub1. We noticed the presence of some large-budded cells and detectable Pds1 protein expression in dam1-3D mutant after α factor treatment, which is likely due to the difficulty in SAC silencing.
Figure 2.
bub1-∆K and sgo1Δ mutants suppress the anaphase entry delay in dam1-3D cells. (A) The delayed Pds1 degradation in dam1-3D cells is suppressed by bub1-∆K. G1-arrested WT and mutant cells with Pds1-18myc were released into YPD medium at 30°. α-factor was added after budding. Cell lysates were prepared at the indicated times for western blotting with anti-myc antibody. The budding index and Pds1 protein levels are shown. Pgk1, loading control. (B) The delay of Mad1 dephosphorylation in dam1-3D cells is suppressed by bub1-∆K. G1-arrested WT and mutant cells with Mad1-3HA were released into YPD medium at 30°. Cell lysates were prepared at the indicated time points for Western blotting with anti-HA antibody. The Mad1 modification is show in the left panel and the budding index is shown in the right panel. (C) The delay of Mad1 dephosphorylation in dam1-3D cells is suppressed by sgo1Δ. The cells were treated as described above. The Mad1 modification and the budding index are shown.
The SAC component Mad1 is phosphorylated in a SAC-dependent manner and its dephosphorylation indicates SAC silencing (Hardwick and Murray 1995; Mirchenko and Uhlmann 2010). dam1-3D cells exhibited persistent Mad1 phosphorylation, indicating compromised SAC silencing (Jin and Wang 2013). We compared Mad1 dephosphorylation kinetics in synchronized dam1-3D and dam1-3D bub1-ΔK cells. The delay of Mad1 dephosphorylation in dam1-3D was eliminated in the double mutant cells (Figure 2B). Similarly, sgo1Δ mutation also suppressed this delay (Figure 2C). Taken together, the premature SAC silencing in bub1-ΔK cells and the suppression of SAC silencing defect in dam1-3D mutants by bub1-ΔK support the conclusion that the Bub1 kinase activity is required to prevent premature SAC silencing.
The phosphorylation of H2A by Bub1 prevents SAC silencing
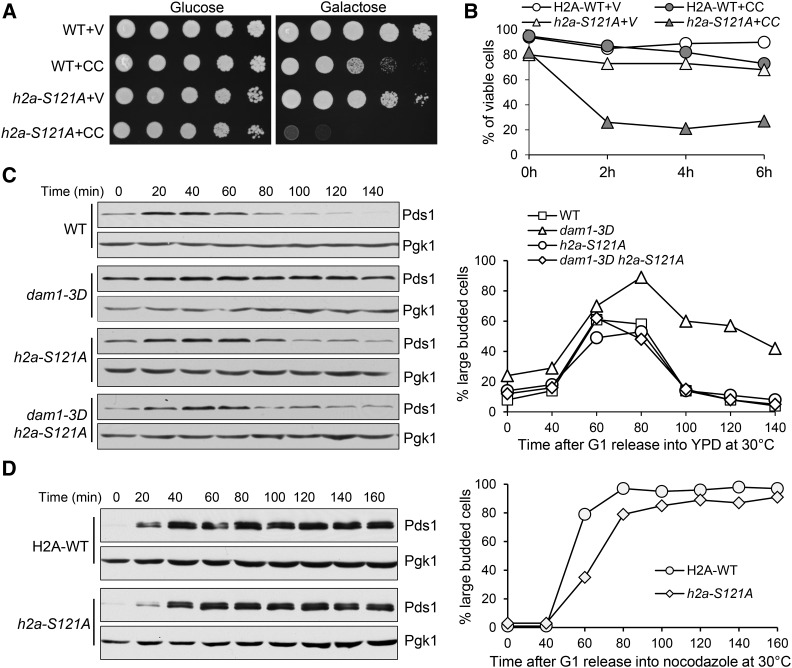
The phosphorylation of budding yeast H2A at serine 121 by Bub1 is essential for centromere localization of Sgo1, and phospho-deficient h2a-S121A mutant fails to recruit Sgo1 to centromeres (Kawashima et al. 2010; Nerusheva et al. 2014). Thus, we postulated that h2a-S121A mutants would show premature SAC silencing similar to bub1-ΔK and sgo1Δ mutants. To test the idea, we first examined the growth of h2a-S121A mutant cells overexpressing CIK1-CC. The growth defect of h2a-S121A cells with a control vector on galactose plates was noticeable but minor. The mutant cells with a PGALCIK1-CC plasmid, however, failed to grow on galactose plates (Figure 3A). Moreover, after 2 hr incubation in galactose medium, only 26% of h2a-S121A cells remained viable (Figure 3B). The viability loss is likely caused by chromosome missegregation, but the multiple selection markers in the h2a-S121A strain make it difficult to construct strains and examine chromosome segregation.
