Abstract
Background
We reviewed studies that analyzed cysticercosis (CC), neurocysticercosis (NCC) and epilepsy across Latin America, Asia and Sub-Saharan Africa, to estimate the odds ratio and etiologic fraction of epilepsy due to CC in tropical regions.
Methodology
We conducted a systematic review of the literature on cysticercosis and epilepsy in the tropics, collecting data from case-control and cross-sectional studies. Exposure criteria for CC included one or more of the following: serum ELISA or EITB positivity, presence of subcutaneous cysts (both not verified and unverified by histology), histology consistent with calcified cysts, and brain CT scan consistent with NCC. A common odds-ratio was then estimated using meta-analysis.
Principal findings
37 studies from 23 countries were included (n = 24,646 subjects, 14,934 with epilepsy and 9,712 without epilepsy). Of these, 29 were case-control (14 matched). The association between CC and epilepsy was significant in 19 scientific articles. Odds ratios ranged from 0.2 to 25.4 (a posteriori power 4.5–100%) and the common odds ratio was 2.7 (95% CI 2.1–3.6, p <0.001). Three subgroup analyses performed gave odds ratios as: 2.2 (EITB-based studies), 3.2 (CT-based studies), 1.9 (neurologist-confirmed epilepsy; door-to-door survey and at least one matched control per case). Etiologic fraction was estimated to be 63% in the exposed group among the population.
Significance
Despite differences in findings, this meta-analysis suggests that cysticercosis is a significant contributor to late-onset epilepsy in tropical regions around the world, and its impact may vary depending on transmission intensity.
Author summary
Cysticercosis is a helminthic infection of the central nervous system (CNS) and the leading cause of late onset epilepsy in low-and middle-income countries. This neurological disease is a public health problem in Sub-Saharan Africa, Asia and Latin America, affecting impoverished rural and peri-urban populations where sanitation is inadequate. Diagnostic criteria for NCC vary according to regional availability and access to imaging and serological tests. This systematic review highlights the lack of appropriate methodology in most observational studies, with few studies including control groups, a basic epidemiological criteria needed to demonstrate an association. EITB for cysticercosis was widely used to measure exposure, and brain CT scan was a non-invasive alternative used to identify cysts. Neurocysticercosis (NCC) is a preventable neurological condition in the tropics despite resource limitations in LMIC regions. Well-designed studies are needed to provide quality evidence to support control interventions and surveillance systems for this important zoonotic disease.
Introduction
Cysticercosis (CC) is a parasitic infection caused by the larva stage (cysticercus) of the tapeworm Taenia solium. It has been a major public health problem since historical times [1], and remains so, particularly in the developing world (low-and middle-income countries; LMIC), due to inadequate hygiene, rudimentary pig management and slaughter, and poor waste water management [2]. Developed regions such as Europe and North America are considered to be virtually free of endemic transmission, although there remains a substantial disease burden in these regions due to migration [3]. Neurocysticercosis (NCC) is considered a common helminthic infection of the central nervous system (CNS) across Latin America, Sub-Saharan Africa and Asia [4–7], and a common cause of late-onset epilepsy in LMIC [4,5,8]. For instance, a study in Burundi showed a strong link between CC and epilepsy, with an etiologic fraction of 50% (95% CI: 42–57) and an odds ratio of 3.8 (95% CI: 2.5 to 5.1) [6]. It was estimated in a recent meta-analysis that people infected with CC in Sub-Saharan Africa (SSA) are at 3.4–3.8 fold greater risk of having epilepsy [7]. It is noted that despite the importance of these diseases at an individual and population level, there are still discrepancies in the literature about their precise impact [4]. Moreover, earlier reviews focused on specific regions alone [7]. We conducted a review of studies that analyzed CC, NCC, and epilepsy across Latin America, Asia and Africa, to estimate the probability and etiologic fraction of epilepsy due to CC in tropical regions.
Methods
Literature search
Systematic searches were conducted for articles in English and French using the following databases: Medline, Scopus, Science Direct, Ingenta Connect, Refdoc (formerly Article Science). We also searched for articles and theses in the bibliographic database of the Institut d’Epidemiologie et de Neurologie Tropicale http://www.unilim.fr/ient/. Keywords used were (cysticercosis OR Taenia solium OR neurocysticercosis) AND epilepsy. Logical operators (AND, OR, NOT) were used. Bibliographies of published reviews and meta-analyses were also searched.
Data extraction
Two reviewers (GD and PMP) extracted data using methodology applied in previous meta-analyses [7] that focused on sub-Saharan Africa. Data types collected included; General: authors, year of publication, country, and study design used. Epilepsy: case sources, definition used, how and who confirmed epilepsy, source of people without epilepsy i.e. controls, and matching criteria. CC: methods used to evaluate CC and NCC (serological tests including enzyme-linked immunoelectrotransfer blot-EITB and enzyme-linked immunosorbent assay-ELISA, as well as neuroimaging) Methods: sample size for the following four groups: people with epilepsy affected by cysticercosis (PWE + CC), people with epilepsy not affected by cysticercosis (PWE—CC), people without epilepsy affected by cysticercosis (PWOE + CC), people without epilepsy unaffected by cysticercosis (PWOE—CC).
Eligible studies included those that 1) had epilepsy as a disease of interest and cysticercosis as exposure, 2) estimated sample size using appropriate techniques, 3) included detailed methods for diagnosis and determination of exposure, and 4) included a control group in the analysis. Case-reports, notes, letters to the editor, scientific reviews and other meta-analyses were excluded at this stage. For manuscripts in which authors presented results for multiple methods, we followed an order of priority. For instance, we considered computed tomography (CT) results more relevant than EITB or ELISA assays, and we retained EITB in preference to ELISA.
Subgroup analyses
We conducted three subgroup analyses by taking into account those studies that used specific diagnostic tools for CC or NCC and epilepsy. The first group comprised studies that used EITB to determine CC exposure. The second analysis included studies that used brain CT scan to assess NCC exposure. The third analysis involved studies that had used standardized diagnostic methods to confirm epilepsy in population-based studies, such as neurological surveys applied in a door-to-door fashion with evaluation by well-trained general practitioners and /or neurological evaluation to confirm cases, and including at least one matched control per case. Finally, we performed an analysis by continent.
Statistical analysis
For each of the selected studies, the odds ratio (OR) and its 95% confidence interval was determined using Epi-Info 6 (Centers for Disease Control and Prevention, Atlanta, USA). A meta-analysis was used to estimate the risk of developing epilepsy when exposed to CC, applying a random-effects model using Stata software, version 10.1 (Stata-Corp, College Station, TX, USA) to account for the variance of each included study [9]. Odds ratios (OR) and 95% confidence intervals (95% CIs) were determined. Homogeneity was assessed by I squared tests. Subgroup analyses were also conducted for studies ascertaining CC by EITB assays, CT scan and those studies that followed certain requirements for determining epilepsy (as mentioned under Methods, above). Because epilepsy has multiple causes and associated factors, we calculated the etiologic fraction (EF) i.e. the proportion of cases “attributable” to cysticercosis, by comparing the prevalence among exposed and the unexposed. The EF provided an unadjusted estimate of the proportion of cases of epilepsy that could be prevented if exposure to CC were eliminated. The etiologic fraction was based on the pooled estimate of risk, rather than single risk estimates for individual studies, by using the following formula: proportion exposed (common OR-1)/proportion exposed (common OR-1) +1.
