Abstract
Background:
Nutraceuticals containing cis-9-cetylmyristoleate (CMO) are used to improve knee pain despite the lack of placebo-controlled studies in humans. The aim of the study was to explore the minimal effective dose of CMO for relieving knee joint pain.
Methods:
Twenty-eight subjects with mild degree arthritic knee joint pain were randomized into 4 groups; groups A, B, and C that contained 100%, 80%, and 62.4% of fatty acid component with 12.5% of CMO, and control group D (starch 100%). The pain intensity, functional disability, and the Patient Global Impression of Change (PGIC) were assessed for a 12-week ingestion period.
Results:
Compared to group D (n = 6), there were significant differences in pain score in group A (n = 7, P = 0.005) and group C (n = 7, P = 0.012), but not significant in group B (n = 6, P = 0.180). Western Ontario and McMaster Universities Arthritis (WOMAC) score decreased significantly in groups A and C. The PGIC was positive in the majority (>50%) in groups A, B, and C, whereas negative in 83.3% in group D (control).
Conclusion:
CMO is effective in alleviating knee pain in persons with mild degree arthritis of the knee joint, at an effective dose of 62.4%.
Keywords: arthritis, cis-9-cetylmyristoleate, fatty acid complex, health supplements, knee joint pain
1. Introduction
Knee joint pain is one of the most common ailments in adults. The prevalence of self-reported knee pain was 30.9% of adult population ≥40 years in a cross-sectional survey,[1] and has increased significantly over 20 years.[2] Although knee joint pain is most often due to osteoarthritis (OA), any of the knee joint structures including ligaments, tendons, cartilage, and bursae can be sources of pain that can hinder work and diminish quality of life.[3]
Various modalities to manage knee joint pain range from conservative management, such as exercise, oral medication including nonsteroidal antiinflammatory agents, and joint injection, to surgical treatment. Health products also have been widely used, and efforts to find novel effective products with less side effects have increased. The efficacy of the 2 most popular health products, glucosamine and chondroitin sulfate, remains contentious despite extensive research,[4–11] and various guidelines have deferred conclusion on their appropriateness for knee joint pain. In search of a substitute, other agents including collagen derivatives, ginger, and herbal medicines have been studied and are in use, along with beef tallow extract including cis-9-cetylmyristoleate (CMO).[12–14]
CMO is a naturally occurring fatty acid complex (FAC) existing in an oleate form. It was first extracted from National Institutes of Health Swiss albino mice resistant to arthritis. Subsequent animal studies and clinical trials showed conflicting results for CMO in preventing and alleviating symptoms of arthritis.[15–20] CMO improves joint pain and may help prevent further joint destruction by alleviating joint inflammation by suppressing cyclooxygenase and lipoxygenase pathway of arachidonic acid metabolism, and subsequently via reduced generation of the pain mediators, such as prostaglandin and leukotriene.[20]
Although nutraceuticals containing CMO have been used for pain and inflammation relief, there have only been a few studies or randomized controlled trials (RCTs) comparing its efficacy with placebo in humans.[17–19,21] The current recommended daily CMO dose has been based on only 1 previous RCT,[18] which used an arbitrary dose likely based on anecdotal evidence in humans with those provided in existing health supplements.
This explorative pilot study sought to determine the minimal effective dose of CMO by comparing the pain reduction effect of different doses in subjects with mild degree arthritic knee joint pain.
2. Methods
This small pilot, double-blind, RCT was approved by the Institutional Review Board of the Seoul National University Hospital (No. 1307-098-506), a large civilian teaching institution in South Korea, and was registered in ClinicalTrials. gov (NCT02800759). It was conducted in accordance with the ethical principles of the Declaration of Helsinki, and written informed consent was obtained from all participants before initiating study.
Inclusion criteria were subhealthy persons >18 years of age and knee joint pain with a 0–10 numerical rating scale (NRS) pain score ≤4. Exclusion criteria were: current medication use related to arthritis; current use of FAC containing products; clinical or radiological diagnosis as moderate degree arthritis accompanied by periarticular spur formation, irregular joint margin, and/or subchondral cyst; previous history of knee surgery; pregnant, breastfeeding, or practicing contraception with reliable methods, or not accepting our guidelines during the research periods; major pain other than knee joint pain; current treatment of gastritis or gastric ulcer; abnormal screening laboratory results; major cardiac, renal disease, or disability that could affect adverse effect assessment or interfere with study completion when enrolled; history of major procedures or operations that might affect study results; enrolment in another clinical trial or human application testing; and judged as unsuitable for human application testing.
