Abstract
Background and Purpose
Peri-hematomal edema (PHE) is a marker of secondary injury in intracerebral hemorrhage (ICH). PHE measurement on computed tomography (CT) is challenging and the principles used to detect PHE have not been described fully. We developed a systematic approach for CT-based measurement of PHE.
Methods
Two independent raters measured PHE volumes on baseline and 24-hour post-ICH CT scans of twenty primary supratentorial ICH subjects. Boundaries were outlined with an edge-detection tool and adjusted after inspection of the three orthogonal planes. PHE was delineated with the additional principle that it should be (a) more hypodense than the corresponding area in the contralateral hemisphere and (b) most hypodense immediately surrounding the hemorrhage. We examined intra- and interrater reliability using intraclass correlation coefficients (ICCs) and Bland-Altman plots for interrater consistency. CT-based PHE was also compared using MRI-based PHE detection for eighteen subjects.
Results
Median PHE volumes were 22.7 cc at baseline and 20.4 cc at 24-hours post-ICH. There were no statistically significant differences in PHE measurements between raters. Interrater and intrarater reliability for PHE were excellent. At baseline and 24-hours, interrater ICCs were 0.98 (0.96–1.00) and 0.98 (0.97–1.00); intrarater ICCs were 0.99 (0.99–1.00) and 0.99 (0.98–1.00). Bland-Altman analysis showed the bias for PHE measurements at baseline and 24 hours, −0.5 cc (SD, 5.4) and −3.2 cc (SD, 5.0), was acceptably small. PHE volumes determined by CT and MRI were similar (23.9 ± 16.9 cc vs. 23.9 ± 16.0 cc, R2 = 0.98, p < 0.0001).
Conclusions
Our method measures PHE with excellent reliability at baseline and 24-hours post-ICH.
Keywords: intracerebral hemorrhage, peri-hematomal edema, computed tomography
Introduction
Intracerebral hemorrhage (ICH) accounts for 15% of all adult strokes and is the deadliest stroke subtype.1 Poor outcome after ICH is due to both primary and secondary injury.2, 3 The former refers to the mechanical effects from the hemorrhage and the latter includes the effects of the inflammatory response to ICH, the toxicity of blood breakdown products and peri-hematomal edema (PHE).1
Volumetric measurements have been performed to explore whether PHE is an independent predictor of outcome.4 PHE has also been used as a surrogate endpoint in trials of neuroprotective agents targeting secondary injury.5 Most studies use computed tomography (CT) because magnetic resonance imaging (MRI) is not feasible in unstable patients. MRI has been described as superior for PHE quantification given the high contrast between the peri-hematomal hyperintensity thought to represent PHE and neighboring brain tissue.6 However, there is evidence that MRI may not be the best modality for PHE quantification; one study reported that the change in peri-hematomal MRI hyperintensity correlated poorly with the change in ipsilateral hemispheric volume.7
On CT, the hyperdense region of hemorrhage contrasts with neighboring tissue and is measured relatively easily. However, PHE manifests as a peri-hematomal hypodensity that can be challenging to distinguish from normal tissue and other entities that are also hypodense (e.g. infarction). Furthermore, the boundaries of PHE become less clear over time. Previous studies have used threshold-based or edge-detection algorithms to measure PHE, yet the principles used to trace PHE have not been explicitly clarified (Table I online Data Supplement).4, 8–10 Perhaps for this reason midline shift (MLS) is still used in some ICH studies in place of volumetric measurements.11 Unfortunately, MLS correlates with total hemispheric swelling, not exclusively PHE.12
Here we outline an approach for CT-based measurement of PHE that combines an edge-detection algorithm and considers the pathophysiology of PHE formation. We determined the interrater and intrarater reliability of the method. We also examined the correspondence between PHE determined by CT and MRI in a group of subjects in which both neuroimaging modalities were obtained.
Methods
Subject Identification
Subjects were retrospectively identified from an Institutional Review Board-approved prospective cohort study of ICH performed at Massachusetts General Hospital between 2000 and 2013. Inclusion criteria were age over 18 years with primary spontaneous supratentorial ICH confirmed by CT. Exclusion criteria were infratentorial hemorrhage, primary intraventricular hemorrhage (IVH), subsequent surgery, and any suspected cause of secondary ICH. Twenty randomly selected subjects with baseline and 24-hour post-ICH CT scans were included. Eighteen subjects with both CT and MRI performed in close association were also included.
