Abstract
Background
Acute kidney injury (AKI) remains a treatment-limiting toxicity of colistin. Recently developed clinical practice guidelines from the Kidney Disease: Improving Global Outcomes (KDIGO) group have harmonized definitions of AKI, but have not been widely applied to patients receiving colistin.
Methods
We retrospectively defined AKI by KDIGO definitions among adult patients receiving intravenous colistin for ≥ 3 days. Risk factors for AKI within 48 hours and 7 days of initiating colistin were determined by multivariable logistic regression.
Results
Among 249 patients treated with colistin, rates of AKI were 12% and 29% at 48 hours and 7 days, respectively. At 48 hours, patients in the intensive care unit were at increased risk for AKI. Within 7 days, colistin daily doses >5mg/kg, chronic liver disease, and concomitant vancomycin were independent predictors. Seven percent of patients required renal replacement therapy at a median of 5 days (range: 3–7) following colistin initiation.
Conclusion
Safe use of colistin is promoted by early detection of AKI with KDIGO criteria, avoiding nephrotoxins, and limiting duration of therapy.
Introduction
Colistin use has resurged due to the emergence of infections caused by extensively drug-resistant (XDR) Gram-negative bacteria. The drug’s efficacy is dependent upon achieving adequate exposures in patients, which requires higher doses than initially prescribed [1]. Ongoing pharmacokinetic (PK) studies have further underscored the importance of a loading dose to mitigate the slow conversion of colistimethate to its active form, colistin [1]. Taken together, these approaches have led to improved PK target attainment [2]; however, the resulting effect on colistin tolerability is largely unknown [3]. Indeed, escalating daily doses of colistin may be associated with a greater risk for acute kidney injury (AKI) [4, 5]. It is unclear if colistin loading doses potentiate this risk, particularly within the first 48 hours of treatment.
AKI remains a treatment-limiting adverse effect of colistin. Colistin-induced AKI appears to be due to the d-aminobutyric and fatty acid components of the drug, which increase cell membrane permeability resulting in cell lysis and acute tubular necrosis [6]. In the modern era, rates of AKI following colistin therapy range from 21–76% [3–5, 7–11]. Rates vary by predisposing conditions and severity of illness, but also criteria used to define AKI [6, 12]. To this end, a recently developed clinical practice guideline from the Kidney Disease: Improving Global Outcomes (KDIGO) group represents a landmark effort in harmonizing AKI definitions [13, 14]. The KDIGO clinical practice guidelines were informed by an exhaustive literature review and recognition that even small changes in absolute serum creatinine levels are associated with adverse outcomes [15, 16]. Indeed, defining AKI with KDIGO criteria is more predictive of in-hospital mortality than the commonly-used RIFLE criteria [17]. By unifying criteria for AKI, clinicians and researchers are armed with improved tools to elucidate risk factors for drug-induced AKI within specific populations. Notably, the KDIGO criteria have been rarely applied to patients receiving colistin [18]. Our objective was to determine the incidence and risk factors for colistin-induced AKI by KDIGO criteria during an era of PK-driven colistin dosing.
Materials and methods
The protocol was reviewed by the University of Pittsburgh Institutional Review Board (IRB) and determined to meet the necessary criteria for exemption under section 45 of the Code of Federal Regulations. Per local policies and through consultation with the IRB, written patient consent was not required and formal ethical approval was reviewed and waived.
We performed a retrospective, cohort study of adult patients receiving intravenous colistimethate (colistin) for ≥ 3 days at the University of Pittsburgh Medical Center (UPMC) from January 2009 to September 2013. For patients who received more than one course of colistin therapy, only the first was included in the analysis. Those who required hemodialysis (HD) or other types of renal replacement therapy (RRT) at the time of colistin initiation were excluded.
