Supplemental Digital Content is available in the text
Keywords: Cox regression, Kaplan–Meier analysis, plasmablastic lymphoma, prognostic factor
Abstract
Background:
Plasmablastic lymphoma (PBL) is a B-cell malignancy associated with human immunodeficiency virus (HIV). PBL could also influence the HIV-negative patients. The study aimed to identify prognostic factors for survival among Chinese PBL patients.
Materials and methods:
Eligible patients from literature and Peking Union Medical College Hospital (PUMCH) were included in this study. Clinical characteristics and immunophenotypic data were extracted. Kaplan–Meier curve was used to describe the survival status. Cox regression was used for multivariate analysis.
Results:
A total of 60 Chinese PBL patients were included, including 54 patients from 36 published articles and 6 new patients that have not been reported. The median overall survival was 7 months (95% confidence interval 3.853–10.147 months). An overwhelming majority (79.31%) of the included cases were Ann Arbor stage IV patients. All the Chinese PBL patients were HIV-negative; 46.81% were Epstein-Barr virus-positive. CD38, CD138, or MUM1 was positively expressed in more than 80% of patients; CD20 expression was also found in 22.03% of cases. Kaplan–Meier curve revealed obvious differences in patient survival between patients in primary stages and advanced stages, as well as between patients with kidney involvement and those without kidney involvement. Cox regression analysis indicated that stage and age were 2 prognostic factors for patient survival.
Conclusions:
Advanced stage might be associated with poor prognosis among PBL HIV-negative patients in Chinese.
1. Introduction
Plasmablastic lymphoma (PBL) is an aggressive B-cell malignancy that highly correlated with human immunodeficiency virus (HIV).[1] Recently, PBL is also identified as a subtype of non-Hodgkin lymphoma (NHL), and it is estimated incidence of PBL accounts for approximately 5% of all HIV-positive NHL cases.[2] Despite the strong relationship with HIV infection, PBL could also influence the HIV-negative patients.[3] Previously, a case report has recorded the characteristics of the first PBL case in an HIV-negative individual.[4] It is speculated the HIV-negative PBL cases might derive from previous lymphoproliferative or autoimmune disorders.[5] However, incidence of HIV-negative PBL is still unclear.
With regard to the management of PBL, the common treatments are chemotherapy, radiotherapy with or without surgical excision, or the combination of chemotherapy and radiotherapy. Despite these advances, PBL has a poor prognosis, with a relapse rate of approximately 60% in the first year.[6] Therefore, many studies have been conducted to identify prognostic factors in PBL patients, mostly in HIV-positive patients.[7,8] Several factors such as CD4+ count, HIV infection, Epstein-Barr virus (EBV) status, clinical stage, and the response to chemotherapy have been suggested to be associated with survival in PBL patient[7,9–11]; however, effects of the above factors on the prognosis are not consistent in different individual reports. Thus, further investigation on patient survival is imperatively required.
Currently, most of the data available on PBL are from case reports and case series owing to the low incidence.[8] Furthermore, different populations might generate different outcomes.[8,12] In the present study, we systematically analyzed the demographic, clinicopathological, etiological, and immunophenotypic characteristics of Chinese PBL patients by reviewing the published cases and using several new cases that have never been reported. In addition, we also tried to identify prognostic factors for patient's survival. This study aimed to provide a more comprehensive understanding of this rare disease in China.
2. Patients and methods
2.1. Literature retrieval and patient collection
Electronic databases including PubMed and Web of Science online database were retrieved from 1997 to March 2015, using the key search terms: “plasmablastic lymphomaor” AND “Chinese”; meanwhile, Chinese CNKI, Wanfang, and Vip databases were also retrieved using the key search term: “jiang mu xi bao lin ba liu” to search studies published in Chinese.
In addition, we also collected the eligible patients in Peking Union Medical College Hospital (PUMCH).
2.2. Informed consent and ethical approval
The enrolled patient information was approved by the Ethics Committee of PUMCH, and all the patients signed the informed consent.