Figure 3.
The abolishment of Bub1-dependent H2A phosphorylation leads to premature SAC silencing. (A) h2a-S121A mutant cells are sensitive to CIK1-CC overexpression. WT and h2a-S121A cells with a vector (V) or a PGALCIK1-CC (CC) plasmid were serial 10-fold diluted and then plated onto glucose and galactose plates for further incubation at 30° for 2 days. (B) h2a-S121A mutants lose viability after CIK1-CC overexpression. Cells with the indicated genotypes in raffinose medium were released into galactose medium at 30°. Cells were collected at 0, 2, 4, and 6 hr and spread onto YPD plates to examine the plating efficiency after overnight growth at 25° (n ≥ 200). (C) h2a-S121A mutation abolishes the anaphase entry delay in dam1-3D mutants. Cells with the indicated genotypes were arrested in G1 phase with α-factor and then released into cell cycle in YPD. Cells were collected over time to examine the Pds1 protein levels. Budding index and Pds1 protein levels are shown. Pgk1, loading control. (D) h2a-S121A mutant cells show efficient metaphase arrest in response to nocodazole treatment. G1-arrested PDS1-18myc and h2a-S121A PDS1-18myc cells were released into YPD medium containing 20 μg/ml of nocodazole and incubated at 30°. Pds1 protein levels were determined after Western blotting. The Pds1 levels and budding index are shown. Pgk1, loading control.
We have shown that bub1-ΔK and sgo1Δ mutations alleviate the anaphase entry delay in dam1-3D mutants (Figure 2). Therefore, we tested if this is also true for the h2a-121A mutant. G1-synchronized dam1-3D and dam1-3D h2a-S121A cells were released into the cell cycle and the Pds1 protein levels were examined. It was clear that h2a-S121A suppressed the delayed Pds1 degradation in dam1-3D mutants (Figure 3C). Since this suppression could be caused by the failure of SAC activation, we further analyzed SAC function in h2a-S121A mutant cells. For this purpose, G1-arrested WT and h2a-S121A mutant cells were released into a medium containing 20 µl/ml nocodazole, which activates the SAC to prevent Pds1 degradation. In both WT and h2a-S121A mutant, Pds1 protein levels were persistent in the presence of nocodazole, indicating that the SAC is competent in h2a-S121A mutant cells (Figure 3D). Therefore, our data support the conclusion that Bub1-dependent phosphorylation of H2A at S121 in budding yeast is required to prevent premature SAC silencing in the absence of kinetochore tension. This phosphorylation regulates SAC silencing likely through centromere recruitment of Sgo1.