Results
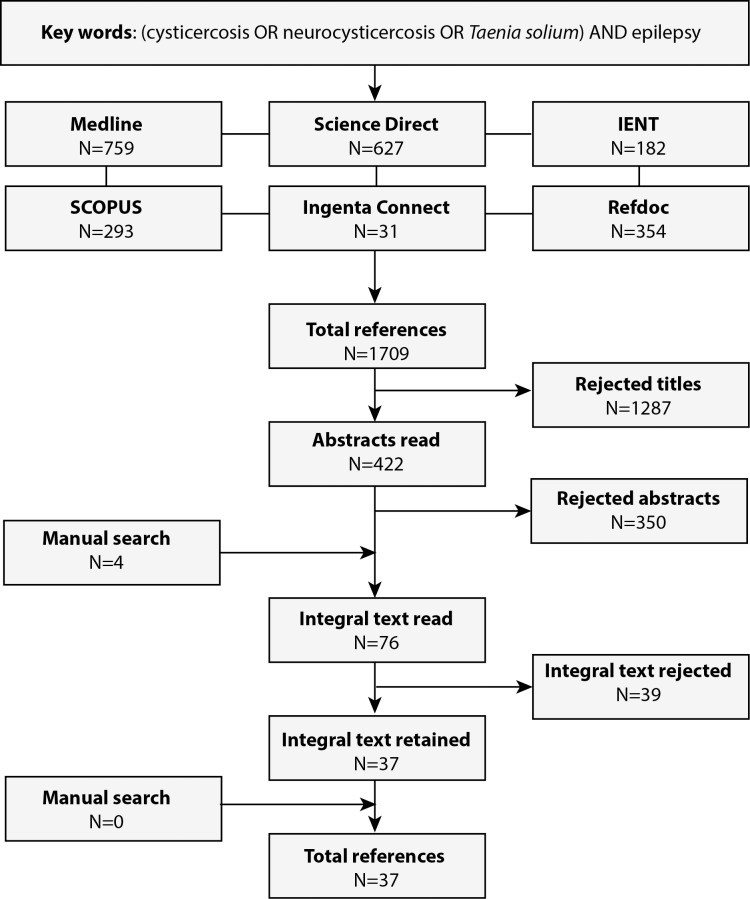
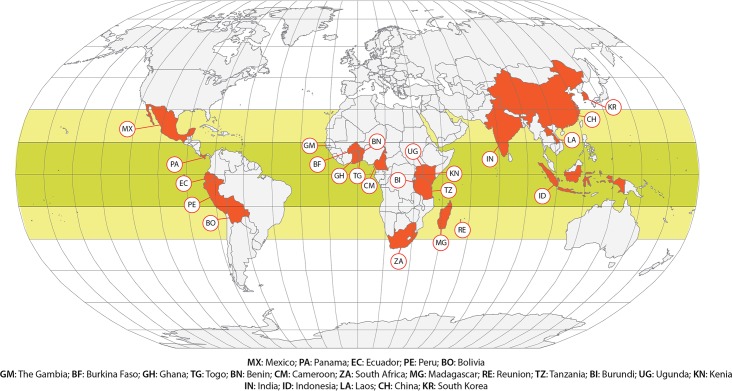
In total, our searches identified 1709 publications. Of these, 1287 articles were excluded at the title level and 350 at the abstract level because they did not meet the inclusion criteria. Seventy-six articles were read in entirety; 37 were found to meet inclusion criteria and were included in the analysis (see Fig 1). These 37 studies (see Fig 2) were conducted in 23 different countries (five countries each from Asia and Latin America, and 13 from Africa). In total, these 37 studies covered 24,646 subjects (14,934 PWOE and 9,712 PWE). Seventy eight percent (29/37) were case-control studies, of which 14/29 (48.27%) were matched studies, (see Table 1).
Fig 1. Flowchart of literature search.
Fig 2. Locations of studies that evaluated association of cysticercosis and epilepsy.
Table 1. Description of the methods used in studies seeking an association between cysticercosis and epilepsy, classified by year of publication.
| People with epilepsy | People without epilepsy | Exposure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors (year) | Country | Continent | Sub-continent | Design | Sources | Definition | Conf | Sources | criteria | Examination | Criteria/CC | Criteria/NCC |
| Chopra, 1981 [22] | India | Asia | Southern Asia | CCS | Hospital | NS | NS | GenPop | None | Cranial X-Ray, HAT | (+) HAT | (+)Cranial X-ray |
| Maldonado, 1986 [11] | La Réunion | Africa | Eastern Africa | CCS | Hospital | NS | NS | Hospital | None | X-Ray st | Calcified Lesion | None |
| Mignard, 1986 [23] | La Réunion | Africa | Eastern Africa | CCS | Hospital | NS | CK | Hospital | None | ELISA, CT | (+) ELISA | (+) CT |
| Dumas, 1989 [24] | Togo | Africa | Western Africa | CSS | GenPop | NS | Neurol | GenPop | House a | Bx, Cranial X-ray, ELISA | (+) ELISA/His | (+) Cranial X-ray |
| Gracia, 1990 [25] | Panama | America | Central America | CCS | GenPop | ILAE 1970 | Neurol | GenPop | sex, age >5 | WB | (+)WB | None |
| Dansey, 1992 [26] | South Africa | Africa | South Africa | CCS | Miners | NS | CE | Miners | None | CT | None | (+) CT |
| Nzisabira, 1992 [27] | Burundi | Africa | Eastern Africa | CCS | GenPop | NS | Neurol | GenPop | None | ELISA (CSF), CT,Bx | (+)ELISA | (+)ELISA/(+) CT |
| Sarti, 1992 [28] | Mexico | America | North America | CSS | GenPop | NS | NS | GenPop | None | EITB | (+)EITB | None |
| García, 1993 [29] | Peru | America | South America | CCS | Hospital | NS | Neurol | Hospital | None | EITB | (+)EITB | None |
| Kong, 1993 [30] | Korea | Asia | Eastern Asia | CCS | GenPop* | NS | NS | GenPop | None | ELISA | (+)ELISA | None |
| Bouteille, 1994 [31] | Benin | Africa | Western Africa | CSS | GenPop a | ILAE 1993 | Neurol | GenPop | None | Bx, ELISA | (+)ELISA/His | None |
| Theis, 1994 [32] | Indonesia | Asia | South-Eastern Asia | CCS | Hospital | NS | NS | GenPop | None | ELISA, EITB | (+) Elisa/EITB | None |
| Aranda-Alvarez, 1995 [33] | Mexico | America | North America | CSS | GenPop b | NS | NS | GenPop b | None | ELISA-Ag | (+)ELISA-Ag | None |
| Grill, 1996 [34] | Madagascar | Africa | Eastern Africa | CCS | Hospital a | NS | Neurol | Hospital | None | CT, ELISA /EITB (CSF) | (+) ELISA/EITB | (+) ELISA /EITB /CT |
| Andriantsimahavandy, 1997 [8] | Madagascar | Africa | Eastern Africa | CCS | Hospital | OMS 1981 | CK | Hospital | Province, sex, age>10 | EITB (CSF/ser) | (+)EITB | (+)EITB (CSF) |
| Handali, 1997 [35] | Indonesia | Asia | South-Eastern Asia | CSS | GenPop | NS | NS | GenPop | None | Bx | Cyst presence | None |
| Newell, 1997 [12] | Burundi | Africa | Eastern Africa | CCS | MR | NE | MD | Family | Household | ELISA-Ag, EITB | (+)ELISA-Ag/EITB | None |
| Correa, 1999 [36] | Mexico | America | North America | CCS | GenPop | NS | NS | GenPop | None | ELISA-Ag, EITB | (+)ELISA-Ag/EITB | None |
| Cruz, 1999 [37] | Ecuador | America | South America | CCS | GenPop | ILAE 1993 | Neurol | GenPop | None | EITB, CT | (+)EITB | (+) CT |
| Balogou, 2000 [38] | Togo | Africa | Western Africa | CSS | GenPop | ILAE 1993 | Neurol | GenPop | None | Bx, Cranial X-ray, ELISA | (+) ELISA/His | (+) Cranial X-ray |
| Mittal, 2001 [39] | India | Asia | Southern Asia | CCS | Hospital | NS | NS | NE | None | ELISA | (+)ELISA | None |
| Nicoletti, 2002 [13] | Bolivia | America | South America | CCS | GenPop | ILAE 1993 | Neurol | GenPop | Villa, sex, age >5 | EITB | (+)EITB | None |
| Macharia, 2002 [40] | Kenya | Africa | Eastern Africa | CCS | Hospital | ILAE 1993 | CK | Hospital | Province,age,sex | ELISA | (+)ELISA | None |
| Rakatobe, 2002 [41] | Madagascar | Africa | Eastern Africa | CCS | GenPop | ILAE 1989 | Neurol | GenPop | Family | ELISA, WB | (+) WB | None |