2.3. Data extraction
From each included study, the following information in each PBL patient was extracted and recorded: demographic data (such as age, sex), clinicopathological data (clinical stage, organ involvement), expression profiles of immunophenotypic markers (CD38, CD138, MUM1, CD20, CD79α, CD56, Ki-67, Bcl-6, CD3, PAX-5, and ALK), etiological factors (EBV infection, hepatitis B virus infection, herpesvirus-8 (HHV-8) infection, immunosuppression), treatment strategy (chemotherapy, radiotherapy, and surgery), and overall survival (defined as the period from diagnosis to death or latest follow-up).
2.4. Statistical analysis
Kaplan–Meier curve was used to describe the survival status.[13] Cox regression was used for multivariate analysis.[14] The software Stata 12.0 (Stata Corp, College Station, TX) was used for data analysis. P value <0.05 was considered as the cut-off for statistical significance.
3. Results
3.1. Study selection and case inclusion
A total of 54 PBL eligible patients in 36 studies were included in this study, including 45 cases in 27 Chinese studies, and 9 cases in 9 English studies (Supplemental Table 1). Moreover, 6 additional PBL cases from PUMCH that have not yet been published were also included (Supplement Table 2). Thus, there included a total of 60 cases in this study.
3.2. Clinical characteristics of the patients
The demographic data and clinicopathological characteristics of the 60 Chinese PBL patients are presented in Table 1. Sex information is provided in 59 PBL patients, and the ratio of male (38) to female (21) was 1.8:1. Patients younger than 30 years had a very low proportion (5.00%), whereas middle-age patients (30–60 years’ old) accounted for a high percentage (60%). Majority (79.31%) of the included patients were in stage IV based on Ann Arbor classification.
Table 1.
Demographic, clincipathological, etiological, and genetic characteristics, as well as treatments of the included plasmablastic lymphoma cases in this study.
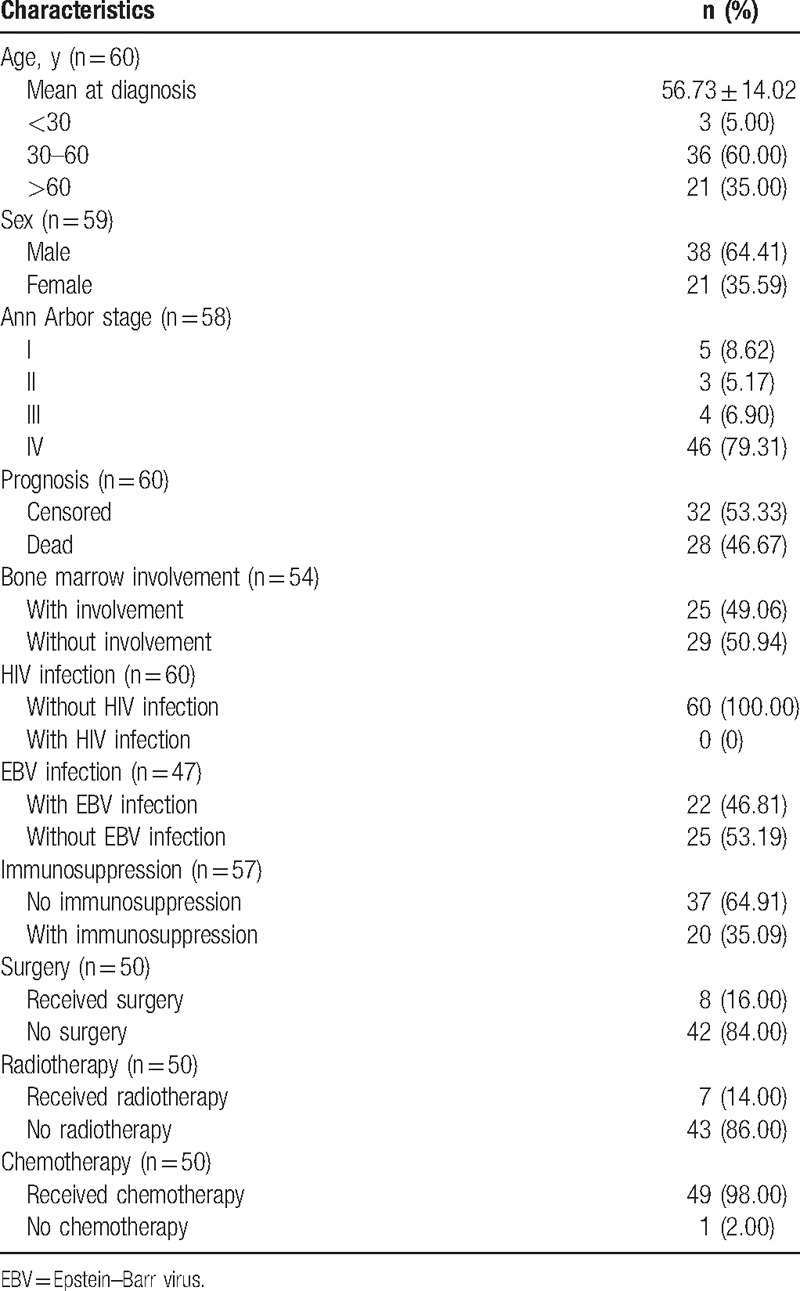
Notably, all the included PBL patients were HIV-negative. Meanwhile, 22 of 47 cases (46.81%) were infected by EBV (Table 1). All the patients included were HHV-8-negative.