Rts1 prevents SAC silencing in the presence of syntelic attachment
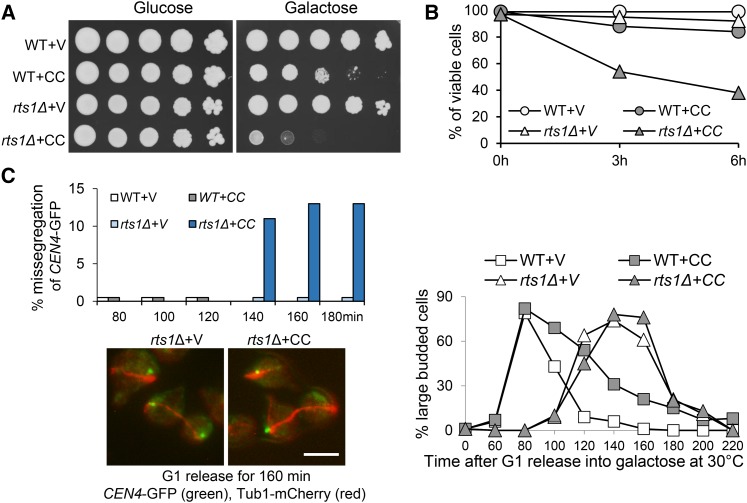
Rts1 and Cdc55 are the two PP2A B-regulatory subunits (Shu et al. 1997). Centromere-localized Sgo1 recruits PP2ARts1, condensin, and Ipl1 kinase complex to the centromere region (Riedel et al. 2006; Peplowska et al. 2014; Verzijlbergen et al. 2014). Although we found that Ipl1 is required to prevent premature SAC silencing in response to tension defects (Jin and Wang 2013), previous work shows that the centromere localization of Ipl1 is dispensable for this function (Campbell and Desai 2013). One possibility is that Sgo1’s function in SAC silencing depends on its role in centromere recruitment of PP2ARts1, but not Ipl1 kinase. Thus, we first examined the sensitivity of rts1Δ mutants to CIK1-CC overexpression. rts1Δ cells harboring PGALCIK1-CC plasmids grew very poorly on galactose plates (Figure 4A), which is consistent with a previous observation (Peplowska et al. 2014). Moreover, after 6-hr incubation in galactose medium, 62% of rts1Δ cells with PGALCIK1-CC plasmids were inviable, whereas only 8% of rts1Δ cells with a vector lost viability. The viability loss for WT cells with PGALCIK1-CC plasmids was much less significant (16%) (Figure 4B). We further assessed CEN4-GFP separation in rts1Δ cells overexpressing CIK1-CC. G1-arrested cells were released into galactose medium and CEN4-GFP segregation was examined in cells with an elongated spindle. More than 10% of rts1Δ cells overexpressing CIK1-CC showed CEN4-GFP cosegregation at 140, 160, and 180 min; but no missegregation was observed in WT cells (Figure 4C). We noticed delayed cell cycle progression of rts1Δ cells in the synthetic galactose medium, which is consistent with a previous observation that loss of Rts1 causes delayed initiation of bud growth (Artiles et al. 2009). These results indicate that rts1Δ mutants are sensitive to the induction of syntelic attachment due to chromosome missegregation. However, the rate of missegregation is less significant compared to sgo1Δ mutant cells, indicating the presence of redundant pathways.
Figure 4.
rts1Δ mutants are sensitive to CIK1-CC overexpression. (A) Overexpression of CIK1-CC is toxic to rts1Δ cells. WT and rts1Δ cells with a vector (V) or a PGALCIK1-CC (CC) plasmid were 10-fold serial diluted and spotted onto glucose and galactose plates for further incubation at 30° for 2 days. (B) rts1∆ cells lose viability after CIK1-CC overexpression. WT and rts1∆ cells with a vector or a PGALCIK1-CC plasmid were grown to log phase in raffinose medium at 30° and then galactose was added into the medium. Cells were collected at 0, 3, and 6 hr and spread onto YPD plates to assess the plating efficiency (n ≥ 200). (C) CIK1-CC overexpression leads to chromosome missegregation in rts1Δ cells. CEN4-GFP TUB1-mCherry and rts1Δ CEN4-GFP TUB1-mCherry cells with a vector or a PGALCIK1-CC plasmid were arrested in G1 phase in raffinose medium and then released into galactose medium at 30°. Cells were collected at indicated time points and fixed for the examination of fluorescence signals. The percentage of cells with missegregated sister CEN4-GFPs among all anaphase cells is shown on the top left (n > 100). The localization of CEN4-GFP as well as spindle morphology in some representative cells is shown at the bottom. The budding index is shown on the right. Bar, 5 µm.
Next we asked if rts1Δ mutation suppresses the failure of SAC silencing in dam1-3D cells as sgo1Δ and bub1-∆K do. dam1-3D and dam1-3D rts1Δ cells synchronized in G1 were released into the cell cycle and Pds1 levels were monitored over time. The stabilized Pds1 protein level and cell cycle delay in dam1-3D cells were largely abolished by RTS1 deletion (Figure 5A). To test if the suppression is due to defective SAC function in rts1Δ cells, we also examined the cell cycle arrest in rts1Δ cells treated with nocodazole, which activates the SAC. As a result, both WT and rts1Δ cells exhibited stabilized Pds1 protein levels in the presence of nocodazole, indicating a competent SAC in rts1Δ cells (Figure 5B). These results indicate that Rts1 is also required to prevent anaphase entry in the presence of tension defects, but Rts1 is dispensable for SAC activation in response to spindle damage.