Measurements
ICH, PHE and IVH volumes were determined by two independent raters (S.U. and D.W.G.) using Analyze 11.0 (AnalyzeDirect, Overland Park, KS, USA) (Figures I–VII Online Supplement). The raters were blinded to clinical data and to each other’s measurements. Measurements were performed by outlining the hemorrhage and rim of PHE on axial slices in the software’s Region of Interest (ROI) module with a semi-automated edge detection tool. This tool calculates boundaries for each lesion based on Hounsfield Units in the area selected by the rater, the most optimal of which is selected by the rater. Next, boundaries were adjusted after inspection of the three orthogonal planes in the software’s Volume Edit module, which allows for better assessment of the location and distribution of each lesion beyond the two-dimensional axial plane. The three-dimensional view is especially helpful to visualize the extent of PHE throughout the brain in large hemorrhages. To delineate PHE the additional principles that it should be (a) more hypodense than the corresponding region in the contralateral hemisphere and (b) most hypodense immediately surrounding the hemorrhage were employed. The rationale for the latter is that following ICH, transendothelial water flux from the intravascular to the interstitial compartments, both before and after frank blood-brain barrier disruption, occurs initially immediately adjacent to the hematoma.13 Therefore, if a less hypodense area adjacent to the hematoma is followed by a more hypodense area further outward, the latter could represent another entity like an old infarct. Measurements were repeated by one rater (S.U.) after a four-month interval to determine intrarater reliability. All measurements were reviewed by two stroke neurologists (L.A.B. and K.N.S.). Figures 1 depicts a representative example of a subject’s ICH and PHE measurements. PHE volumes were determined on MRI (fluid-attenuated inversion recovery sequence, FLAIR) using the semi-automated edge detection tool to delineate the peri-hematomal hyperintensity.
Figure 1.
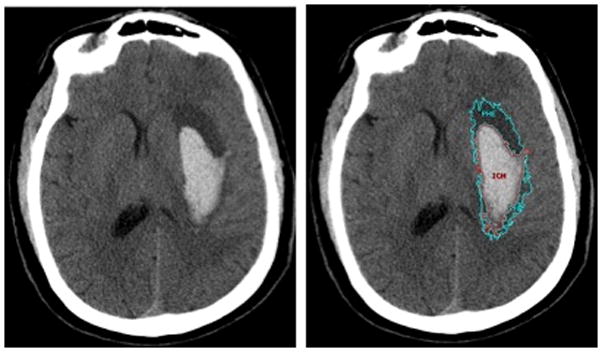
Example of Region of Interest object maps used to measure intracerebral hemorrhage (ICH) and peri-hematomal edema (PHE) volumes. Left panel, left putaminal hemorrhage. Right panel, ICH (red) and PHE (cyan) delineated with an edge-detection algorithm.
Statistical Analysis
Statistical analysis was performed using STATA Version 12.1 (Stata Corp, College Station, Texas). Intrarater and interrater reliability were measured with intraclass correlation coefficients (ICCs) using one-way analysis of variance. An ICC was considered moderate agreement if 0.41–0.60, substantial agreement if 0.61–0.80, and almost perfect (excellent) if 0.81–1.00.14 Wilcoxon rank-sum tests were used to determine whether measurements differed significantly. Bland-Altman plots were constructed to verify that there was no systematic bias using GraphPad Prism 6.00 (GraphPad Software, La Jolla, California). Linear regression analysis was performed to compare PHE volumes on CT and MRI; the parameter R2 was reported as the measure of concurrent validity.
Results
ICH was lobar in 8 and deep hemispheric in 12 subjects (Table II Data Supplement). ICH (Table III Data Supplement), PHE (Table 1), and IVH (Table IV Data Supplement) volumes at both baseline and 24-hours post-ICH for the two raters did not differ. Intrarater measurements also did not differ (Table 1, Data Supplement Tables III and IV).
Table.