Detailed treatments were reported among 50 patients: 8 of them underwent surgery (16%), 7 of them received radiotherapy (14%), and 49 (98%) received chemotherapy (Table 1). For those receiving chemotherapy, most of them underwent regimens like CHOPE (etoposide, cyclophosphamide, doxorubicin, vincristine, prednisone) or CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone). Other regimens were also applied in some patients, such as HyperCVAD (high-dose cyclophosphamide + vincristine + doxorubicin + dexamethasone or methotrexate + cytosine arabinoside), R-CHOP (rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone), and ESHAP (etoposide + cis-platinum + methylprednisolone + cytosine arabinoside). Based on description of PBL cases in the original article, immunosuppression was empirically speculated in 20 of 57 (35.09%) PBL patients, including 7 hepatitis B-infected patients, 7 elder patients (older than 70 years) and 6 patients receiving radiotherapy or chemotherapy because of other tumors (e.g., breast cancer, cervical cancer).
3.3. Immunohistochemical examination
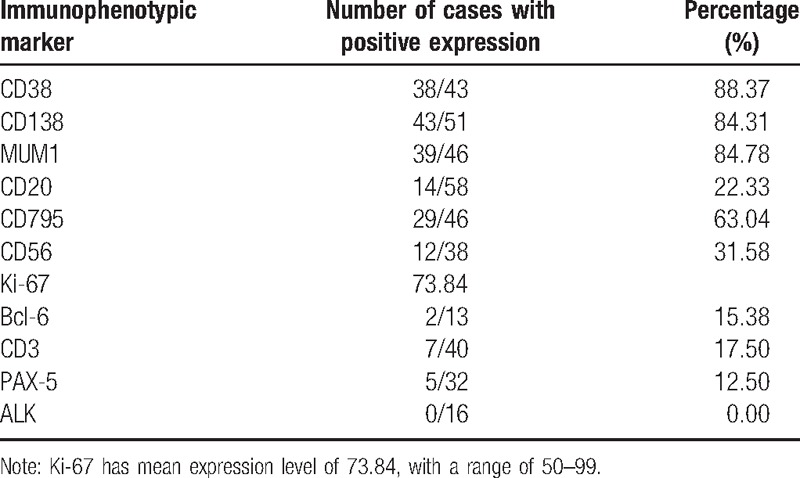
Immunohistochemistry revealed that CD38, CD138, and MUM1 proteins were positively expressed in >80% of the enrolled patients; CD20, CD79α, CD56, BCL-6, CD3, and PAX-5 were positively expressed in >10% patients. No patient had positive expression with ALK among the 16 cases undergoing examination. The mean expression level of Ki-67 was 73.84, with a range of 50 to 99 (Table 2).
Table 2.
Immunophenotyping of the included plasmablastic lymphoma cases.