AKI was defined by applying the KDIGO recommendations within 48 hours or 7 days from the initiation of colistin treatment. Specifically, AKI was defined as a ≥0.3 mg/dL increase in SCr from baseline at 48 hours, or a 1.5x increase within 7 days [13, 14]. Creatinine clearance was calculated using the Cockcroft-Gault equation [19]. Continuous and categorical variables were compared with the Mann Whitney U and χ2 (or Fisher’s exact) tests, respectively. To determine risk factors for AKI during colistin treatment, we first identified covariates with a P-value <0.10 on univariate analysis. Next, we applied multivariable logistic regression to identify independent predictors of AKI using backward selection procedures (STATA SE v.13.1, College Station, TX) Two-tailed P-values <0.05 were considered statistically significant.
Results
Three-hundred and sixty patients received ≥ 3 days of colistin during the study period; 111 patients required RRT prior to colistin treatment and were excluded. Among the remaining 249 patients, the median age was 59 years (inter-quartile range [IQR]: 21–86), 53% (132/249) were male and the median Charlson Comorbidity index was 2 (IQR: 2–6). At the time of colistin initiation, the majority of the patients resided in ICU (64%, 160/249). XDR Acinetobacter baumannii was the most commonly targeted pathogen (38%, 94/249), followed by Pseudomonas aeruginosa (19%, 48/249), Klebsiella pneumoniae (18%, 45/249), and other Enterobacteriaceae (5%, 13/249). Sixteen percent of patients were infected by >1 XDR pathogen (39/249) and 4% (10/249) were treated empirically. The median duration of colistin treatment was 8 days (IQR: 5–14). Colistin was employed as part of combination therapy in 86% (215/249) of patients. Twenty-four percent (60/249) of patients received aerosolized colistin as adjunctive therapy and 47% (118/249) received a loading dose. Ninety percent of patients received concomitant nephrotoxic agents, most commonly, vancomycin (56%, 140/249) and loop diuretics (45%, 112/249).
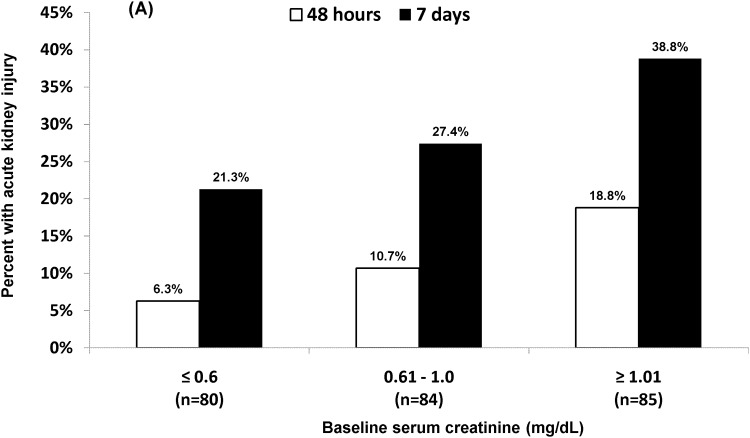
Criteria for AKI were met within 48 hours and 7 days of colistin initiation in 12% (30/249) and 29% (73/249) of patients, respectively (Table 1). Ninety-eight percent (243/249) and 100% of patients had ≥ 2 SCr values measured within 48 hours and 7 days, respectively. Median baseline SCr values were higher among patients with AKI at 48 hours (1.1 vs. 0.8 mg/dL; P = 0.006) and 7 days (1.0 vs. 0.8 mg/dL; P = 0.003). Indeed, rates of AKI were associated with increasing baseline SCr values and decreasing creatinine clearance (Fig 1). Median daily doses of colistin did not differ among patients with or without AKI; however, receipt of a loading dose or a total daily dose >5 mg/kg were more common among patients with AKI at 7 days (Table 1; P = 0.039 and 0.044, respectively).