| Nsengiyumva, 2003 [6] | Burundi | Africa | Eastern Africa | CCS | Hospital | ILAE 1993 | Neurol | Hospital | Province,age | ELISA | (+)ELISA | None |
| Dongmo, 2004 [42] | Cameroun | Africa | Middle Africa | CCS | GenPop a | ILAE 1993 | Neurol | GenPop | Age > 5 | ELISA | (+)ELISA | None |
| Del Brutto, 2005 [43] | Ecuador | America | South America | CCS | GenPop | ILAE 1989 | Neurol | GenPop | Sex, age > 5 | WB, CT | (+)WB | (+) CT |
| Montano, 2005 [44] | Peru | America | South America | CSS | GenPop | ILAE 1989 | Neurol | GenPop | None | EITB, CT | (+)EITB | (+) CT |
| Li, 2006 [45] | China | Asia | Eastern Asia | CSS | GenPop | NS | MD | GenPop | None | ELISA | (+)ELISA | None |
| Tran, 2007 [46] | Laos | Asia | South-Eastern Asia | CCS | GenPop | ILAE 1993 | Neurol | GenPop | Villa, sex, age > 5 | ELISA, WB | (+) ELISA/WB | None |
| Prasad, 2008 [47] | India | Asia | Southern Asia | CCS | GenPop | ILAE 1993 | NS | Family | Family | EITB, MRI | (+)EITB | (+) MRI |
| Winkler, 2009 [10] | Tanzanie | Africa | Eastern Africa | CCS | Hospital b | Winkler 2007 | Neurol | Hospital | None | ELISA (CSF,Ser), CT | (+)ELISA | (+)ELISA(CSF)/ CT |
| Secka, 2010 [48] | Gambia | Africa | Western Africa | CCS | Hospital, MR | ILAE 1989 | NS | GenPop | Villa, sex, age > 5 | ELISA-Ag, EITB, CT | (+)ELISA-Ag/EITB | (+) CT |
| Nitiéma, 2012 [49] | Burkina Faso | Africa | Western Africa | CCS | GenPop c | ILAE 2006 | MD | GenPop c | None | ELISA-Ag | (+)ELISA-Ag | None |
| Singh, 2012 [50] | India | Asia | Southern Asia | CCS | GenPop a | ILAE 1989 | Neurol | GenPop a | Sex, age > 2 | EITB | (+)EITB | None |
| Elliott, 2013 [51] | Cameroun | Africa | Middle Africa | CCS | MR | ILAE 1989 | MD | GenPop | Sex, age | EITB | (+)EITB | None |
| Ngugi, 2013 [52] | Kenya * | Africa | Eastern Africa | CCS | GenPop d | ILAE 1989 | MD | GenPop d | Age | WB | (+)WB | None |
Source, NS: non-specific, GenPop: general population, GP*: population from Charity centers, GenPop a: General population older than 5 years old, GenPop b: General Population older than 14 years old. GenPop c: General Population older than 7 years old, GenPop d: General Population followed by centers for surveillance of health and demographic, Hospital a: Hospital Population older than 1 year old, Hospital b: Hospital Population older than 5 years old, MR: Medical Records.
Criteria, House a: House & Neighborhood. Confirmation, CK: cases know from local health centers, CE: clinical evaluation, MD: medical doctor, Neurol: neurologist.
HAT: hemagglutination test, X-Ray st: X-Ray soft Tissue, WB: Western Blot, Bx: Biopsy, His: Histology, Elisa-Ag: Elisa Antigen, Ser: serum, CSF: Cerebro Spinal Fluid
CT: computed tomography of the brain, MRI: Magnetic Resonance of the Brain
Kenia
*: Kenya, Sud-Africa, Uganda, Tanzania, Ghana.
CSS: cross-sectional study, CCS: case-control studies, Conf: confirmation, Criteria: selection criteria
Epilepsy, CC and NCC
Twenty studies defined epilepsy, of which 18/20 (90%) followed at least one definition recommended by the International League against Epilepsy (ILAE, 1981, 1989, 1993, 2006). One study each used definitions proposed by the World Health Organization and that recommended for LMICs [10]. As noted in Table 1, there was great variability in the tools used for assessing exposure to CC, ranging from physical examination of subcutaneous nodules to Computed Tomography of the brain (CT), MRI images, cyst histology, and bioassays in serum or cerebrospinal fluid (CSF).
A total of 21/37 (56.75%) studies determined exposure to CC by detecting antibodies or antigens in serum using ELISA or ELISA-Ag. Seven studies used EITB to confirm or refute the results of ELISA and 12 studies used only EITB to determine CC exposure. One study used a hemagglutination test with sheep red blood cells sensitized to cysticercus antigens to determine exposure to CC. NCC exposure was determined by measuring antibodies in the CSF, but only 5/37 studies (13.51%) did so, by using ELISA (n = 3) or EITB (n = 2). CT was used in 14 studies, including 13 to assess NCC, and one [11] focused on the soft parts of the thigh.
Association between CC and epilepsy
As shown in Table 2, the association between CC and epilepsy was statistically significant in 19 studies, leaving 18 with a non-significant association. The association was in fact nearly significant for two studies [12,13]. Odds ratios ranged from 0.2 to 25.4 and the a posteriori statistical power ranged from 4.5% to 100.0%.
Table 2. Results obtained in studies looking for an association between cysticercosis and epilepsy classified by year of publication.