3.4. Cytogenetic examination
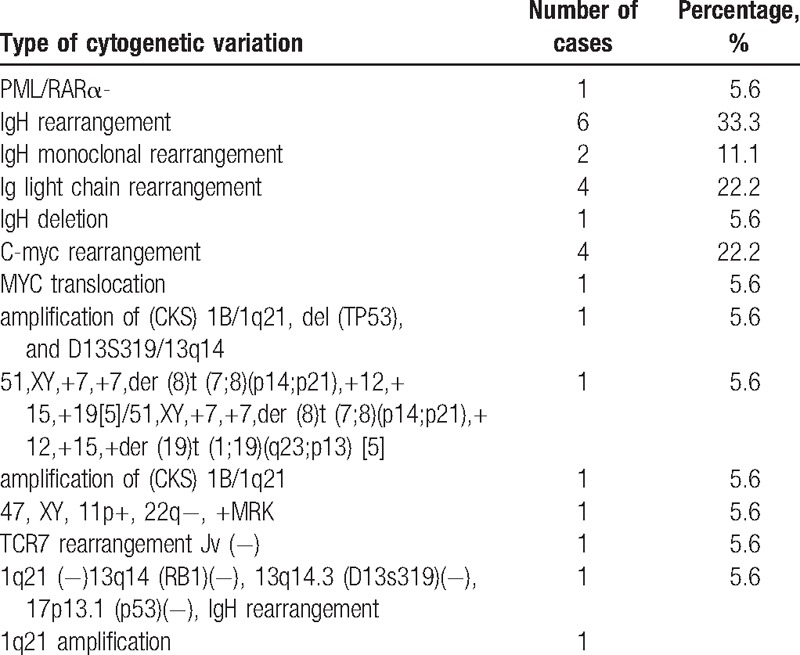
Among the 60 Chinese cases, 18 underwent cytogenetic examination. There were a total of 14 types of cytogenetic variations, and the type of IgH rearrangement was predominant, accounting for 33.3% for all the cases (Table 3).
Table 3.
Types of cytogenetic variations and percentage of cases with each variation type among all the 18 cases undergoing cytogenetic examination.
3.5. Prognostic analysis
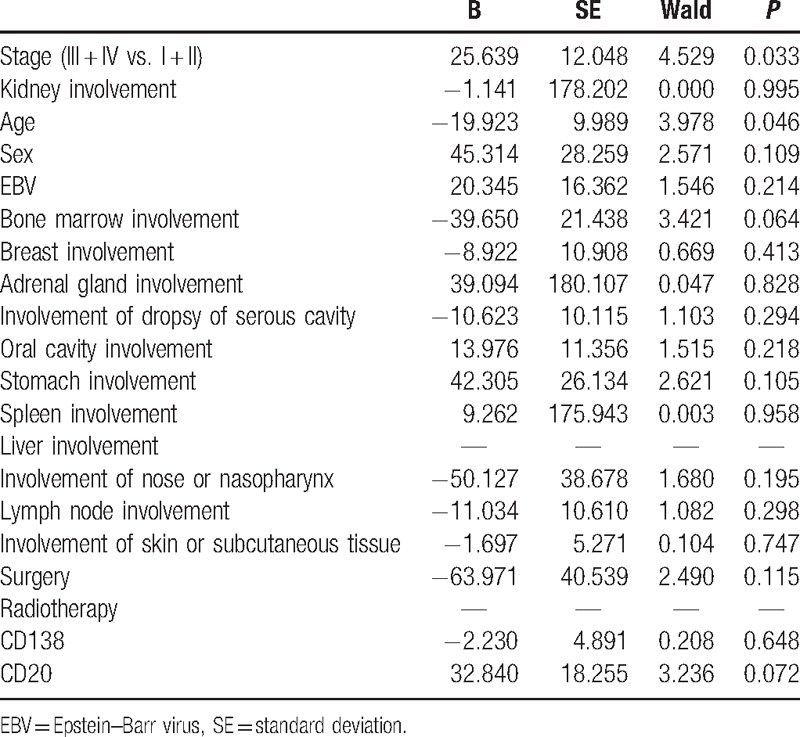
Overall, 28 of the 60 PBL patients were found dead (Table 1). The median overall survival was 7 months (95% confidence interval 3.853–10.147). Kaplan–Meier curves showed that the survival between PBL patients in primary stages (I and II) and those in advanced stages (III and IV) (Fig. 1A), and between patients with and without kidney involvement were obviously different (Fig. 1B). Cox regression analysis indicated that stage and age were 2 significant prognostic factors for patient survival (P < 0.05, Table 4).
Figure 1.