Table 1. Factors associated with colistin-induced Acute Kidney Injury (AKI).
| Variable | All patients (n = 249) | AKI at 48 hours (≥ 0.3 mg/dL increase in SCr) | AKI within 7 days (≥ 1.5x baseline SCr) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AKI | No AKI | Univariate | Multivariate | AKI | No AKI | Univariate | Multivariate | ||
| (n = 30) | (n = 219) | P-value | P-value (OR, 95% CI) | (n = 73) | (n = 176) | P-value | P-value (OR, 95% CI) | ||
| Median age (range), years | 59 (18–91) | 63 (41–77) | 59 (18–91) | 0.151 | 62 (23–81) | 57.5 (18–91) | 0.034 | 0.138 | |
| Male sex, no. (%) | 132 (53) | 18 (60) | 114 (52) | 0.414 | 45 (62) | 87 (49) | 0.079 | 0.347 | |
| Caucasian, no. (%) | 198 (80) | 21 (70) | 177 (81) | 0.168 | 58 (79) | 140 (80) | 0.987 | ||
| Comorbidities, no. (%) | |||||||||
| • Chronic respiratory disease | 114 (46) | 10 (33) | 104 (47) | 0.206 | 30 (41) | 84 (48) | 0.414 | ||
| • Congestive heart failure | 105 (42) | 11 (28) | 94 (43) | 0.650 | 34 (47) | 71 (40) | 0.444 | ||
| • Chronic kidney disease | 33 (13) | 3 (10) | 30 (14) | 0.575 | 9 (12) | 24 (14) | 0.782 | ||
| • Chronic liver disease | 29 (12) | 6 (20) | 23 (11) | 0.134 | 14 (19) | 15 (9) | 0.028 | 0.011 (3.09, 1.30–7.34) | |
| • Diabetes mellitus | 77 (31) | 8 (27) | 69 (32) | 0.591 | 21 (29) | 56 (32) | 0.655 | ||
| • Obesity (BMI >30) | 8 (3) | 1 (3) | 7 (32) | 0.968 | 3 (4) | 5 (3) | 0.696 | ||
| Median Charlson Score (range) | 4 (0–11) | 3 (0–8) | 4 (0–11) | 0.353 | 4 (0–10) | 3 (0–11) | 0.537 | ||
| Solid-organ transplant recipient, no. (%) | 46 (18) | 7 (23) | 39 (18) | 0.465 | 17 (23) | 29 (16) | 0.207 | ||
| Intensive care unit at start of therapy, no. (%) | 160 (64) | 26 (87) | 134 (61) | 0.007 | 0.024 (3.57, 1.18–10.8) | 52 (71) | 108 (61) | 0.139 | |
| Requirement for vasopressors, no. (%) | 89 (36) | 14 (47) | 75 (34) | 0.183 | 29 (40) | 60 (34) | 0.398 | ||
| Serum albumin < 2 g/dL, no. (%) | 92 (37) | 10 (33) | 82 (37) | 0.662 | 28 (38) | 64 (36) | 0.767 | ||
| Median baseline SCr (range) | 0.8 (0.1–3.7) | 1.1 (0.4–2.9) | 0.8 (0.1–3.7) | 0.006 | 0.115 | 1.0 (0.2–3.6) | 0.8 (0.1–3.7) | 0.003 | 0.094 |
| Median colistin dose in mg/kg/day (range)* | 3.45 (0.5–7.69) | 3.49 (1.18–6.06) | 3.44 (0.5–7.69) | 0.751 | 3.45 (0.85–7.69) | 3.48 (0.5–6.56) | 0.802 | ||
| Colistin dose > 5mg/kg/day, no. (%) | 37 (15) | 6 (20) | 31 (14) | 0.413 | 16 (22) | 21 (12) | 0.044 | 0.017 (2.58, 1.18–5.61) | |
| Colistin loading dose, no. (%) | 118 (47) | 17 (57) | 101 (46) | 0.278 | 42 (58) | 76 (43) | 0.039 | 0.284 | |
| Concomitant inhaled colistin, no. (%) | 60 (24) | 6 (20) | 54 (25) | 0.656 | 19 (26) | 41 (23) | 0.630 | ||
| Concomitant nephrotoxins, no. (%) | |||||||||
| • Intravenous contrast dye | 36 (14) | 3 (10) | 33 (15) | 0.588 | 7 (10) | 29 (16) | 0.159 | ||
| • Aminoglycoside | 60 (24) | 8 (27) | 52 (24) | 0.726 | 19 (26) | 41 (23) | 0.646 | ||
| • Vancomycin | 140 (56) | 21 (70) | 119 (54) | 0.105 | 50 (68) | 90 (51) | 0.012 | 0.03 (1.98, 1.07–3.66) | |
| • Amphotericin B | 13 (5) | 4 (13) | 9 (4) | 0.057 | 0.088 | 5 (7) | 8 (5) | 0.533 | |
| • Calcineurin inhibitor | 45 (18) | 7 (23) | 38 (17) | 0.425 | 17 (23) | 28 (16) | 0.168 | ||
| • Angiotensin converting enzyme inhibitor | 12 (5) | 0 (0) | 12 (5) | 0.370 | 1 (1) | 11 (6) | 0.189 | ||
| • Loop diuretic | 112 (45) | 13 (43) | 99 (45) | 0.847 | 39 (53) | 73 (41) | 0.085 | 0.151 | |
| • NSAID | 44 (18) | 2 (7) | 42 (19) | 0.125 | 11 (15) | 33 (19) | 0.488 | ||