| PWE | PWOE | PWE CC+ | PWOE CC+ | SP | |||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | n | n | n | % | n | % | (%) | OR (95%IC) | P value |
| Chopra, 1981 [22] | 771 | 98 | 267 | 25.7 | 2 | 2 | 100 | 25.4 (6.2–104.0) | < 0.001 |
| Maldonado, 1986 [11] | 240 | 385 | 40 | 16.7 | 19 | 5 | 99.9 | 3.9 (2.2–6.8) | < 0.001 |
| Mignard, 1986 [23] | 242 | 385 | 45 | 18.6 | 32 | 8.3 | 96.9 | 2.5 (1.6–4.1) | < 0.001 |
| Dumas, 1989 [24] | 88 | 1439 | 27 | 30.7 | 98 | 6.8 | 100 | 6.1 (3.7–10.0) | < 0.001 |
| Gracia, 1990 [25] | 19 | 36 | 1* | 5.3 | 1* | 2.8 | 6.6 | 1.9 (0.1–32.9) | 0.772 |
| Dansey, 1992 [26] | 165 | 138 | 63 | 38.2 | 20 | 14.5 | 99.5 | 3.6 (2.1–6.4) | < 0.001 |
| Nzisabira, 1992 [27] | 98 | 30 | 40 | 40.8 | 1* | 3.3 | 96.9 | 20.0 (2.6–152.9) | < 0.001 |
| Sarti, 1992 [28] | 16 | 1533 | 5 | 31.3 | 162 | 10.6 | 67.8 | 3.9 (1.3–11.2) | 0.245 |
| Kong, 1993 [30] | 189 | 309 | 22 | 11.6 | 8 | 2.6 | 98.6 | 1.9 (1.1–3.3) | 0.018 |
| Garcia, 1993 [29] | 2667 | 750 | 108 | 4.1 | 16 | 2.1 | 66.4 | 5.0 (2.2–11.4) | < 0.001 |
| Bouteille, 1994 [31] | 22 | 1421 | 2 | 9.1 | 55 | 3.9 | 21.2 | 2.5 (0.6–10.9) | 0.49 |
| Theis, 1994 [32] | 74 | 746 | 10 | 13.5 | 94 | 12.6 | 4.5 | 1.1 (0.5–2.2) | 0.967 |
| Aranda-Alvarez, 1995 [33] | 46 | 854 | 3 | 6.5 | 6 | 0.7 | 92.7 | 9.9 (2.4–40.8) | 0.002 |
| Grill, 1996 [34] | 256 | 113 | 153 | 59.8 | 30 | 26.5 | 100 | 4.1 (2.5–6.7) | < 0.001 |
| Andriantsimahavandy, 1997 [8] | 104 | 104 | 33 | 31.7 | 14 | 13.5 | 88.6 | 3.0 (1.5–6.0) | 0.003 |
| Handali, 1997 [35] | 241 | 260 | 163 | 67.6 | 215 | 82.7 | 99.2 | 0.4 (0.3–0.7) | < 0.001 |
| Newell, 1997 [12] | 103 | 72 | 12 | 11.7 | 2 | 2.8 | 56.8 | 4.6 (1.0–21.3) | 0.065 |
| Cruz, 1999 [37] | 26 | 118 | 14 | 53.8 | 17 | 14.4 | 99.4 | 7.0 (2.7–17.5) | < 0.001 |
| Correa, 1999 [36] | 68 | 133 | 15 | 22.1 | 17 | 12.8 | 38 | 1.9 (0.9–4.2) | 0.134 |
| Balogou, 2000 [38] | 115 | 1343 | 12 | 10.4 | 37 | 2.8 | 99.1 | 4.1 (2.1–8.1) | < 0.001 |
| Mittal, 2001 [39] | 1881 | 50 | 196 | 10.4 | 1* | 2 | 96.1 | 5.7 (0.8–41.5) | 0.088 |
| Nicoletti, 2002 [13] | 113 | 233 | 22 | 19.5 | 27 | 11.6 | 47 | 1.8 (1.0–3.4) | 0.071 |
| Macharia, 2002 [40] | 99 | 124 | 5 | 5.1 | 3 | 2.4 | 19.1 | 2.2 (0.5–9.2) | 0.492 |
| Rakatobe, 2002 [41] | 99 | 107 | 2 | 2 | 13 | 12.1 | 72.4 | 0.2 (0.1–0.7) | 0.011 |
| Nsengiyumva, 2003 [6] | 324 | 648 | 193 | 59.6 | 204 | 31.5 | 100 | 3.2 (2.4–4.2) | < 0.001 |
| Dongmo, 2004 [42] | 93 | 81 | 17 | 18.3 | 12 | 14.8 | 9.3 | 1.3 (0.6–2.9) | 0.683 |
| Del Brutto, 2005 [43] | 19 | 19 | 5 | 26.3 | 1 | 5.3 | 42.8 | 6.4 (0.7–61.5) | 0.182 |
| Montano, 2005 [44] | 39 | 111 | 15 | 38.5 | 26 | 23.4 | 41.8 | 2.0 (0.9–4.5) | 0.109 |
| Li, 2006 [45] | 55 | 445 | 9 | 16.4 | 11 | 2.5 | 99.9 | 7.7 (3.0–19.6) | < 0.001 |
| Tran, 2007 [46] | 31 | 124 | 1* | 3.2 | 6 | 4.8 | 5.2 | 0.7 (0.1–5.7) | 0.923 |
| Prasad, 2008 [47] | 60 | 107 | 29 | 48.3 | 31 | 28.9 | 70.9 | 2.3 (1.2–4.4) | 0.02 |
| Winkler, 2009 [10] | 212 | 198 | 38 | 17.9 | 10 | 5.1 | 98.2 | 4.1 (2.0–8.5) | < 0.001 |
| Secka, 2010 [48] | 210 | 420 | 1* | 0.5 | 1* | 0.2 | 6.6 | 2.0 (0.1–32.2) | 0.802 |
| Nitiéma, 2012 [49] | 39 | 814 | 5 | 12.9 | 28 | 3.4 | 67.1 | 4.1 (1.5–11.4) | 0.022 |
| Singh, 2012 [50] | 106 | 106 | 27 | 25.5 | 13 | 12 | 77.7 | 2.4 (1.2–5.1) | 0.011 |
| Elliott, 2013 [51] | 249 | 245 | 11 | 4.4 | 13 | 53 | 7.6 | 0.8 (0.4–1.9) | 0.803 |
| Ngugi, 2013 [52] | 533 | 835 | 15 | 28.1 | 18 | 21.5 | 52.7 | 1.3 (0.7–2.6) | 0.533 |
*Result = 0 in the study, modified to calculate the odds ratio, otherwise OR independent, SP: a posteriori statistical power
OR: Odds ratio, PWE + CC: people with epilepsy affected by cysticercosis, PWE—CC: people with epilepsy not affected by cysticercosis, PWOE + CC: people without epilepsy affected by cysticercosis, PWOE–CC: people without epilepsy unaffected by cysticercosis
Meta-analysis and subgroup analyses
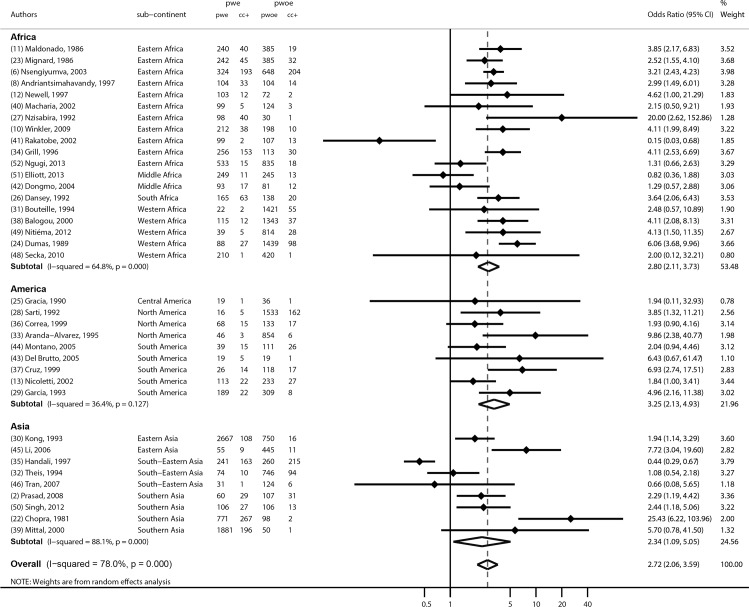
A meta-analysis of 37 studies based on the determination of exposure through detection of antibodies by ELISA or EITB, antigen detection by ELISA, or CT findings, is shown in Fig 3. The common odds ratio was estimated to be 2.7 (95% CI 2.1–3.6), p<0.001. Heterogeneity was substantial with a I squared at 78% (p<0.0001).
Fig 3. Meta-analysis assessing the association between CC and epilepsy globally and by continents: OR (odds ratio) of each study and common OR estimated using a random effects model.