Figure 5.
rts1Δ mutation suppresses the anaphase entry delay in dam1-3D cells without compromising SAC activation. (A) The delay of Pds1 degradation in dam1-3D cells is suppressed by rts1Δ. G1-arrested WT and mutant cells with Pds1-18myc were released into YPD medium at 30°. α-factor was added back after budding. Cell lysates were prepared at the indicated time points for Western blotting with anti-myc antibody. The Pds1 levels and budding index are shown. Pgk1, loading control. (B) rts1∆ cells show intact SAC function. G1-arrested PDS1-18myc and rts1Δ PDS1-18myc cells were released into YPD medium containing 20 μg/ml of nocodazole and incubated at 30°. Cells were collected every 20 min for the budding index and the determination of Pds1 protein levels. Pgk1, loading control. The Pds1 levels and the budding index are shown.
The interaction of Sgo1 with either H2A or PP2A is required to prevent premature SAC silencing
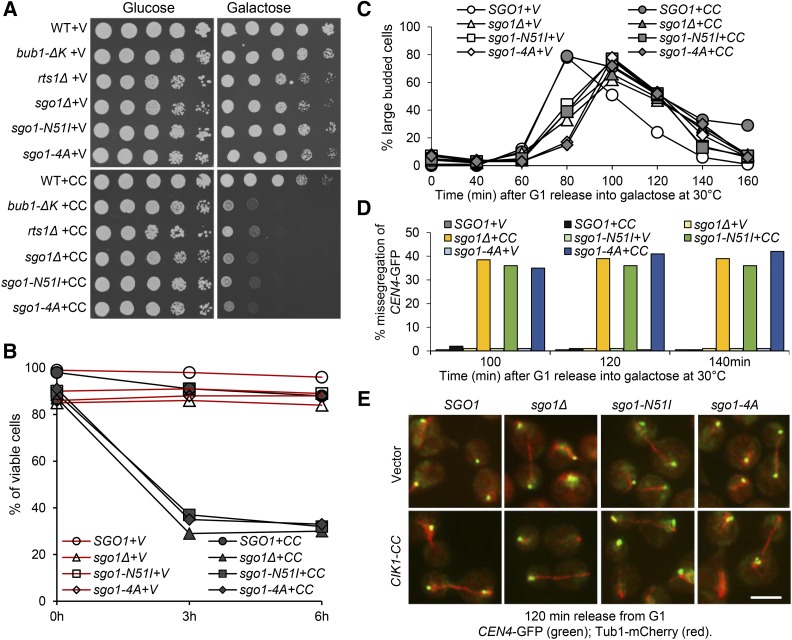
Our observations support the possibility that the recruitment of Sgo1-PP2ARts1 to the centromere region through Sgo1-H2A interaction prevents premature SAC silencing. To test this possibility, we examined the SAC silencing process in cells with disrupted association of Sgo1 with either H2A or PP2A. The Sgo1-H2A interaction depends on a conserved basic motif KMRR in Sgo1 (Kitajima et al. 2004). Thus, we mutated this motif to four alanine residues, generating a sgo1-4A plasmid. Previous works define the PP2A-binding domain in Sgo1 as well, and sgo1-N51I mutation abolishes this interaction (Xu et al. 2009; Peplowska et al. 2014). We also generated a sgo1-N51I plasmid. To construct sgo1-4A and sgo1-N51I yeast mutant strains, we inserted the integrating plasmids containing SGO1, sgo1-4A, and sgo1-N51I genes into the endogenous locus in a sgo1Δ strain. To compare the sensitivity of these sgo1 mutants to CIK1-CC overexpression with rts1 and bub1 mutants, we performed the 10-fold dilution of all the mutants on the same plates. sgo1-N51I and sgo1-4A mutant cells harboring a control vector grew well on galactose plates, but the mutant cells with PGALCIK1-CC plasmids failed to grow on galactose plates. Their sensitivity to CIK1-CC overexpression is similar to sgo1Δ and bub1-ΔK cells, but rts1Δ cells are slightly less sensitive (Figure 6A). After CIK1-CC induction for 6 hr in galactose medium, 88% of cells with SGO1 were viable, but the viability of sgo1-4A and sgo1-N51I mutants reduced to 32 and 33%, respectively, which is comparable to the viability of sgo1Δ cells (30%) (Figure 6B).
