Abstract
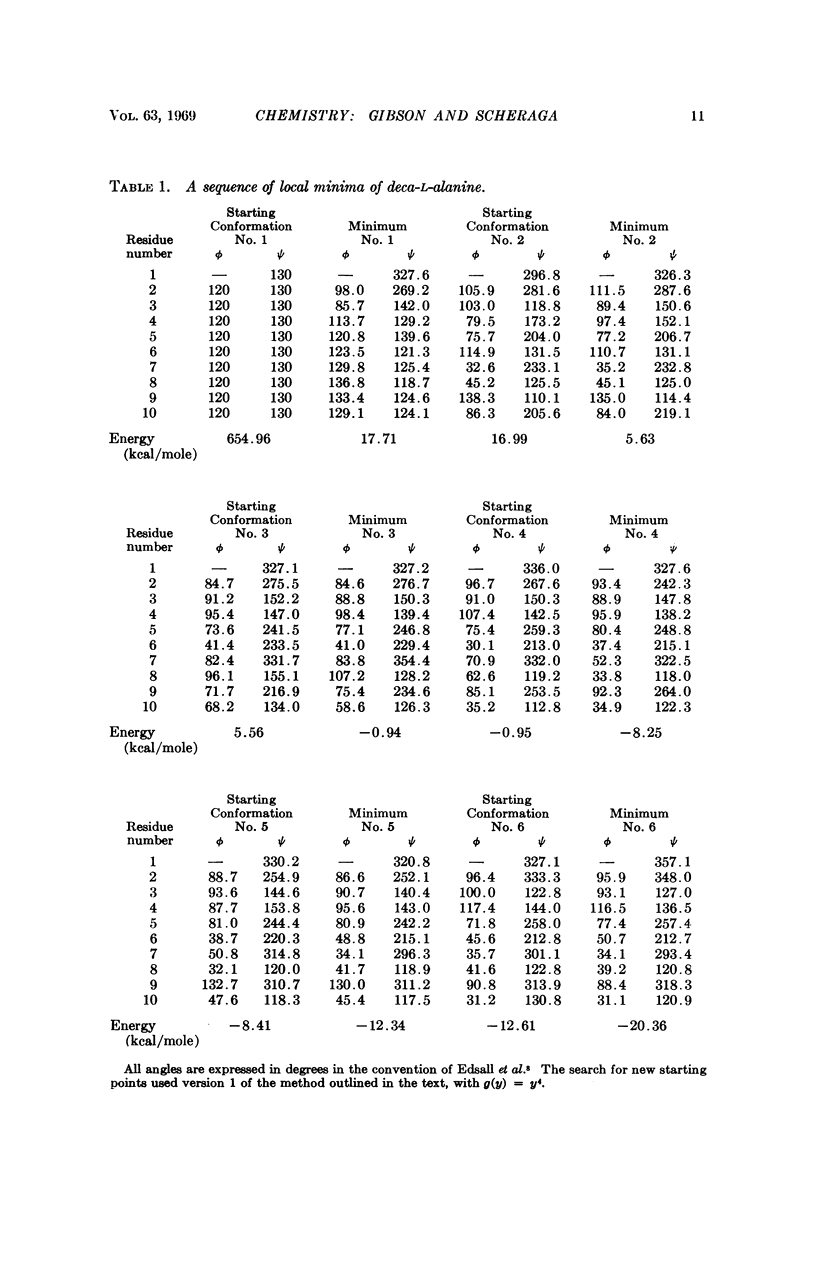
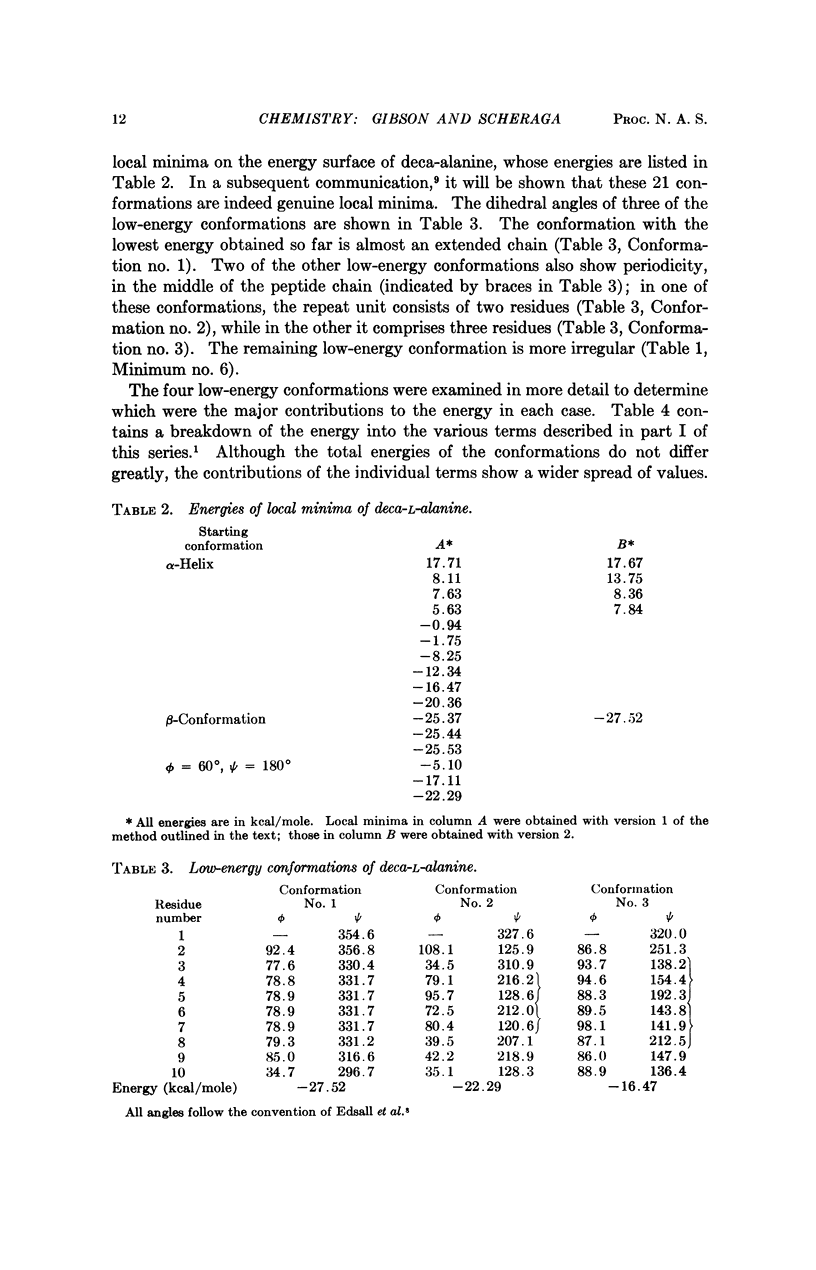
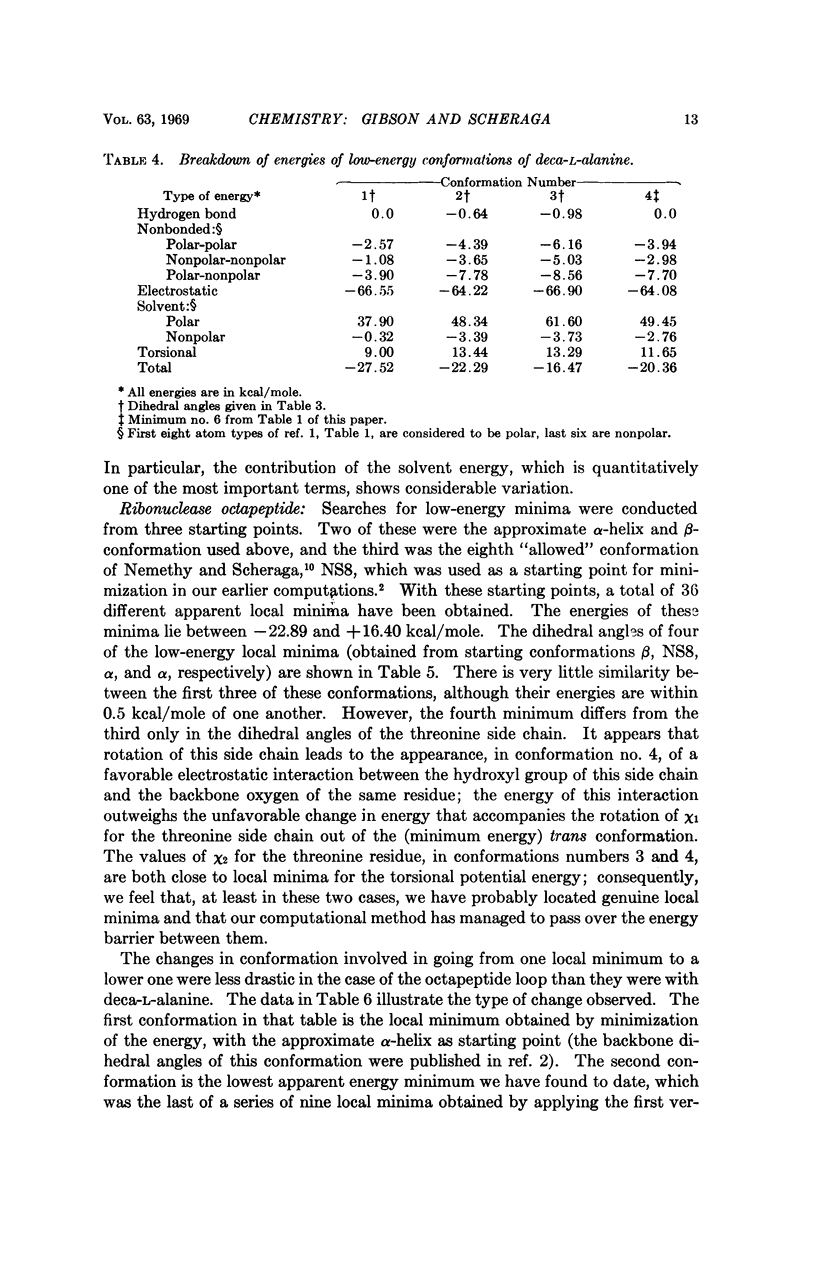
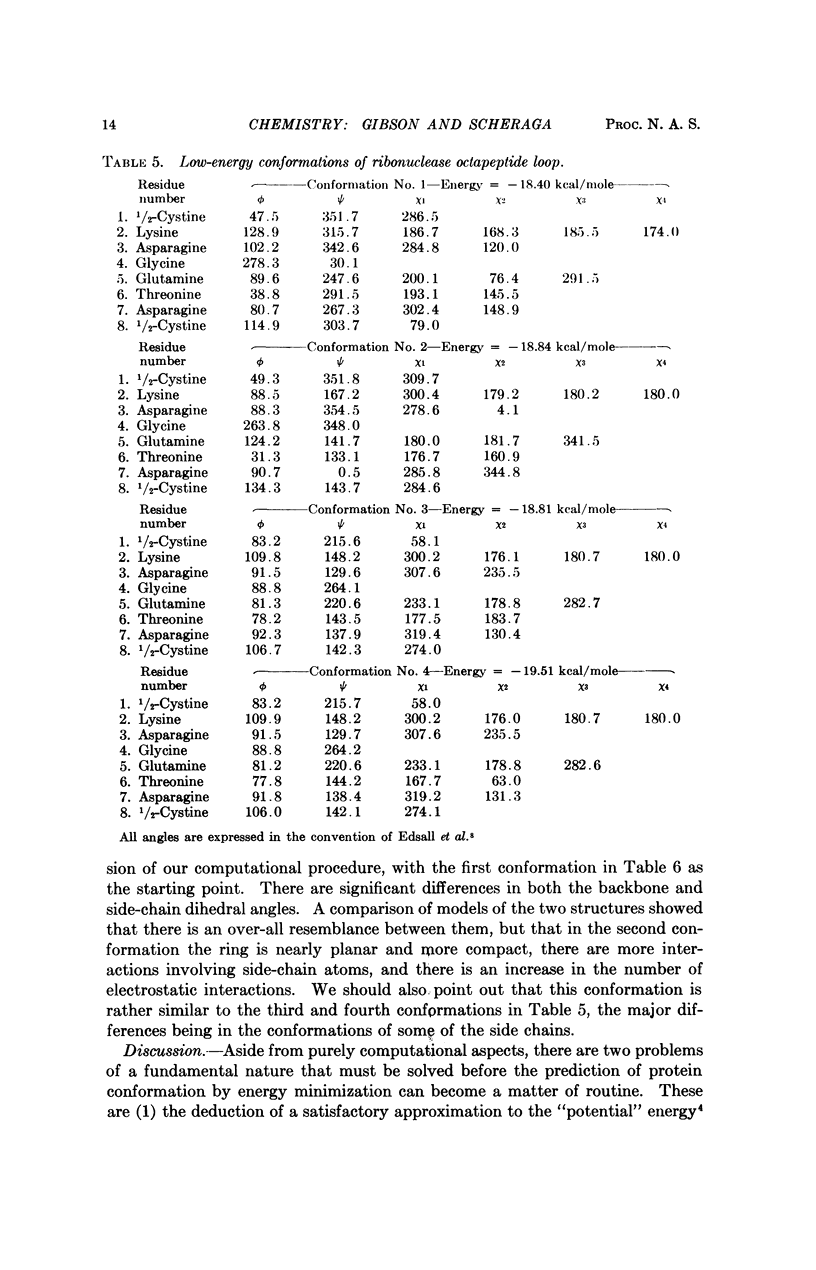
A succession of apparent local energy minima, in which the energies decrease successively, have been located for deca-L-alanine and the octapeptide loop of ribonuclease. The lowest energy minima were approximately 40 kcal/mole lower than the highest ones. The results suggest that the computational methods used here might be of considerable use in searching for the global energy minimum of larger polypeptides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edsall J. T., Flory P. J., Kendrew J. C., Liquori A. M., Nemethy G., Ramachandran G. N., Scheraga H. A. A proposal of standard conventions and nomenclature for the description of polypeptide conformation. J Biol Chem. 1966 Feb 25;241(4):1004–1008. [PubMed] [Google Scholar]
- Gibson K. D., Scheraga H. A. Minimization of polypeptide energy. I. Preliminary structures of bovine pancreatic ribonuclease S-peptide. Proc Natl Acad Sci U S A. 1967 Aug;58(2):420–427. doi: 10.1073/pnas.58.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelchuck D., Scheraga H. A. The influence of short-range interactions on protein conformation. I. Side chain-backbone interactions within a single peptide unit. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1163–1170. doi: 10.1073/pnas.61.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]