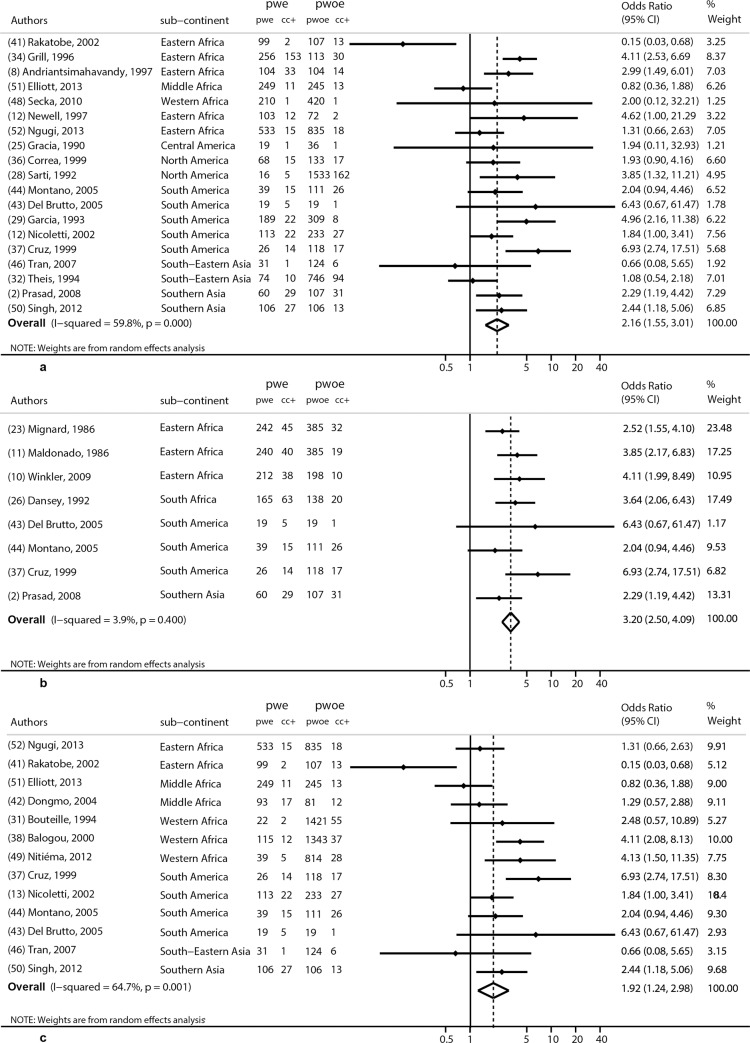
Three subgroup analyses were also performed as detailed in the methods section above. The first was based on studies that used EITB (n = 19), Fig 4A. The common odds ratio obtained was 2.2 (95% CI 1.6–3.0), p<0.001. Another subgroup analysis (Fig 4B) was based on studies that used brain computer tomography (n = 8). This gave a common odds-ratio of 3.2, (95% CI 2.5–4.1, p<0.001). The third subgroup analysis, Fig 4C, was based on the methods used to confirm epilepsy (n = 13), and gave a common odds ratio of 1.9 (95% CI 1.2–3.0), p<0.001. We also performed an analysis by continent, showing that the effect was quite similar around the world (Fig 3).
Fig 4.
Various subgroup meta-analyses (EITB: Fig 4A, CT scan: Fig 4b; best studies: Fig 4c) assessing the association between CC and epilepsy in Latin America, Asia and Africa: OR (odds ratio) of each study and common OR estimated using a random effects model.
Etiologic fraction
The etiologic fraction was estimated to be 63.0% (95% CI: 61.4–64.5) in the exposed group among the population. In other words, 63% of epilepsies were reportedly due to CC.
Discussion
Our work was based on 37 studies conducted in many regions of Latin America, Asia and Africa. Particular efforts were made to identify studies by searching many databases and sources, including those that do not have a large international readership or were not in English. However, published information was available from only 23 countries, suggesting an evident information gap (see Fig 2).
A substantial proportion of these publications (n = 11) reported hospital-based studies, four were performed in health centers or medical clinics and another in a very specific population of mine-workers. There is a need to conduct well-designed interventions with appropriate methodology and to use validated tools to improve data quality, thereby reducing basic epidemiological biases. The lack of a control group, even in analytic cross-sectional studies, made it impossible to probe the association between this CNS helminthic infection and late-onset epilepsy by itself (i.e. ELISA or EITB for cysticercosis tested in PWE vs general population). This is one reason why several wide-scale or hospital studies were not included in this meta-analysis [14,15,16]. This type of study is also vulnerable to selection bias, particularly in rural areas, as epilepsy is stigmatized and may not also be visible (partial, mild seizures) or reported [17]. The remaining 21 studies were population-based that did include a control group.
The association between CC and epilepsy was statistically significant in only 19 studies, (Tables 1 and 2) and nearly significant in two studies. The odds ratios ranged from 0.2–25.4, and the a posteriori statistical power from 4.5% to 100.0% (Table 3). This wide variability could in part be due to non-adjustment of one or several other factors responsible for epilepsy occurrence. Many other factors, such as family predisposition, childbirth problems or head trauma, could lead to epilepsy and almost all studies failed to take into account all of these possible factors. Data elsewhere also support evidence that while in some populations there is a positive association between CC and epilepsy [12], in other studies conducted at a similar point of time these associations are absent [18]. Moreover, several studies with positive association between CC and epilepsy have their fair share of inconsistencies as well. For instance, one study in Burundi used an unmatched case-control study design [12] in which there were fewer control subjects than cases and controls were recruited from the same families as the cases.
Table 3. Results obtained in studies looking for an association between cysticercosis and epilepsy classified by continent and sub-continent.
| PWE | PWOE | PWE CC+ | PWOE CC+ | SP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | continent | sub-continent | n | n | n | % | n | % | (%) | OR (95%IC) |
| Chopra, 1981 [22] | Asia | Southern Asia | 771 | 98 | 267 | 25.7 | 2 | 2 | 100 | 25.4 (6.2–104.0) |
| Maldonado, 1986 [11] | Africa | Eastern Africa | 240 | 385 | 40 | 16.7 | 19 | 5 | 99.9 | 3.9 (2.2–6.8) |
| Mignard, 1986 [23] | Africa | Eastern Africa | 242 | 385 | 45 | 18.6 | 32 | 8.3 | 96.9 | 2.5 (1.6–4.1) |
| Dumas, 1989 [24] | Africa | Western Africa | 88 | 1439 | 27 | 30.7 | 98 | 6.8 | 100 | 6.1 (3.7–10.0) |
| Gracia, 1990 [25] | America | Central America | 19 | 36 | 1 | 5.3 | 1 | 2.8 | 6.6 | 1.9 (0.1–32.9) |
| Dansey, 1992 [26] | Africa | South Africa | 165 | 138 | 63 | 38.2 | 20 | 14.5 | 99.5 | 3.6 (2.1–6.4) |
| Nzisabira, 1992 [27] | Africa | Eastern Africa | 98 | 30 | 40 | 40.8 | 1 | 3.3 | 96.9 | 20.0 (2.6–152.9) |
| Sarti, 1992 [28] | America | North America | 16 | 1533 | 5 | 31.3 | 162 | 10.6 | 67.8 | 3.9 (1.3–11.2) |
| Garcia, 1993 [29] | America | South America | 2667 | 750 | 108 | 4.1 | 16 | 2.1 | 66.4 | 5.0 (2.2–11.4) |
| Kong, 1993 [30] | Asia | Eastern Asia | 189 | 309 | 22 | 11.6 | 8 | 2.6 | 98.6 | 1.9 (1.1–3.3) |
| Bouteille, 1994 [31] | Africa | Western Africa | 22 | 1421 | 2 | 9.1 | 55 | 3.9 | 21.2 | 2.5 (0.6–10.9) |
| Theis, 1994 [32] | Asia | South-Eastern Asia | 74 | 746 | 10 | 13.5 | 94 | 12.6 | 4.5 | 1.1 (0.5–2.2) |
| Aranda-Alvarez, 1995 [33] | America | North America | 46 | 854 | 3 | 6.5 | 6 | 0.7 | 92.7 | 9.9 (2.4–40.8) |
| Grill, 1996 [34] | Africa | Eastern Africa | 256 | 113 | 153 | 59.8 | 30 | 26.5 | 100 | 4.1 (2.5–6.7) |
| Andriantsimahavandy, 1997 [8] | Africa | Eastern Africa | 104 | 104 | 33 | 31.7 | 14 | 13.5 | 88.6 | 3.0 (1.5–6.0) |
| Handali, 1997 [35] | Asia | South-Eastern Asia | 241 | 260 | 163 | 67.6 | 215 | 82.7 | 99.2 | 0.4 (0.3–0.7) |
| Newell, 1997 [12] | Africa | Eastern Africa | 103 | 72 | 12 | 11.7 | 2 | 2.8 | 56.8 | 4.6 (1.0–21.3) |
| Cruz, 1999 [37] | America | South America | 26 | 118 | 14 | 53.8 | 17 | 14.4 | 99.4 | 7.0 (2.7–17.5) |
| Correa, 1999 [36] | America | North America | 68 | 133 | 15 | 22.1 | 17 | 12.8 | 38 | 1.9 (0.9–4.2) |
| Balogou, 2000 [38] | Africa | Western Africa | 115 | 1343 | 12 | 10.4 | 37 | 2.8 | 99.1 | 4.1 (2.1–8.1) |