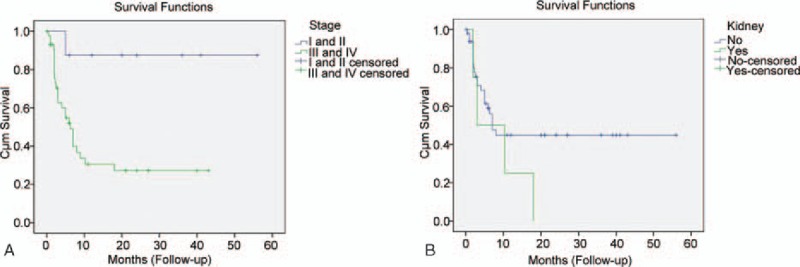
Kaplan–Meier analysis of patient survival with plasmablastic lymphoma stage (A) and kidney involvement (B).
Table 4.
Prognostic factors by multivariate Cox regression analysis.
4. Discussion
As far as we know, this is the first systematical review to summarize the PBL characteristics and identify the prognostic factors among Chinese population. In this study, we collected 60 Chinese PBL cases from literature retrieval or hospital, and analyzed the demographic and clinicopathological characteristics. Furthermore, we also performed survival analysis with some of the demographic and clincipathological characteristics, and found that the overall survival of PBL patients in stages I and II dramatically differed with those in stages III and IV, indicating that disease stage was a prognostic factor of PBL.
A weak male predominance (male/female ratio of 1.8) was noticed upon the PBL cases included here, which was inconsistent with a previous finding in HIV-associated PBL that reports a male/female ratio of 4:1.[7] More strikingly, all the Chinese PBL cases included here were HIV-negative, suggesting HIV might be more easily affected in male patients with PBL. Actually, most PBL cases are HIV-positive. Furthermore, Castillo et al[10] have compared the clinicopathological characteristics between 157 HIV-positive and 71 HIV-negative PBL cases, and found that HIV-positive patients had a better response to chemotherapy, as well as longer survival. This finding suggests that HIV might influence the survival rate of PBL. However, as all the Chinese PBL cases included here were HIV-negative, we needed more evidence to confirm the role of HIV among Chinese population. In addition to HIV, both of EBV and HHV-8 may also play key roles in the pathogenesis of PBL. Infection with these 2 viruses has been reported in many PBL patients, especially EBV.[15] Therefore, it could be understandable that the EBV infection rate in this study was as high as 47.92%. However, HHV-8 infection was not investigated in our study. Meanwhile, hepatitis B virus infection was observed in 11.7% (7 cases) of the total PBL cases, accounting for more than one-third of the patients with immunosuppression (20 cases). Previously, 2 HIV-negative PBL patients have been reported with a history of hepatitis B.[16,17] Another study also supposes that hepatitis B or C may also have a more important role than HIV-induced immunosuppression in the development of non-AIDS-defining cancers.[18] Thus, our finding might validate their speculation that the hepatitis B-induced immunosuppression has an important role in the development of HIV-negative PBL in Chinese.
PBL cells are known to be positive to plasma cell markers, such as CD138 (syn-1, a member of the transmembrane heparin sulphate proteoglycan family),[19–21] MUM1/IRF4 (multiple myeloma oncogene 1/interferon regulatory factor 4),[19,22] and CD38 (cyclic adenosine diphosphate ribose hydrolase),[19,22] whereas they are negative or weakly negative to B-cell markers as CD20 or CD45.[8,23] Many patients included in the present study have shown the immunophenotype. In accordance with these previous findings, the immunophenotypic markers as CD38, CD138, or MUM1 were positively expressed in >80% of PLB cases, whereas CD20 expression was only found in 22.03% of cases.
Based on Cox regression analysis, the radiotherapy and surgery had no different effect on patient survival. Therefore, an overwhelming majority of patients underwent chemotherapy and most of them received CHOPE or CHOP, which is the most commonly used regimen at present.[24] Additionally, other regimens were also employed in some cases.
Here, various types of cytogenetic variations were also detected in PBL cases. Among them, IgH gene rearrangement occurred in the most cases, and c-Myc gene rearrangement also had a relatively high incidence. Actually, IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas.[25] In addition, gene fusion (PML-RARα), robertsonian translocation (der (8)t(7;8)(p14;p21), der(19)t(1;19)(q23;p13) were also found in the PBL cases in this study. MYC translocation has been reported in plasmablastic lymphoma.[25,26]PML/RARα fusion gene has an essential role in the leukemogenesis,[27,28] and here it was reported in PBL for the first time.