Figure 6.
The binding of Sgo1 to H2A and PP2A is essential to prevent premature SAC silencing. (A) Overexpression of CIK1-CC is toxic to sgo1 mutants with abolished binding to either H2A or PP2A. Saturated cells with a vector (V) or a PGALCIK1-CC plasmid (CC) were 10-fold serial diluted and spotted onto glucose and galactose plates, which were incubated at 30° for 2 days. (B) The viability loss of SGO1, sgo1Δ, sgo1-N51I, and sgo1-4A cells after CIK1-CC overexpression. Log-phase cells in raffinose were released into galactose medium at 30°. Cells were collected at 0, 3, and 6 hr and spread onto YPD plates to determine the plating efficiency (n ≥ 200). (C–E) CIK1-CC overexpression causes chromosome missegregation in sgo1 mutants. A vector or a PGALCIK1-CC plasmid was introduced into WT, sgo1Δ, sgo1-N51I, and sgo1-4A mutants with CEN4-GFP TUB1-mCherry. G1-arrested cells were released into galactose medium at 30°. α-factor was restored after budding. Cells were collected at the indicated time points for the examination of fluorescence signals. (C) The budding index . (D) The percentage of anaphase cells that show cosegregated CEN4-GFP. (E) The distribution of CEN4-GFP and spindle morphology in some representative cells. Bar, 5 µm.
To investigate whether the viability loss of these sgo1 mutants resulted from chromosome missegregation, a vector and PGALCIK1-CC plasmid were introduced into SGO1, sgo1Δ, sgo1-N51I, and sgo1-4A cells with CEN4-GFP and Tub1-mCherry. G1-arrested cells were released into galactose medium. CIK1-CC overexpression caused a cell cycle delay in cells with WT SGO1, but failed to do so in sgo1Δ, sgo1-4A, and sgo1-N51I mutant cells (Figure 6C). After G1 release for 100 min, 36% of sgo1-N51I and 35% of sgo1-4A cells with an anaphase spindle showed CEN4-GFP cosegregation, which is similar to sgo1Δ cells (39%). The same is true for cells at 120 and 140 min. In clear contrast, no CEN4-GFP cosegregation was observed in cells with WT SGO1 during CIK1-CC overexpression (Figure 6, D and E). These results suggest that abrogation of the interaction of Sgo1 with H2A or PP2A completely abolishes Sgo1’s function in preventing premature SAC silencing.
Discussion and Conclusion
Using budding yeast as a model organism, recent studies indicate that centromere-associated Sgo1 protein is required to prevent SAC silencing until tension is generated at kinetochores by bipolar attachment (Keating et al. 2009; Jin and Wang 2013; Wang et al. 2014). Here we show that components acting up- and downstream of Sgo1 are also required to prevent premature SAC silencing. We first found that the kinase domain of Bub1 is essential to maintain SAC activation in cells lacking tension. Yeast Bub1 phosphorylates H2A at S121 to trigger the recruitment of Sgo1 to centromeres (Kawashima et al. 2010). We further demonstrated that mutation of the Bub1 phosphorylation site in H2A (h2a-S121A) or the H2A binding site in SGO1 (sgo1-4A) resulted in SAC silencing in the presence of tensionless syntelic attachment. Therefore, Bub1-dependent centromere binding of Sgo1 is essential to prevent premature SAC silencing. In addition, we found that abolishment of Sgo1-PP2A binding in sgo1-N51I mutants as well as the deletion of RTS1, which encodes one of the PP2A regulatory subunits, also leads to premature SAC silencing. These results support the conclusion that Bub1-dependent centromere recruitment of Sgo1-PP2ARts1 through H2A prevents premature SAC silencing. Therefore, our results uncover the role of the Bub1-H2A-Sgo1-PP2ARts1 axis in the maintenance of a prolonged checkpoint arrest in response to tensionless chromosome attachment.