Summary of PHE Measurements
| Median Volume cc (IQR) | Wilcoxon Rank-Sum P Value | Interrater ICC (95% CI) | Intrarater ICC (95% CI) | |
|---|---|---|---|---|
| Baseline | ||||
| R1, R2 | 22.7 (9.2–37.2), 19.6 (10.1–40.6) | 0.96 | 0.98 (0.96–1 .00) | NA |
| R1 retest | 18.5 (9.2–37.5) | 0.70 | NA | 0.99 (0.99–1.00) |
| 24-hours post-ICH | ||||
| R1, R2 | 20.4 (11.5–40.5), 23.2 (12.4–46.5) | 0.67 | 0.98 (0.97–1 .00) | NA |
| R1 retest | 21.6 (11.7–42.4) | 0.67 | NA | 0.99 (0.98–1.00) |
| CT, MRI (FLAIR) | 16.9 (9.8–37.1), 16.4 (11.8–36.1) | 0.80 | NA | 0.99 (0.98–1.00) |
cc indicates cubic centimeters; CI, confidence interval; CT, computed tomography; FLAIR, fluid-attenuated inversion recovery sequence; ICC, intraclass correlation coefficient; IQR, interquartile range; MRI, magnetic resonance imaging; NA, not applicable; R1, Rater 1; and R2, Rater 2.
All interrater and intrarater ICCs were excellent at both baseline and 24-hours post-ICH (Table 1, Data Supplement Tables III and IV). For PHE, at baseline and 24-hours post-ICH, interrater ICCs were 0.98 (0.96–1.00) and 0.98 (0.97–1.00); intrarater ICCs were 0.99 (0.99–1.00) and 0.99 (0.98–1.00).
The Bland-Altman plots of interrater consistency of PHE measurements revealed a bias of −0.5 cc (SD, 5.4) and −3.2 cc (SD, 5.0) at baseline and 24-hours post-ICH, respectively (Figure 2, Data Supplement Figures VIII and IX). There was a single outlier at both baseline and 24 hours that corresponded to a 118 cc frontal lobe hemorrhage, which was the most difficult to measure due to marked irregularity and diffuse swelling that made delineation of boundaries challenging.
Figure 2.
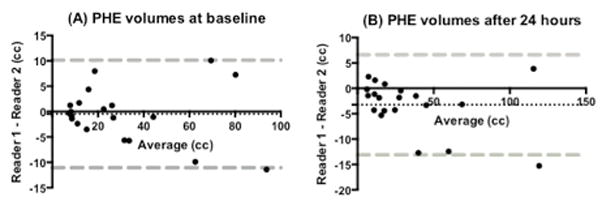
Bland-Altman plots of interrater consistency of PHE measurements at baseline and 24-hours post-ICH. Dashed black line represents the bias (mean of the difference between measurements). Dashed gray lines represent the limits of agreement (mean ± 1.96 SD).
In the eighteen subjects with both CT and MRI scans available, median time between scans was 17 hours (8–37 hours). PHE volumes on CT and MRI did not differ (Table 1). There was a high correlation between modalities. In regression analysis, R2 for the CT and MRI measurements was 0.98, slope 1.05 (95 CI%, 0.97–1.12), intercept −1.00, and p<0.0001. The ICC between the CT and MRI measurements was 0.99 (95%CI 0.98–1.00). Bland-Altman analysis revealed that CT and MRI-based PHE measurements were consistent, with a small bias of 0.07 cc (SD, 2.4) (Figure X Data Supplement).
Discussion
Our approach for CT-based PHE measurement capitalizes on a quantitative edge-detection algorithm plus knowledge of the pathophysiology of PHE formation to delineate boundaries. The method has excellent interrater and intrarater reliability and measurements are comparable to those obtained on MRI. We observed an acceptably small bias in measuring PHE, considering a difference in 10 cc of PHE on admission tripled the odds of poor discharge outcome in one study.4 This could represent a method for researchers to explore further the prognostic significance of PHE and test therapies that reduce secondary injury.
Our data show decreased interrater consistency in PHE measurements for very large hemorrhages in which there is diffuse swelling. Appelboom et al. showed PHE volume was an independent predictor of outcome only in patients with ICH volumes ≤ 30 cc.4 Therefore, secondary injury and PHE may be less likely to be clinically relevant in ICH patients with very large hemorrhages. These patients are likely to have a poor prognosis regardless as a result of the direct mechanical effects from the large hemorrhage (primary injury). In this pool of subjects with moderate ICH volumes our method had excellent interrater and intrarater consistency. Our findings should be confirmed in a larger, prospective dataset.