| Mittal, 2001 [39] | Asia | Southern Asia | 1881 | 50 | 196 | 10.4 | 1 | 2 | 96.1 | 5.7 (0.8–41.5) |
| Nicoletti, 2002 [13] | America | South America | 113 | 233 | 22 | 19.5 | 27 | 11.6 | 47 | 1.8 (1.0–3.4) |
| Macharia, 2002 [40] | Africa | Eastern Africa | 99 | 124 | 5 | 5.1 | 3 | 2.4 | 19.1 | 2.2 (0.5–9.2) |
| Rakatobe, 2002 [41] | Africa | Eastern Africa | 99 | 107 | 2 | 2 | 13 | 12.1 | 72.4 | 0.2 (0.1–0.7) |
| Nsengiyumva, 2003 [6] | Africa | Eastern Africa | 324 | 648 | 193 | 59.6 | 204 | 31.5 | 100 | 3.2 (2.4–4.2) |
| Dongmo, 2004 [42] | Africa | Middle Africa | 93 | 81 | 17 | 18.3 | 12 | 14.8 | 9.3 | 1.3 (0.6–2.9) |
| Del Brutto, 2005 [43] | America | South America | 19 | 19 | 5 | 26.3 | 1 | 5.3 | 42.8 | 6.4 (0.7–61.5) |
| Montano, 2005 [44] | America | South America | 39 | 111 | 15 | 38.5 | 26 | 23.4 | 41.8 | 2.0 (0.9–4.5) |
| Li, 2006 [45] | Asia | Eastern Asia | 55 | 445 | 9 | 16.4 | 11 | 2.5 | 99.9 | 7.7 (3.0–19.6) |
| Tran, 2007 [46] | Asia | South-Eastern Asia | 31 | 124 | 1 | 3.2 | 6 | 4.8 | 5.2 | 0.7 (0.1–5.7) |
| Prasad, 2008 [47] | Asia | Southern Asia | 60 | 107 | 29 | 48.3 | 31 | 28.9 | 70.9 | 2.3 (1.2–4.4) |
| Winkler, 2009 [10] | Africa | Eastern Africa | 212 | 198 | 38 | 17.9 | 10 | 5.1 | 98.2 | 4.1 (2.0–8.5) |
| Secka, 2010 [48] | Africa | Western Africa | 210 | 420 | 1 | 0.5 | 1 | 0.2 | 6.6 | 2.0 (0.1–32.2) |
| Nitiéma, 2012 [49] | Africa | Western Africa | 39 | 814 | 5 | 12.9 | 28 | 3.4 | 67.1 | 4.1 (1.5–11.4) |
| Singh, 2012 [50] | Asia | Southern Asia | 106 | 106 | 27 | 25.5 | 13 | 12 | 77.7 | 2.4 (1.2–5.1) |
| Elliott, 2013 [51] | Africa | Middle Africa | 249 | 245 | 11 | 4.4 | 13 | 53 | 7.6 | 0.8 (0.4–1.9) |
| Ngugi, 2013 [52] | Africa | Eastern Africa | 533 | 835 | 15 | 28.1 | 18 | 21.5 | 52.7 | 1.3 (0.7–2.6) |
*Result = 0 in the study, modified to calculate the odds ratio, otherwise OR independent, SP: a posteriori statistical power
OR: Odds ratio, PWE + CC: people with epilepsy affected by cysticercosis, PWE—CC: people with epilepsy not affected by cysticercosis,
PWOE + CC: people without epilepsy affected by cysticercosis, PWOE–CC: people without epilepsy unaffected by cysticercosis
Overall, the global OR from 37 studies was estimated to be 2.7 with a 95% confidence interval of 2.1 to 3.6. This degree of association conforms to individual studies conducted elsewhere [8]. Another review from SSA yielded an OR of 3.4 [7].
Although we did not conduct any analyse based on the type of epilepsy, the literature suggests, although again not without exceptions, a stronger association of CC with late-onset epilepsy and partial seizures[4]. Another issue that can be raised is the temporality. We cannot be sure if seizures actually predated infection as several of our studies (see above) were cross-sectional surveys. Given the challenges in the availability of reliable patient records in most LMICs and excessive reliance on backward patient reporting about exposures to risk factors, even within case-control studies, it is not always and possible to confidently assess the temporality of this exposure before epilepsy becomes visible. [19].
Two different serological tests to detect antibodies T solium in serum were applied in 27/37 (72.97%) studies. In field conditions, EITB-LLGP (known as western blot or immunoblot) is a useful tool to identify exposure, but does not discriminate between active or inactive lesions. In the clinical setting, a positive EITB-LLGP can support a diagnosis of NCC when there are suggestive images on brain CT scan or MRI. The sensitivity of this test is reported to be 98% with 100% specificity [20]; however, the sensitivity is much lower for NCC with less than 2 parenchymal cysts or for calcified NCC. This is contrast to ELISA, which is specific to viable cyst infection (93.7%) but much less sensitivity in single-lesion[53]. The prevalence of viable NCC cases are low in field conditions (most of them asymptomatic) making this tool unhelpful for epidemiological interventions. Detection of CC would, therefore, depend on the type, accuracy, cost and availability of these tests. Studies that used EITB antibody detection gave a common OR of 1.9, much lower than the global OR obtained by taking into account all 37 studies. Other factors may also reduce the strength of any association between serologically-defined CC and other disease conditions, including a) high background seroprevalence in the general population (usually considered to be 10–25%), and b) many individuals with calcified CC become seronegative over the years [5].
The gold standard tool for determining CC exposure is to demonstrate the parasite in the CNS, by biopsy, although this is not without risk. Modern neuroimaging can provide strong evidence of NCC and should be done for both cases and controls. As shown above, many studies do not include neuroimaging due to cost, radiation exposure, and guidelines. Of the 37 studies reviewed, only eight used CT in both cases and controls; in these, the common OR reached 3.2, a value close to, but higher than, that obtained by considering all studies.
Based on our 37 studies, the etiologic fraction was estimated to be 63% among the exposed group in the population. This indicates an excellent opportunity to prevent a large fraction of late onset epilepsy given that CC can be prevented by controlling transmission of T. solium [21]. This study suggesst that adequate control measures and surveillance of CC in endemic regions should be key issues in preventing late-onset epilepsy in tropical regions.
Perspectives
We propose that future field interventions should meet basic requirements to be more useful:
Adequate design and use of validated surveys in community-based studies
Case-control studies with high levels of exposure to CC
Sufficient statistical power by recruiting adequate numbers of people with epilepsy and controls
Matching of controls by sex, age and location
Computed tomography of the brain without contrast and serological assays (Ag-ELISA and EITB) should be performed for all included subjects.
Use of International League Against Epilepsy guidelines for epidemiological studies to standardized concepts of classification of epilepsy (ILAE 1993).