2.1. Materials
The product containing FAC with 12.5% CMO in the form of capsules (JOINTRUS) was provided by SFC Bio, Inc (Seoul, South Korea). The test products I, II, and III were composed of 62.4%, 80%, and 100% of FAC containing 12.5% CMO, mixed with 37.6%, 20%, and 0% of starch, respectively. Test product III (100% FAC with 12.5% CMO) contained 62.5 mg CMO. Detailed training regarding study medication packaging, dispensing, administration, storage, and accountability was provided in a separately required training module by the Medical Research Collaborating Center in the study hospital.
2.2. Participant enrollment and randomization
This study included a 7-day baseline screening period (visit 1) and was followed by randomization (visit 2), a 12-week ingestion period with clinic visits at week 4 (visit 3), 8 (visit 4), and 12 (visit 5) after starting ingestion, and a 4-week postingestion safety follow-up.
At visit 1, 1 pain physician managed primary recruitment of subject on his or her 1st visit, according to the inclusion criteria. Participant information with past medical history, vital signs, physical examination, 12-lead electrocardiography, and laboratory tests including pregnancy was obtained. During the screening period, enrolled subjects had to record the NRS pain score each day in a diary that was kept. We only enrolled the participants who recorded their diary at least 4 days a week while having an average NRS pain score ≤4, which was used as a baseline NRS pain score. Two pain physicians who did not participate in treatment determined the final enrollment by crosschecking the exclusion criteria.
At visit 2, each subject was blindly randomized into 1 of the 4 groups (group A, B, C, or control group D), using the table of random sampling numbers that were created and managed by the Medical Research Collaborating Center in the hospital. Group A was provided with test product III (FAC [12.5% CMO] 100%, starch 37.6%); group B test product II (FAC [12.5% CMO] 80%, starch 20%); group C test product I (FAC [12.5% CMO] 62.4%); and group D control product (starch 100%). Subjects were encouraged to record their daily diary throughout the whole study period. They were instructed to take the product, 2 capsules at a time, twice daily (morning and evening) 30 minutes after each meal. The daily dose of test product III was 2 capsules at a time, twice daily; product III contained 250 mg of CMO and was equivalent to the current recommended daily dose determined by a previous study.[18] Corresponding products were distributed at visit 2 (randomization and registration day), and visits 3 and 4. The residual product was collected at visits 3, 4, and 5. Subject compliance was calculated (%) as (actual ingestion number/[last ingestion date − registration date]). We included the subjects with ≥80% compliance according to the formula in our final analysis.
The study proceeded in a double-blinded manner; hence, participants and physicians could not be aware which group they belong to, because all capsules were identical in size, shape, and color. Information about the group randomization was unavailable during the study period.
2.3. Assessment of pain, function, satisfaction, and safety
To evaluate the pain relieving effect of group B (FAC, 80%) and C (FAC, 62.4%) after 3 months of ingestion in participants, we hypothesized that pain reduction in group B and C would not be inferior compared to group A (FAC, 100%), and would be significantly superior than the control group (group D). The primary outcome measure of this study was to compare the pain relieving effect of 80% and 62.4% FACs with 100% FAC or placebo, by evaluating the decrease (%) of pain at weight bearing, after 3 month (visit 5) of ingestion compared to baseline. Daily pain was assessed with the NRS score, composed of an 11-point scale from 0 (no pain) to 10 (the worst pain possible); pain intensity at weight bearing was self-checked every evening, from visit 1 to 5 throughout the 12-week drug ingestion period. This self-checked scoring was reviewed at 3 4-week visits (visits 3, 4, and 5). At least 4 entries in the daily diaries were needed within the past week to calculate a mean score.
Secondary endpoints included difference in NRS pain score from baseline within groups, change of functional disability state, and Global Impression of Change at visit 5. For evaluating functional disability state, the Korean version of Western Ontario and McMaster Universities Arthritis (WOMAC)[22] was conducted at each visit. The WOMAC score measures 5 items for pain (score range 0–20), 2 for stiffness (score range 0–8), and 17 for functional limitation (score range 0–68).[23] At visit 5, Clinical Global Impression of Change by the physician and Patient Global Impression of Change (PGIC) by the patients were used to evaluate the subjective perception of decrease in pain intensity. Clinical Global Impression of Change and PGIC are self-administered instruments that measure change in a subject's overall status on a scale ranging from 1 (very much improved) to 7 (very much worse). The recall period was from the start of study medication.[24]
During the ingestion period, all subjects were advised to return at week 4 (visit 3), 8 (visit 4), and 12 (visit 5) from treatment initiation, while keeping a constant visiting interval with an error range of less than ±3 days. Monitoring for concomitant medication use and side effects was performed during the study period and finally checked at 4 weeks after the final ingestion of test products as a postingestion safety follow-up via telephone.