sgo1Δ cells display more significant viability loss than bub1-ΔK after the induction of syntelic attachment by CIK1-CC. However, mutation of the Bub1 phosphorylation site in H2A (h2a-S121A) causes comparable viability loss as sgo1∆ after CIK1-CC overexpression. One possibility is that the h2a-S121A mutation, but not the deletion of the kinase domain of BUB1, abolishes Sgo1 centromere localization completely. Although we are unable to analyze chromosome missegregation in h2A-S121A strains due to multiple selection markers, we expect a high rate of missegregation. In addition, the sgo1-4A mutants with abolished Sgo1-H2A interaction exhibited a similar frequency of chromosome missegregation as sgo1Δ cells after CIK1-CC overexpression. These observations support the conclusion that the association of Sgo1 with H2A is essential for its function in preventing SAC silencing.
An important question is how Sgo1 regulates SAC silencing. Previous studies indicate that Sgo1 recruits Ipl1/Aurora B kinase complex to centromeres (Yamagishi et al. 2010; Peplowska et al. 2014). Because Ipl1 kinase prevents SAC silencing in response to tension defects (Biggins and Murray 2001; Jin et al. 2012; Jin and Wang 2013), one speculation is that Sgo1 prevents SAC silencing through Ipl1 kinase. However, the observation that the tension sensing by Ipl1 is independent of the centromere localization of Ipl1 complex argues against this possibility (Campbell and Desai 2013). Consistently, overexpression of the Ipl1 cofactor SLI15 restores centromere Ipl1 localization in sgo1∆ mutants, but this fails to suppress sgo1∆’s sensitivity to CIK1-CC overexpression (Peplowska et al. 2014). Here, we further showed that sgo1Δ mutation alleviated the anaphase entry delay in the phospho-mimetic mutant dam1-3D. Because Dam1 is a well-defined substrate of Ipl1 kinase (Cheeseman et al. 2002), Sgo1 likely acts downstream of Ipl1.
In addition to Ipl1, Sgo1 also recruits PP2A and condensin to centromeres (Nerusheva et al. 2014; Peplowska et al. 2014), and the presence of PP2ARts1 at centromeres prevents cohesin cleavage by separase (Riedel et al. 2006). It is unlikely that centromere cohesion prevents SAC silencing, because cohesin mutants show delayed SAC silencing (Biggins and Murray 2001; Jin and Wang 2013). Since some condensin mutants are sensitive to CIK1-CC overexpression (Peplowska et al. 2014), another untested possibility is that Sgo1 regulates SAC silencing through condensin; but recent work supports the possibility that the condensin facilitates chromosome biorientation (Verzijlbergen et al. 2014). Our results support the notion that Sgo1 recruits PP2A to centromeres to prevent premature SAC silencing. First, mutation of the PP2A-binding motif in Sgo1 causes premature SAC silencing. Moreover, the absence of the PP2A regulatory subunit Rts1 also leads to premature SAC silencing. It is likely that PP2ARts1 dephosphorylates kinetochore proteins to prevent SAC silencing, and it will be our future interest to identify these PP2A substrates.
sgo1-N51I mutants show abolished interaction with PP2A (Xu et al. 2009). Of interest, we noticed that sgo1-N51I mutants exhibited more pronounced viability loss and chromosome missegregation than rts1∆ mutants after CIK1-CC overexpression. Because PP2A has two regulatory subunits, Rts1 and Cdc55, our explanation is that PP2ACdc55 and PP2ARts1 may show redundant function, which is supported by the demonstrated interaction of Sgo1 with both Rts1 and Cdc55 (Verzijlbergen et al. 2014). The sgo1-N51I mutation may abolish its interaction with both of them, thereby showing more pronounced phenotypes. Indeed, we found that the cdc55 mutant also exhibits chromosome missegregation after CIK1-CC overexpression (Bokros et al. 2016), but further experiments are needed to test if this phenotype in cdc55 mutant is independent of its role in mitotic exit (Wang and Ng 2006).