Include family trees to assess familial history of seizures.
Efforts should be made to assess all other possible risk factors for epilepsy.
Conclusions
Cysticercosis is an active helminthic infection common in tropical regions. Many questions are still unanswered and there are still many limitations in epidemiological base-studies. Based on the current data, NCC is significantly associated with symptomatic epilepsy in low and middle-income countries. However, the strength of this association certainly varies depending on the transmission intensity (rural areas, poor sanitation, lack of potable water, etc). More meta-analyses that are meaningful require good quality studies in tropical regions following certain basic methodological requirements listed above. Finally, epilepsy attributable to CC is preventable. There is a need to focus our efforts on research, control and prevention of CC to avoid increased costly neurological morbidity of this zoonotic disease.
Supporting information
(PDF)
Acknowledgments
We acknowledge copyediting of the final manuscript by Mr. William Francis (France) and Dr. Seth O´Neal (US, Oregon Health & Science University).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
LMM thanks the support received by the scholarship of the Franco Peruvian School of Life Sciences, the faculties and fellow students from Ph.D. program of Life Sciences from Universidad Peruana Cayetano Heredia and University of Limoges (France). LMM received support from FIC/NIH Training Grant TW001140. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Armstrong H (1888) A case of Cysticercus cellulosae of brain in a native coolly. Ind Med Gaz 23: 252. [Google Scholar]
- 2.Prasad KN, Prasad A, Verma A, Singh AK (2008) Human cysticercosis and Indian scenario: a review. J Biosci 33: 571–582. [DOI] [PubMed] [Google Scholar]
- 3.EU (2000) Opinion of the scientific committee on veterinary measures relating to Public Health on the control of taeniosis/cysticercosis in man and animals Belgium: European Commission; 1–31. [Google Scholar]
- 4.Garcia HH, Del Brutto OH (2005) Cysticercosis Working Group in Peru. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol 4: 653–661. 10.1016/S1474-4422(05)70194-0 [DOI] [PubMed] [Google Scholar]
- 5.Garcia HH, Talley A, Gilman RH, Zorrilla L, Pretell J (1999) Epilepsy and neurocysticercosis in a village in Huaraz, Peru. Clin Neurol Neurosurg 101: 225–228. [DOI] [PubMed] [Google Scholar]
- 6.Nsengiyumva G, Druet-Cabanac M, Ramanankandrasana B, Bouteille B, Nsizabira L, et al. (2003) Cysticercosis as a major risk factor for epilepsy in Burundi, east Africa. Epilepsia 44: 950–955. [DOI] [PubMed] [Google Scholar]
- 7.Quet F, Guerchet M, Pion SD, Ngoungou EB, Nicoletti A, et al. (2010) Meta-analysis of the association between cysticercosis and epilepsy in Africa. Epilepsia 51: 830–837. 10.1111/j.1528-1167.2009.02401.x [DOI] [PubMed] [Google Scholar]
- 8.Andriantsimahavandy A, Lesbordes JL, Rasoaharimalala B, Peghini M, Rabarijaona L, et al. (1997) Neurocysticercosis: a major aetiological factor of late-onset epilepsy in Madagascar. Trop Med Int Health 2: 741–746. [DOI] [PubMed] [Google Scholar]
- 9.Cucherat M, Boissel JP, Leizorovicz A (1997) Manuel pratique de méta-analyse des essais thérapeutiques Lyon, France: University of Lyon. [Google Scholar]
- 10.Winkler AS, Blocher J, Auer H, Gotwald T, Matuja W, et al. (2009) Epilepsy and neurocysticercosis in rural Tanzania-An imaging study. Epilepsia 50: 987–993. 10.1111/j.1528-1167.2008.01867.x [DOI] [PubMed] [Google Scholar]
- 11.Maldonado P, Dandelot JB, Wolga J, Ambroise-Thomas P (1986) Prévalence de la cysticercose calcifiée dans l’île de La Réunion—étude corrélative à l’épilepsie chez 625 malades hospitalisés, par la radiographie des parties molles. Médecine et Maladies Infectieuses 16: 393–395. [Google Scholar]
- 12.Newell E, Vyungimana F, Geerts S, Van Kerckhoven I, Tsang VC, et al. (1997) Prevalence of cysticercosis in epileptics and members of their families in Burundi. Trans R Soc Trop Med Hyg 91: 389–391. [DOI] [PubMed] [Google Scholar]
- 13.Nicoletti A, Bartoloni A, Reggio A, Bartalesi F, Roselli M, et al. (2002) Epilepsy, cysticercosis, and toxocariasis: a population-based case-control study in rural Bolivia. Neurology 58: 1256–1261. [DOI] [PubMed] [Google Scholar]
- 14.Goel D, Dhanai JS, Agarwal A, Mehlotra V, Saxena V (2011). Neurocysticercosis and its impact on crude prevalence rate of epilepsy in an Indian community. Neurol India. Jan-Feb;59(1):37–40. 10.4103/0028-3886.76855 [DOI] [PubMed] [Google Scholar]
- 15.Rajshekhar V, Raghava MV, Prabhakaran V, Oommen A, Muliyil J (2006). Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology 67:2135–9. 10.1212/01.wnl.0000249113.11824.64 [DOI] [PubMed] [Google Scholar]
- 16.Prabhakaran V, Raghava MV, Rajshekhar V, Muliyil J, Oommen A (2008). Seroprevalence of Taenia solium antibodies in Vellore district, south India. Trans R Soc Trop Med Hyg. 102:246–50. 10.1016/j.trstmh.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 17.Jacoby A, Austin JK (2007) Social stigma for adults and children with epilepsy. Epilepsia 48 Suppl 9: 6–9. [DOI] [PubMed] [Google Scholar]
- 18.Druet-Cabanac M, Preux PM, Bouteille B, Bernet-Bernady P, Dunand J, et al. (1999) Onchocerciasis and epilepsy: a matched case-control study in the Central African Republic. Am J Epidemiol 149: 565–570. [DOI] [PubMed] [Google Scholar]
- 19.Diwan AR, Coker-Vann M, Brown P, Subianto DB, Yolken R, et al. (1982) Enzyme-linked immunosorbent assay (ELISA) for the detection of antibody to cysticerci of Taenia solium. Am J Trop Med Hyg 31: 364–369. [DOI] [PubMed] [Google Scholar]
- 20.Proano-Narvaez JV, Meza-Lucas A, Mata-Ruiz O, Garcia-Jeronimo RC, Correa D (2002) Laboratory diagnosis of human neurocysticercosis: double-blind comparison of enzyme-linked immunosorbent assay and electroimmunotransfer blot assay. J Clin Microbiol 40: 2115–2118. 10.1128/JCM.40.6.2115-2118.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales J, Martinez JJ, Manoutcharian K, Hernandez M, Fleury A, et al. (2008) Inexpensive anti-cysticercosis vaccine: S3Pvac expressed in heat inactivated M13 filamentous phage proves effective against naturally acquired Taenia solium porcine cysticercosis. Vaccine 26: 2899–2905. 10.1016/j.vaccine.2008.03.042 [DOI] [PubMed] [Google Scholar]
- 22.Chopra JS, Kaur U, Mahajan RC (1981) Cysticerciasis and epilepsy: a clinical and serological study. Trans R Soc Trop Med Hyg 75: 518–520. [DOI] [PubMed] [Google Scholar]
- 23.Mignard C, Mignard D, Dandelot JB, Polydor JP, Laporte JP, et al. (1986) Enquête épidémiologique sur l’endémie cysticerquienne à la Réunion. Rev Neurol (Paris) 142: 635–637. [PubMed] [Google Scholar]
- 24.Dumas M, Grunitzky E, Deniau M, Dabis F, Bouteille B, et al. (1989) Epidemiological study of neuro-cysticercosis in northern Togo (West Africa). Acta Leiden 57: 191–196. [PubMed] [Google Scholar]
- 25.Gracia F, de Lao SL, Castillo L, Larreategui M, Archbold C, et al. (1990) Epidemiology of epilepsy in Guaymi Indians from Bocas del Toro Province, Republic of Panama. Epilepsia 31: 718–723. [DOI] [PubMed] [Google Scholar]
- 26.Dansey RD, Hay M, Cowie RL (1992) Seizures and neurocysticercosis in black men. S Afr Med J 81: 424–425. [PubMed] [Google Scholar]
- 27.Nzisabira L, Nsengiyumva G, Bouteille B, Ndayiragije A, Niyongabo T, et al. (1992) La cysticercose dans la provinde dde Kayanza (Burundi). Bull Soc Pathol Exot 85: 374–377. [PubMed] [Google Scholar]
- 28.Sarti E, Schantz PM, Plancarte A, Wilson M, Gutierrez IO, et al. (1992) Prevalence and risk factors for Taenia solium taeniasis and cysticercosis in humans and pigs in a village in Morelos, Mexico. Am J Trop Med Hyg 46: 677–685. [DOI] [PubMed] [Google Scholar]
- 29.Garcia HH, Gilman R, Martinez M, Tsang VC, Pilcher JB, et al. (1993) Cysticercosis as a major cause of epilepsy in Peru. The Cysticercosis Working Group in Peru (CWG). Lancet 341: 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong Y, Cho SY, Cho MS, Kwon OS, Kang WS (1993) Seroepidemiological observation of Taenia solium cysticercosis in epileptic patients in Korea. J Korean Med Sci 8: 145–152. 10.3346/jkms.1993.8.2.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouteille B, Preux PM, Grunitzky EK, Avode DG, Hegbe M, et al. (1994) Approche épidémiologique de l'association cysticercose-épilepsie au Togo et au Bénin, Afrique de l'Ouest. 19ème Réunion Scientifique de l'Association des Epidémiologistes de Langue Française (ADELF). Rennes, France.