2.4. Sample size
The sample size was based on a previous study[25] and was calculated to detect >20% difference of a decrease of the NRS pain scores, which was significant between groups after the 3-month ingestion of CMO, with a significance level (α) of 0.05 and an effect size (d) of 0.8. For these, 4 between-group comparisons were necessary (group A vs B; group A vs C; group B vs D; and group C vs D), so the significance level was determined at a corrected value of 0.0125 (0.05/4) using the Bonferroni correction. We adjusted the sample size for an estimated follow-up loss rate of 10% at visit 5 and needed 7 participants in each group.
2.5. Statistical analyses
Data represent mean ± standard deviation (95% confidence interval) or (min, max), or frequency (%). Demographics and all primary and secondary efficacy endpoints were analyzed based on the Per-Protocol population, and safety analysis was based on the intention to treat population, using Fisher exact tests, Kruskal–Wallis test, or Wilcoxon singed rank test as appropriately. P < 0.05 was considered statistically significant for all analyses. For between-group analysis, a difference in pain reduction (%) in group A versus group B and group A versus group C was compared to prove the hypothesis that pain reduction in group B and C would not be inferior compared to group A (FAC, 100%) in pain reduction. In addition, comparison of group B versus D and group C versus D was performed to prove the hypothesis that product B and C are superior to product D (control). Mann–Whitney U test was used for between-group analyses, and a corrected P < 0.0125 was considered statistically significant using Bonferroni adjustment.
3. Results
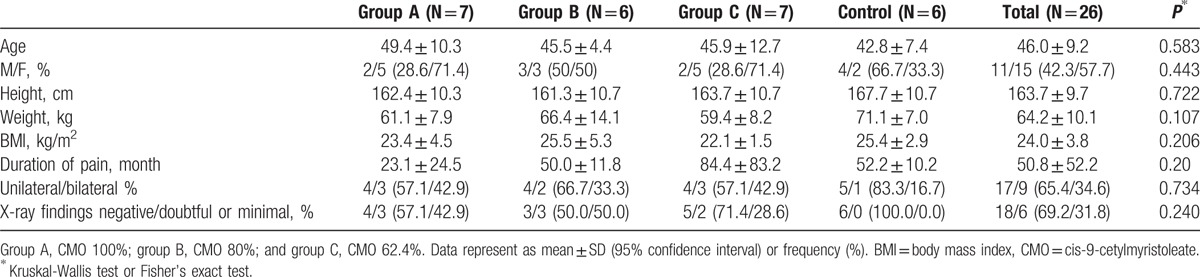
Thirty participants were randomized at visit 2. After the randomization, 6 patients dropped out during the follow-up because of withdraw of consent (n = 1), major violation (n = 3), and other causes (n = 2). Therefore, 26 participants completed the study (group A = 7; group B = 6; group C = 7; and group D = 6) (Fig. 1). There were no statistically significant differences in the demographics (Table 1).
Figure 1.
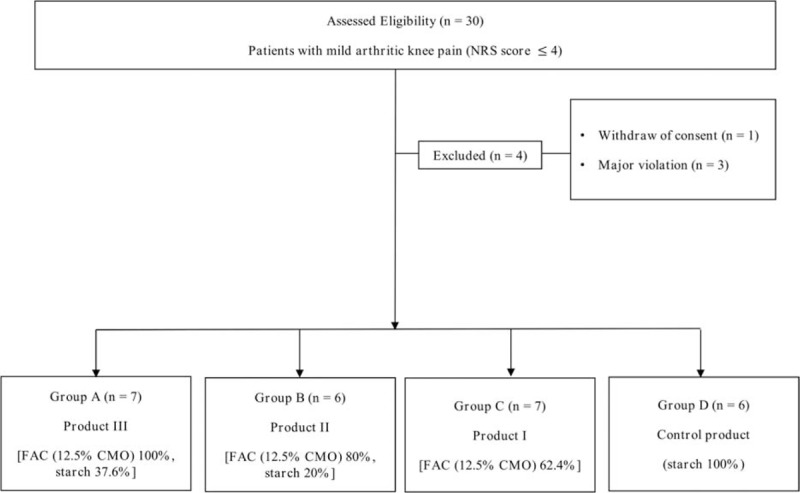
Flow diagram showing random allocation. The diagram shows the number of patients in each group. NRS = numeric rating scale.
Table 1.
Demographics and clinical features.