| Concomitant antibiotics, no. (%) | |||||||||
| • Penicillin | 83 (33) | 15 (50) | 68 (31) | 0.061 | 0.075 | 24 (33) | 59 (34) | 0.922 | |
| • Cephalosporin | 64 (26) | 8 (27) | 56 (26) | 0.898 | 21 (29) | 43 (24) | 0.476 | ||
| • Carbapenem | 184 (74) | 22 (73) | 162 (74) | 0.940 | 56 (77) | 128 (73) | 0.515 | ||
| • Fluoroquinolone | 41 (16) | 3 (10) | 38 (17) | 0.433 | 11 (15) | 30 (17) | 0.702 | ||
| • Tetracycline | 37 (15) | 5 (17) | 32 (15) | 0.785 | 9 (12) | 28 (16) | 0.470 | ||
| • Macrolide | 31 (12) | 2 (7) | 29 (13) | 0.391 | 5 (7) | 26 (15) | 0.095 | ||
AKI = Acute kidney injury, BMI = Body mass index, NSAID = Non-steroidal anti-inflammatory drug, SCr = Serum creatinine
* Defined as the average daily dose administered over the first 72 hours of colistin therapy divided by total body weight.
Fig 1. Rates of acute kidney injury by baseline (A) serum creatinine value and (B) creatinine clearance.
Note (A). Rates of acute kidney injury were significantly higher for patients with a baseline serum creatinine value ≥1.01 mg/dL compared to ≤0.6 mg/dL at 48 hours (P = 0.01) and 7 days (P = 0.01).
In multivariate analyses (Table 1), after controlling for baseline SCr, residence in the ICU at the time of colistin initiation (OR = 3.57, 95% CI: 1.18–10.8; P = 0.024) was an independent predictor of AKI at 48 hours. By 7 days, colistin daily doses >5 mg/kg (OR = 2.58, 95% CI: 1.18–5.61; P = 0.017), receipt of concomitant vancomycin (OR = 1.98, 95% CI: 1.07–3.66; P = 0.03) and chronic liver disease (OR = 3.09, 95% CI: 1.30–7.34; P = 0.011) were associated with AKI. Following colistin initiation, 7% (17/249) of patients required RRT; median time to RRT was 5 days (IQR: 3–7).
Discussion
Our study represents one of the largest cohorts of patients treated with intravenous colistin reported in the literature, and the largest to define AKI using the KDIGO clinical practice guideline criteria [14, 18]. We have applied these criteria to the first 48 hours and 7 days of colistin treatment, which allows for a dynamic assessment of risk factors early, and over time. Indeed, AKI typically occurs within the first 5–7 days of colistin therapy [4, 20, 21], and is associated with higher rates of mortality compared to late-onset (>7 days) AKI [20]. Here, we found the incidence of AKI at 48 hours and 7 days to be 12% and 29%, respectively. Within the first 48 hours of treatment, colistimethate is slowly converted to its active form, colistin. Administration of a loading dose, results in earlier achievement of steady-state concentrations [1, 22]; however, the incidence and risk factors for AKI within these first 48 hours are unknown. In our analysis, we found ICU residence, but not administration of a loading dose, to be predictive of AKI within 48 hours of initiating colistin. These findings support patient severity of illness as an important determinant of AKI [23]. By 7 days, colistin daily dosage, chronic liver disease and vancomycin co-administration were independent predictors of AKI. Taken together, the data highlight that AKI occurs at variable frequencies among highly dynamic patient populations over time, within whom the interplay between predisposing conditions and acute processes is largely indistinguishable [3]. As such, moving towards consensus definitions, such as those proposed by KDIGO, is imperative. Early detection and treatment of AKI improves patient outcomes, which may be an advantage of KDIGO compared to RIFLE definitions [14].