- 32.Theis JH, Goldsmith RS, Flisser A, Koss J, Chioino C, et al. (1994) Detection by immunoblot assay of antibodies to Taenia solium cysticerci in sera from residents of rural communities and from epileptic patients in Bali, Indonesia. Southeast Asian J Trop Med Public Health 25: 464–468. [PubMed] [Google Scholar]
- 33.Aranda-Alvarez JG, Tapia-Romero R, Alcantara-Anguiano I, Meza-Lucas A, Mata-Ruiz O, et al. (1995) Human cysticercosis: risk factors associated with circulating serum antigens in an open community of San Luis Potosi, Mexico. Ann Trop Med Parasitol 89: 689–692. [DOI] [PubMed] [Google Scholar]
- 34.Grill J, Rakotomalala W, Andriantsimahavandy A, Boisier P, Guyon P, et al. (1996) High prevalence of serological markers of cysticercosis among epileptic Malagasy children. Ann Trop Paediatr 16: 185–191. [DOI] [PubMed] [Google Scholar]
- 35.Handali S, Liying H, Lusikoy C, Senis J, Sihombing D (1997) A survey report—July 1993: cysticercosis in the Grand Dani Valley, Jayawijaya District, Irian Jaya Province, Indonesia. Southeast Asian J Trop Med Public Health 28 Suppl 1: 22–25. [PubMed] [Google Scholar]
- 36.Correa D, Sarti E, Tapia-Romero R, Rico R, Alcantara-Anguiano I, et al. (1999) Antigens and antibodies in sera from human cases of epilepsy or taeniasis from an area of Mexico where Taenia solium cysticercosis is endemic. Ann Trop Med Parasitol 93: 69–74. [DOI] [PubMed] [Google Scholar]
- 37.Cruz ME, Schantz PM, Cruz I, Espinosa P, Preux PM, et al. (1999) Epilepsy and neurocysticercosis in an Andean community. Int J Epidemiol 28: 799–803. [DOI] [PubMed] [Google Scholar]
- 38.Balogou AA, Grunitzky KE, Beketi KA, Bouteille B, Dumas M (2000) Cysticercose et épilepsie au nord du Togo dans le Tone. Rev Neurol (Paris) 156: 270–273. [PubMed] [Google Scholar]
- 39.Mittal V, Singh VK, Ichhpujani RL (2001) Detection of antibodies to Taenia solium in sera of patient with epilepsy using ELISA. J Commun Dis 33: 23–27. [PubMed] [Google Scholar]
- 40.Macharia W, Ramanankandrasana B, Druet-Cabanac M, Nsengiyumva G, Bouteille B, et al. (2002) Kenya: A new human cysticercosis focus. Afr J Neurol Sci 21: 46. [Google Scholar]
- 41.Rakatobe D (2002) Lien entre cysticercose et épilepsie: difficultés rencontrées lors de l'analyse d'une enquête cas-témoins réalisée à Ambilobe.
- 42.Dongmo L, Druet-Cabanac M, Moyou SR, Zebaze DRM, Njamnshi AK, et al. (2004) Cysticercose et épilepsie: etude cas-témoins dans la Vallée du Mbam, Cameroun. Bull Soc Pathol Exot 97: 105–108. [PubMed] [Google Scholar]
- 43.Del Brutto OH, Santibanez R, Idrovo L, Rodriguez S, Diaz-Calderon E, et al. (2005) Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia 46: 583–587. 10.1111/j.0013-9580.2005.36504.x [DOI] [PubMed] [Google Scholar]
- 44.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, et al. (2005) Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology 65: 229–233. 10.1212/01.wnl.0000168828.83461.09 [DOI] [PubMed] [Google Scholar]
- 45.Li T, Craig PS, Ito A, Chen X, Qiu D, et al. (2006) Taeniasis/cysticercosis in a Tibetan population in Sichuan Province, China. Acta Trop 100: 223–231. 10.1016/j.actatropica.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 46.Tran DS, Odermatt P, Le Oanh T, Huc P, Phoumindr N, et al. (2007) Risk factors for epilepsy in rural Lao PDR: a case-control study. Southeast Asian J Trop Med Public Health 38: 537–542. [PubMed] [Google Scholar]
- 47.Prasad A, Gupta RK, Pradhan S, Tripathi M, Pandey CM, et al. (2008) What triggers seizures in neurocysticercosis? A MRI-based study in pig farming community from a district of North India. Parasitol Int 57: 166–171. 10.1016/j.parint.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 48.Secka A, Grimm F, Victor B, Marcotty T, De Deken R, et al. (2010) Epilepsy is not caused by cysticercosis in The Gambia. Trop Med Int Health 15: 476–479. 10.1111/j.1365-3156.2010.02470.x [DOI] [PubMed] [Google Scholar]
- 49.Nitiema P, Carabin H, Hounton S, Praet N, Cowan LD, et al. (2012) Prevalence case-control study of epilepsy in three Burkina Faso villages. Acta Neurol Scand 126: 270–278. 10.1111/j.1600-0404.2011.01639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh G, Bawa J, Chinna D, Chaudhary A, Saggar K, et al. (2012) Association between epilepsy and cysticercosis and toxocariasis: a population-based case-control study in a slum in India. Epilepsia 53: 2203–2208. 10.1111/epi.12005 [DOI] [PubMed] [Google Scholar]
- 51.Elliott I, Jerome A, Angwafor SA, Smith ML, Takougang I, et al. (2013) Epilepsy and cysticercosis in north-west Cameroon: a serological study. Seizure 22: 283–286. 10.1016/j.seizure.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 52.Ngugi AK, Bottomley C, Kleinschmidt I, Wagner RG, Kakooza-Mwesige A, et al. (2013) Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol 12: 253–263. 10.1016/S1474-4422(13)70003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez S,Wilkins P, Dorny P.(2012) Immunological and Molecular diagnosis of cysticercosis.Pathogens and Global Health 5: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.