On the contrary, the Kaplan–Meier curve revealed an obvious difference on overall survival between PBL patients in the primary stages (I and II) and those in the advanced stages (III and IV), as well as between PBL patients with kidney involvement and those without kidney involvement. Further Cox regression analysis showed that disease stage was a significant prognostic factor of survival in HIV-negative PBL patients, suggesting different stages might have different survival outcomes. Previously, Castillo et al[7] reported that the early clinical stage was associated with a longer survival in a study of 248 PBL cases (including 157 HIV-positive cases) by literature search, and they further confirmed this finding in a recent study involving 47 HIV-positive cases diagnosed in Europe, United States, and South America.[29] Recently, Liu et al[11] investigate multiple factors that may affect the overall survival in 114 HIV-negative PBL cases, and also find that Ann Arbor stage IV is a poor prognostic factor of overall survival in addition to immunosuppression status, EBV negativity, and refractory to treatment in HIV-negative PBL. However, in an earlier study including 112 HIV-positive PBL cases, Castillo et al[8] reported no association between neither sex, CD4+ count, viral load, clinical stage, EBV status, primary site of involvement, nor use of CHOP chemotherapy regimen with survival. In addition, we should note that in our study, a majority of the patients were in stage IV, which might influence the statistical analysis of this factor and overstress its relationship with the survival. Moreover, the large proportion of censored data may contribute to a few prognostic factors in the present study, and substantial heterogeneity might exist among the included cases, which could probably lead to a bias in the result. Thus, it remains confusing whether clinical stage is associated with survival, and human race, HIV infection status, and number of included cases may contribute to the discrepancies among different studies.
5. Conclusions
Here, we systematically described the demographic characteristics, clinicopathological features, expression profiles of immunophenotypic markers, etiological factors, and treatment strategy of the PBL cases among Chinese population for the first time, and one of our most striking findings was that all the Chinese PBL cases were HIV-negative. Moreover, the advanced clinical stage was associated with the poor survival and identified as a prognostic factor for HIV-negative PBL patients in Chinese.
Supplementary Material
Footnotes
Abbreviations: CHOP = cyclophosphamide, doxorubicin, vincristine, prednisone, CHOPE = etoposide, cyclophosphamide, doxorubicin, vincristine, prednisone, EBV = Epstein-Barr virus, HHV-8 = herpesvirus-8, HIV = human immunodeficiency virus, NHL = non-Hodgkin lymphoma, PBL = plasmablastic lymphoma, PUMCH = Peking Union Medical College Hospital.
Authors’ contributions: LH performed the statistical analysis; XH carried out the study, together with MD, and collected important background information; DZ drafted the manuscript; WZ conceived of this study, and participated in the design and helped to draft the manuscript. All authors read and approved the final manuscript.