Risk factors for colistin-induced AKI, by any criteria, are subject to the population being studied, the dose and duration of colistin treatment, and the co-administration of other nephrotoxic agents [6]. At our center, we corroborated the additional hazard of ICU residence [3, 5] and chronic liver disease [5, 24]. Such patients are subject to numerous physiologic changes making them more susceptible to AKI. Critically-ill patients, for example are at greater risk for AKI due to the presence of septic shock and a greater severity of illness [23]. In patients with chronic liver disease, increased nitric oxide and angiotensin II result in hypoperfusion of the kidneys [25, 26]. We also found concomitant vancomycin increases the risk for AKI within 7 days of starting colistin therapy. The association has been linked to the duration of vancomycin therapy [27], and is noteworthy given the ubiquitous use of vancomycin among critically-ill patients, and the potential synergistic activity of colistin-vancomycin combinations against XDR Gram-negative pathogens [28]. Like other nephrotoxins, the requirement for co-administration of vancomycin should be balanced with the additive risk of AKI when using colistin. Prediction models that incorporate concomitant nephrotoxins may be useful in this regard to estimate the risk of nephrotoxicity in individual patients [29]. In managing individual patients, other factors associated with an increased risk for AKI, like older age, pre-existing renal impairment, and underlying diseases, should be evaluated prior to initiating therapy [3].
As colistin dosing strategies evolve, it will be important to weigh the benefits of improved target attainment with the risk of toxicity. To this end, we demonstrated that administration of a colistin loading dose was associated with an increased risk of AKI within 7 days on univariate, but not multivariate analysis. On the other hand, total daily doses exceeding 5mg/kg were independently linked to a higher rate of AKI, consistent with prior studies [4, 29]. These data extend those from recent reports evaluating the safety of colistin loading and PK-optimized dosing strategies [3, 30, 31]. Elefritz and colleagues did not find a significant difference in rates of AKI by RIFLE criteria among 30 patients who received a 5 mg/kg loading dose compared to 42 patients who did not; however rates of AKI were 58% and 50%, respectively [30]. Rates of AKI were lower (44%) among a prospective, observational cohort study of patients who received a loading dose of colistin, followed by PK-driven maintenance doses, but doses were capped at a maximum of 270mg for patients with normal renal function [3]. Most recently, Rigatto and colleagues found a significantly higher rate of renal failure (by RIFLE criteria) among patients who received a loading dose of colistin (77.3% vs 23.7%; P≤0.001) though post-hoc analysis showed loading dose patients were older, had more comorbidities, and significantly lower creatinine clearance [31]. Taken together with the current study, the safety of colistin loading doses remains unclear. It is worth highlighting, however, that such approaches are necessary to achieve appropriate serum levels [1]. Importantly, new colistin dosing recommendations advocate for higher daily doses than are currently approved by the US Food and Drug Administration [22, 32]. The impact of these recommendations on rates of AKI will need to be determined in future studies. So too, will the role of renal-protective agents like ascorbic acid, proanthocyanidin, vitamin E, and melatonin [3, 33–35]. Until such data become available, the use of colistin in the clinic requires immediate and continuous monitoring as outlined in the KDIGO guidelines [13].