Supplementary Material
Acknowledgments
Sources of Funding
S.U.: Leon Rosenberg, MD Medical Student Research Fund in Genetics (Yale School of Medicine) and 2014 Student Scholarship in Cerebrovascular Disease and Stroke (American Heart Association’s Stroke Council)
L.A.B.: NIH-K12-NS049453
W.T.K.: NINDS K23NS076597
Footnotes
Disclosures
Dr Rosand has a consulting relationship with Boehringer Ingelheim.
References
- 1.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 3.Li N, Liu YF, Ma L, Worthmann H, Wang YL, Wang YJ, et al. Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke. 2013;44:658–663. doi: 10.1161/STROKEAHA.112.673590. [DOI] [PubMed] [Google Scholar]
- 4.Appelboom G, Bruce SS, Hickman ZL, Zacharia BE, Carpenter AM, Vaughan KA, et al. Volume-dependent effect of perihaematomal oedema on outcome for spontaneous intracerebral haemorrhages. J Neurol Neurosurg Psychiatry. 2013;84:488–493. doi: 10.1136/jnnp-2012-303160. [DOI] [PubMed] [Google Scholar]
- 5.Yeatts SD, Palesch YY, Moy CS, Selim M. High dose deferoxamine in intracerebral hemorrhage (hi-def) trial: Rationale, design, and methods. Neurocrit Care. 2013;19:257–266. doi: 10.1007/s12028-013-9861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carhuapoma JR, Hanley DF, Banerjee M, Beauchamp NJ. Brain edema after human cerebral hemorrhage: A magnetic resonance imaging volumetric analysis. J Neurosurg Anesthesiol. 2003;15:230–233. doi: 10.1097/00008506-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Zazulia AR, Videen TO, Diringer MN, Powers WJ. Poor correlation between perihematomal mri hyperintensity and brain swelling after intracerebral hemorrhage. Neurocritical Care. 2011;15:436–441. doi: 10.1007/s12028-011-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansing LH, Kaznatcheeva EA, Perkins CJ, Komaroff E, Gutman FB, Newman GC. Edema after intracerebral hemorrhage: Correlations with coagulation parameters and treatment. J Neurosurg. 2003;98:985–992. doi: 10.3171/jns.2003.98.5.0985. [DOI] [PubMed] [Google Scholar]
- 9.Levine JM, Snider R, Finkelstein D, Gurol ME, Chanderraj R, Smith EE, et al. Early edema in warfarin-related intracerebral hemorrhage. Neurocrit Care. 2007;7:58–63. doi: 10.1007/s12028-007-0039-3. [DOI] [PubMed] [Google Scholar]
- 10.Volbers B, Staykov D, Wagner I, Dorfler A, Saake M, Schwab S, et al. Semi-automatic volumetric assessment of perihemorrhagic edema with computed tomography. Eur J Neurol. 2011;18:1323–1328. doi: 10.1111/j.1468-1331.2011.03395.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, Pan W, Kranz PG, Hailey CE, Williamson RA, Sun W, et al. Predictors of late neurological deterioration after spontaneous intracerebral hemorrhage. Neurocrit Care. 2013;19:299–305. doi: 10.1007/s12028-013-9894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walberer M, Blaes F, Stolz E, Muller C, Schoenburg M, Tschernatsch M, et al. Midline-shift corresponds to the amount of brain edema early after hemispheric stroke--an mri study in rats. J Neurosurg Anesthesiol. 2007;19:105–110. doi: 10.1097/ANA.0b013e31802c7e33. [DOI] [PubMed] [Google Scholar]
- 13.Aksoy D, Bammer R, Mlynash M, Venkatasubramanian C, Eyngorn I, Snider RW, et al. Magnetic resonance imaging profile of blood-brain barrier injury in patients with acute intracerebral hemorrhage. J Am Heart Assoc. 2013;2:e000161. doi: 10.1161/JAHA.113.000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