Recent data in mammalian cells indicate the spatial regulation of Sgo1 during mitosis. Cohesin and phosphorylated H2A specify two distinct pools of Sgo1-PP2A at inner centromere and kinetochores, respectively. The tension at kinetochores triggers the redistribution of Sgo1 from inner centromeres to kinetochores (Liu et al. 2013). It is unclear if yeast cells have a spatial Sgo1 regulation during mitosis. Previous data indicate that Chl4 and Iml3, two inner kinetochore proteins, are essential for the association of Sgo1 with the pericentric region in meiosis I (Kiburz et al. 2005), and a recent study shows the interaction between Sgo1 and these kinetochore proteins (Hinshaw and Harrison 2013). Thus, yeast Sgo1 could bind to both H2A and kinetochore proteins, but further experiments are needed to test this possibility.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.195727/-/DC1.
Acknowledgments
We thank Yoshinori Watanabe for providing h2a-S121A yeast strains. We thank Brian Washburn, Kristina Poduch, and Cheryl Pye from the Molecular Cloning Laboratory at Florida State University for helping us construct plasmids. We thank the yeast community at Florida State University for comments and suggestions. This work was supported by R01 GM-102115 from National Institutes of Health/ National Institute of General Medical Sciences to Y.W.
Footnotes
Communicating editor: S. Biggins
Literature Cited
- Akiyoshi B., Nelson C. R., Ranish J. A., Biggins S., 2009. Quan-titative proteomic analysis of purified yeast kinetochores identifies a PP1 regulatory subunit. Genes Dev. 23: 2887–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudhan P., Goldfarb A. A., Joglekar A. P., 2015. The kinetochore encodes a mechanical switch to disrupt spindle assembly checkpoint signalling. Nat. Cell Biol. 17: 868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiles K., Anastasia S., McCusker D., Kellogg D. R., 2009. The Rts1 regulatory subunit of protein phosphatase 2A is required for control of G1 cyclin transcription and nutrient modulation of cell size. PLoS Genet. 5: e1000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. G., Manning B. D., Snyder M., 2000. The Kar3p kinesin-related protein forms a novel heterodimeric structure with its associated protein Cik1p. Mol. Biol. Cell 11: 2373–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S., Murray A. W., 2001. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15: 3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokros M., Wang Y., 2016. Spindle assembly checkpoint silencing and beyond. Cell Cycle 15: 1661–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokros M., Gravenmier C., Jin F., Richmond D., Wang Y., 2016. Fin1-PP1 helps clear spindle assembly checkpoint protein Bub1 from kinetochores in anaphase. Cell Rep. 14: 1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. S., Desai A., 2013. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature 497: 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., Anderson S., Jwa M., Green E. M., Kang J., et al. , 2002. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111: 163–172. [DOI] [PubMed] [Google Scholar]
- Eshleman H. D., Morgan D. O., 2014. Sgo1 recruits PP2A to chromosomes to ensure sister chromatid bi-orientation during mitosis. J. Cell Sci. 127: 4974–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernius J., Hardwick K. G., 2007. Bub1 kinase targets Sgo1 to ensure efficient chromosome biorientation in budding yeast mitosis. PLoS Genet. 3: e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K. G., Murray A. W., 1995. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J. Cell Biol. 131: 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw S. M., Harrison S. C., 2013. An Iml3-Chl4 heterodimer links the core centromere to factors required for accurate chromosome segregation. Cell Rep. 5: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma Y., Sacristan C., Pachis S. T., Adamopoulos A., Kuijt T., et al. , 2015. CELL DIVISION CYCLE. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 348: 1264–1267. [DOI] [PubMed] [Google Scholar]
- Indjeian V. B., Stern B. M., Murray A. W., 2005. The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307: 130–133. [DOI] [PubMed] [Google Scholar]
- Janke C., Ortiz J., Tanaka T. U., Lechner J., Schiebel E., 2002. Four new subunits of the Dam1-Duo1 complex reveal novel functions in sister kinetochore biorientation. EMBO J. 21: 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z., Gao H., Yu H., 2015. CELL DIVISION CYCLE. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 348: 1260–1264. [DOI] [PubMed] [Google Scholar]
- Jin F., Wang Y., 2013. The signaling network that silences the spindle assembly checkpoint upon the establishment of chromosome bipolar attachment. Proc. Natl. Acad. Sci. USA 110: 21036–21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F., Liu H., Li P., Yu H. G., Wang Y., 2012. Loss of function of the cik1/kar3 motor complex results in chromosomes with syntelic attachment that are sensed by the tension checkpoint. PLoS Genet. 8: e1002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S. A., Yamagishi Y., Honda T., Ishiguro K., Watanabe Y., 2010. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327: 172–177. [DOI] [PubMed] [Google Scholar]