Like all retrospective studies, ours is not without the limitations that stem from this study design, including the availability of clinical and laboratory data. Though serum creatinine values were readily available, and used to define AKI, future studies may be improved by using more sensitive biomarkers of renal injury [36]. Moreover, we were unable to define AKI by urine output, something that future prospective studies could expand upon. Nonetheless, we defined the rate of colistin-induced AKI using the KDIGO guideline recommendations for the first time at 48 hours and 7 days from the initiation of colistin therapy. In doing so, we have highlighted several patient populations that merit closer monitoring for colistin-induced AKI, namely those with pre-existing renal impairment, chronic liver disease, or treated in the ICU. Concomitant nephrotoxins like, amphotericin B and vancomycin should be used judiciously and whenever possible the duration of colistin therapy should be minimized.
Acknowledgments
We are indebted to John Kellum for his assistance in data interpretation. This project was supported by funding provided by the National Institutes of Health (NIH) under award number K08AI114883 awarded to R. K. S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data Availability
All relevant data are within the paper.
Funding Statement
This project was supported by funding provided by the National Institutes of Health (NIH) under award number K08AI114883 awarded to R. K. S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrobial agents and chemotherapy. 2011;55(7):3284–94. Epub 2011/05/11. 10.1128/AAC.01733-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, et al. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalfino L, Puntillo F, Ondok MJ, Mosca A, Monno R, Coppolecchia S, et al. Colistin-associated Acute Kidney Injury in Severely Ill Patients: A Step Toward a Better Renal Care? A Prospective Cohort Study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;61(12):1771–7. [DOI] [PubMed] [Google Scholar]
- 4.Pogue JM, Lee J, Marchaim D, Yee V, Zhao JJ, Chopra T, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis. 2011;53(9):879–84. 10.1093/cid/cir611 [DOI] [PubMed] [Google Scholar]
- 5.Omrani AS, Alfahad WA, Shoukri MM, Baadani AM, Aldalbahi S, Almitwazi AA, et al. High dose intravenous colistin methanesulfonate therapy is associated with high rates of nephrotoxicity; a prospective cohort study from Saudi Arabia. Annals of clinical microbiology and antimicrobials. 2015;14:3 10.1186/s12941-015-0062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ordooei Javan A, Shokouhi S, Sahraei Z. A review on colistin nephrotoxicity. European journal of clinical pharmacology. 2015;71(7):801–10. 10.1007/s00228-015-1865-4 [DOI] [PubMed] [Google Scholar]
- 7.Doshi NM, Mount KL, Murphy CV. Nephrotoxicity associated with intravenous colistin in critically ill patients. Pharmacotherapy. 2011;31(12):1257–64. 10.1592/phco.31.12.1257 [DOI] [PubMed] [Google Scholar]
- 8.Collins JM, Haynes K, Gallagher JC. Emergent renal dysfunction with colistin pharmacotherapy. Pharmacotherapy. 2013;33(8):812–6. 10.1002/phar.1271 [DOI] [PubMed] [Google Scholar]
- 9.Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48(12):1724–8. 10.1086/599225 [DOI] [PubMed] [Google Scholar]
- 10.Tuon FF, Rigatto MH, Lopes CK, Kamei LK, Rocha JL, Zavascki AP. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int J Antimicrob Agents. 2014;43(4):349–52. 10.1016/j.ijantimicag.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 11.Binh NG, Hayakawa K, Co DX, Tuan ND, Anh NH, Thuy NT, et al. The efficacy and nephrotoxicity associated with colistin use in an intensive care unit in Vietnam: Use of colistin in a population of lower body weight. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2015;35:18–23. [DOI] [PubMed] [Google Scholar]
- 12.Fiaccadori E, Antonucci E, Morabito S, d'Avolio A, Maggiore U, Regolisti G. Colistin Use in Patients With Reduced Kidney Function. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2016;68(2):296–306. [DOI] [PubMed] [Google Scholar]
- 13.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013;61(5):649–72. [DOI] [PubMed] [Google Scholar]
- 14.Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical care. 2013;17(1):204 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Archives of internal medicine. 2008;168(6):609–16. 10.1001/archinte.168.6.609 [DOI] [PubMed] [Google Scholar]