MD and LH are co-first authors.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Chapman J, Gentles AJ, Sujoy V, et al. Gene expression analysis of plasmablastic lymphoma identifies down regulation of B cell receptor signaling and additional unique transcriptional programs. Leukemia 2015;29:2270–3. [DOI] [PubMed] [Google Scholar]
- [2].Gong J, Alkan S, Anand S. A case of cutaneous plasmablastic lymphoma in HIV/AIDS with disseminated cryptococcus. Case Rep Oncol Med 2013;2013:862585–1862585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Choi SY, Cho YA, Hong SD, et al. Plasmablastic lymphoma of the oral cavity in a human immunodeficiency virus-negative patient: a case report with literature review. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;117:e115–20. [DOI] [PubMed] [Google Scholar]
- [4].Scheper MA, Nikitakis NG, Fernandes R, et al. Oral plasmablastic lymphoma in an HIV-negative patient: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:198–206. [DOI] [PubMed] [Google Scholar]
- [5].Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood 2015;125:277–81. [DOI] [PubMed] [Google Scholar]
- [6].Rafaniello Raviele P, Pruneri G, Maiorano E. Plasmablastic lymphoma: a review. Oral diseases 2009;15:38–45. [DOI] [PubMed] [Google Scholar]
- [7].Castillo JJ, Winer ES, Stachurski D, et al. Prognostic factors in chemotherapy-treated patients with HIV-associated plasmablastic lymphoma. The oncologist 2010;15:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: Lessons learned from 112 published cases. Am J Hematol 2008;83:804–9. [DOI] [PubMed] [Google Scholar]
- [9].Bohlius J, Schmidlin K, Costagliola D, et al. Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study group. Prognosis of HIV-associated non-Hodgkin lymphoma in patients starting combination antiretroviral therapy. Aids 2009;23:2029–37. [DOI] [PubMed] [Google Scholar]
- [10].Castillo JJ, Winer ES, Stachurski D, et al. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma 2010;51:2047–53. [DOI] [PubMed] [Google Scholar]
- [11].Liu M, Liu B, Liu B, et al. Human immunodeficiency virus-negative plasmablastic lymphoma: A comprehensive analysis of 114 cases. Oncol Rep 2015;33:1615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Toracchio S, Kozinetz CAKillen DE, Sheehan AM, et al. Variable frequency of polyomavirus SV40 and herpesvirus EBV in lymphomas from two different urban population groups in Houston, TX. J Clin Virol 2009;46:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- [14].Cox DR. Regression models and life-tables. J Royal Stat Soc Series B (Methodological) 1972;34:187–220. [Google Scholar]
- [15].Cioc AM, Allen C, Kalmar JR, et al. Oral plasmablastic lymphomas in AIDS patients are associated with human herpesvirus 8. Am J Surg Pathol 2004;28:41–6. [DOI] [PubMed] [Google Scholar]
- [16].Lee OJ, Kim KW, Lee GK. Epstein–Barr virus and human immunodeficiency virus-negative oral plasmablastic lymphoma. J Oral Pathol Med 2006;35:382–4. [DOI] [PubMed] [Google Scholar]
- [17].Scheper MA, Nikitakis NG, Fernandes R, et al. Oral plasmablastic lymphoma in an HIV-negative patient: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2005;100:198–206. [DOI] [PubMed] [Google Scholar]
- [18].Pantanowitz L, Schlecht HP, Dezube BJ. The growing problem of non-AIDS-defining malignancies in HIV. Curr Opin Oncol 2006;18:469–78. [DOI] [PubMed] [Google Scholar]
- [19].Vega F, Chang C-C, Medeiros LJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Modern Pathol 2005;18:806–15. [DOI] [PubMed] [Google Scholar]
- [20].Ridley R, Xiao H, Hata H, et al. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood 1993;81:767–74. [PubMed] [Google Scholar]
- [21].Lin F, Zhang K, Quiery AT, Jr, et al. Plasmablastic lymphoma of the cervical lymph nodes in a human immunodeficiency virus-negative patient: a case report and review of the literature. Arch Pathol Lab Med 2004;128:581–4. [DOI] [PubMed] [Google Scholar]
- [22].Nicol I, Boye T, Carsuzaa F, et al. Post-transplant plasmablastic lymphoma of the skin. Br J Dermatol 2003;149:889–91. [DOI] [PubMed] [Google Scholar]
- [23].Teruya-Feldstein J, Chiao E, Filippa D, et al. CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and-negative patients. Ann Oncol 2004;15:1673–9. [DOI] [PubMed] [Google Scholar]
- [24].Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: Lessons learned from 112published cases †. Am J Hematol 2008;83:804–9. [DOI] [PubMed] [Google Scholar]
- [25].Valera A, Balagué O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol 2010;34:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Taddesse-Heath L, Meloni-Ehrig A, Scheerle J, et al. Plasmablastic lymphoma with MYC translocation: evidence for a common pathway in the generation of plasmablastic features. Modern Pathol 2010;23:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Z-G, Rivi R, Delva L, et al. Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARalpha independent manner. Blood 1998;92:1497–504. [PubMed] [Google Scholar]
- [28].Wang Y, Jin W, Jia X, et al. Transcriptional repression of CDKN2D by PML/RARα contributes to the altered proliferation and differentiation block of acute promyelocytic leukemia cells. Cell Death Dis 2014;5:e1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Castillo JJ, Furman M, Beltrán BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma. Cancer 2012;118:5270–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