- Keating P., Rachidi N., Tanaka T. U., Stark M. J., 2009. Ipl1-dependent phosphorylation of Dam1 is reduced by tension applied on kinetochores. J. Cell Sci. 122: 4375–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiburz B. M., Reynolds D. B., Megee P. C., Marston A. L., Lee B. H., et al. , 2005. The core centromere and Sgo1 establish a 50-kb cohesin-protected domain around centromeres during meiosis I. Genes Dev. 19: 3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T. S., Kawashima S. A., Watanabe Y., 2004. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427: 510–517. [DOI] [PubMed] [Google Scholar]
- Li Y., Bachant J., Alcasabas A. A., Wang Y., Qin J., et al. , 2002. The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev. 16: 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Jia L., Yu H., 2013. Phospho-H2A and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr. Biol. 23: 1927–1933. [DOI] [PubMed] [Google Scholar]
- London N., Biggins S., 2014. Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint. Genes Dev. 28: 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N., Ceto S., Ranish J. A., Biggins S., 2012. Phos-phoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol. 22: 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., III, Demarini D. J., Shah N. G., Wach A., et al. , 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Luo X., Fang G., Coldiron M., Lin Y., Yu H., et al. , 2000. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nat. Struct. Biol. 7: 224–229. [DOI] [PubMed] [Google Scholar]
- Mirchenko L., Uhlmann F., 2010. Sli15(INCENP) dephosphorylation prevents mitotic checkpoint reengagement due to loss of tension at anaphase onset. Curr. Biol. 20: 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerusheva O. O., Galander S., Fernius J., Kelly D., Marston A. L., 2014. Tension-dependent removal of pericentromeric shugoshin is an indicator of sister chromosome biorientation. Genes Dev. 28: 1291–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peplowska K., Wallek A. U., Storchova Z., 2014. Sgo1 regulates both condensin and ipl1/aurora B to promote chromosome biorientation. PLoS Genet. 10: e1004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky B. A., Kung C., Shokat K. M., Biggins S., 2006. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat. Cell Biol. 8: 78–83. [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Nelson C. R., Biggins S., 2009. Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr. Biol. 19: 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primorac I., Weir J. R., Chiroli E., Gross F., Hoffmann I., et al. , 2013. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. eLife 2: e01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel C. G., Katis V. L., Katou Y., Mori S., Itoh T., et al. , 2006. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441: 53–61. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. S., Cross F. R., Funabiki H., 2011. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr. Biol. 21: 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd L. A., Meadows J. C., Sochaj A. M., Lancaster T. C., Zou J., et al. , 2012. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr. Biol. 22: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., Yang H., Hallberg E., Hallberg R., 1997. Molecular genetic analysis of Rts1p, a B′ regulatory subunit of Saccharomyces cerevisiae protein phosphatase 2A. Mol. Cell. Biol. 17: 3242–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenberg P. T., Burke D. J., 2015. Connecting the microtubule attachment status of each kinetochore to cell cycle arrest through the spindle assembly checkpoint. Chromosoma 124: 463–480. [DOI] [PubMed] [Google Scholar]
- Tien J. F., Umbreit N. T., Gestaut D. R., Franck A. D., Cooper J., et al. , 2010. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J. Cell Biol. 189: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijlbergen K. F., Nerusheva O. O., Kelly D., Kerr A., Clift D., et al. , 2014. Shugoshin biases chromosomes for biorientation through condensin recruitment to the pericentromere. eLife 3: e01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ng T. Y., 2006. Phosphatase 2A negatively regulates mitotic exit in Saccharomyces cerevisiae. Mol. Biol. Cell 17: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jin F., Higgins R., McKnight K., 2014. The current view for the silencing of the spindle assembly checkpoint. Cell Cycle 13: 1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Cetin B., Anger M., Cho U. S., Helmhart W., et al. , 2009. Structure and function of the PP2A-shugoshin interaction. Mol. Cell 35: 426–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y., Honda T., Tanno Y., Watanabe Y., 2010. Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330: 239–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.