- 16.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology: JASN. 2005;16(11):3365–70. 10.1681/ASN.2004090740 [DOI] [PubMed] [Google Scholar]
- 17.Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X, et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Critical care. 2014;18(4):R144 10.1186/cc13977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanita MT, Carrilho CM, Garcia JP, Festti J, Cardoso LT, Grion CM. Parenteral colistin for the treatment of severe infections: a single center experience. Revista Brasileira de terapia intensiva. 2013;25(4):297–305. 10.5935/0103-507X.20130051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. Epub 1976/01/01. [DOI] [PubMed] [Google Scholar]
- 20.Ko H, Jeon M, Choo E, Lee E, Kim T, Jun JB, et al. Early acute kidney injury is a risk factor that predicts mortality in patients treated with colistin. Nephron Clinical practice. 2011;117(3):c284–8. 10.1159/000320746 [DOI] [PubMed] [Google Scholar]
- 21.Deryke CA, Crawford AJ, Uddin N, Wallace MR. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrobial agents and chemotherapy. 2010;54(10):4503–5. 10.1128/AAC.01707-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, et al. Dosing guidance for intravenous colistin in critically-ill patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocco M, Montini L, Alessandri E, Venditti M, Laderchi A, De Pascale G, et al. Risk factors for acute kidney injury in critically ill patients receiving high intravenous doses of colistin methanesulfonate and/or other nephrotoxic antibiotics: a retrospective cohort study. Critical care. 2013;17(4):R174 10.1186/cc12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon JA, Lee JE, Huh W, Peck KR, Kim YG, Kim DJ, et al. Predictors of acute kidney injury associated with intravenous colistin treatment. International journal of antimicrobial agents. 2010;35(5):473–7. 10.1016/j.ijantimicag.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 25.Slack A, Yeoman A, Wendon J. Renal dysfunction in chronic liver disease. Critical care. 2010;14(2):214 10.1186/cc8855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48(6):2064–77. 10.1002/hep.22605 [DOI] [PubMed] [Google Scholar]
- 27.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–55. 10.1007/s00228-012-1259-9 [DOI] [PubMed] [Google Scholar]
- 28.O'Hara JA, Ambe LA, Casella LG, Townsend BM, Pelletier MR, Ernst RK, et al. Activities of vancomycin-containing regimens against colistin-resistant Acinetobacter baumannii clinical strains. Antimicrobial agents and chemotherapy. 2013;57(5):2103–8. 10.1128/AAC.02501-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phe K, Johnson ML, Palmer HR, Tam VH. Validation of a model to predict the risk of nephrotoxicity in patients receiving colistin. Antimicrobial agents and chemotherapy. 2014;58(11):6946–8. 10.1128/AAC.03776-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elefritz JL, Bauer KA, Jones C, Mangino JE, Porter K, Murphy CV. Efficacy and Safety of a Colistin Loading Dose, High-Dose Maintenance Regimen in Critically Ill Patients With Multidrug-Resistant Gram-Negative Pneumonia. Journal of intensive care medicine. 2016. [DOI] [PubMed] [Google Scholar]
- 31.Rigatto MH, Oliveira MS, Perdigao-Neto LV, Levin AS, Carrilho CM, Tanita MT, et al. Multicenter Prospective Cohort Study of Renal Failure in Patients Treated with Colistin versus Polymyxin B. Antimicrobial agents and chemotherapy. 2016;60(4):2443–9. 10.1128/AAC.02634-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, et al. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;62(5):552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozkan G, Ulusoy S, Orem A, Ersoz S, Alkanat M, Yucesan FB, et al. Protective effect of the grape seed proanthocyanidin extract in a rat model of contrast-induced nephropathy. Kidney & blood pressure research. 2012;35(6):445–53. [DOI] [PubMed] [Google Scholar]
- 34.Yousef JM, Chen G, Hill PA, Nation RL, Li J. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrobial agents and chemotherapy. 2011;55(9):4044–9. 10.1128/AAC.00328-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghlissi Z, Hakim A, Sila A, Mnif H, Zeghal K, Rebai T, et al. Evaluation of efficacy of natural astaxanthin and vitamin E in prevention of colistin-induced nephrotoxicity in the rat model. Environmental toxicology and pharmacology. 2014;37(3):960–6. 10.1016/j.etap.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 36.Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16(2):51–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.