3.1. Efficacy
Table 2 shows that NRS pain score at visit 5 was significantly different among groups (P = 0.021). In between-group analyses, compared to group D (control), there were statistically significant differences in NRS pain score in group A (corrected P = 0.005) and group C (corrected P = 0.012), but not statistically significant in group B (corrected P = 0.180). Compared to group A (100% FAC), groups B (80% FAC) and C (62.4% FAC) showed no statistically significant difference in decrease (%) of NRS pain score at visit 5 (corrected P = 0.477 and corrected P = 0.181, respectively). For within-group analysis, significant decrease in NRS pain score was observed at visit 5 in group A (100% FAC) and C (62.4% FAC), whereas decreases of NRS from baseline in group B (80% FAC) was statistically insignificant.
Table 2.
Changes in numerical rating scale pain score during 12-week CMO application.
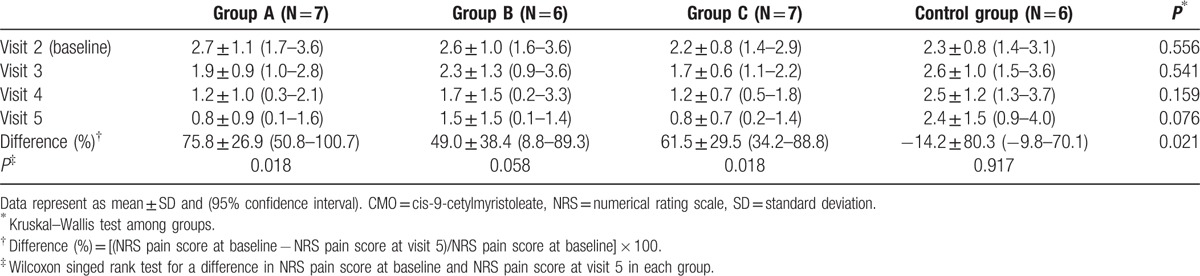
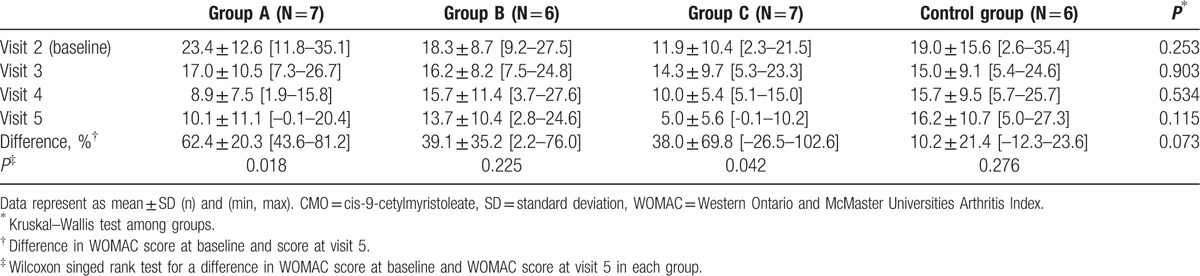
Table 3 shows the change in the Korean version of the WOMAC score during the study period. Difference in total WOMAC score at visit 5 compared to visit 2 did not show any significant intergroup differences (P = 0.073). Changes in the score of each 3 subsections of the WOMAC score including pain, joint stiffness, and physical function were also not significantly different among groups. However, for within-group analysis, decreases in WOMAC score were statistically significant in group A (100% FAC) and group C (62.4% FAC).
Table 3.
Changes in total score of Korean WOMAC during 12-week CMO application.
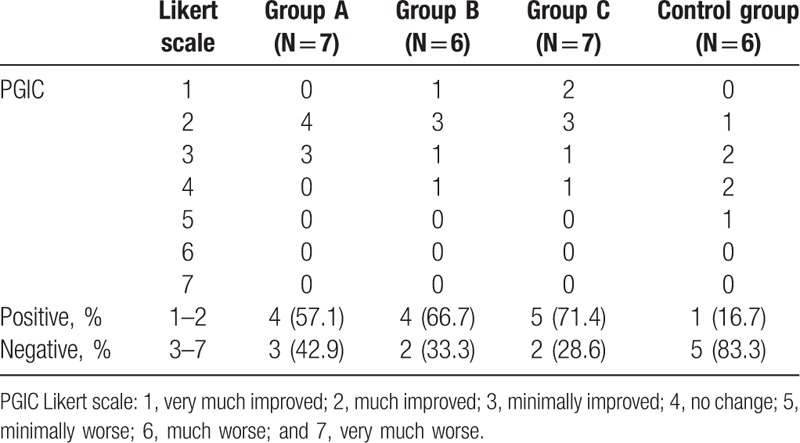
The subjective feeling of the PGIC Likert scale, which describes the general subjective improvement of knee pain at visit 5, was considered positive in more than 50% of the patients in groups A, B, and C, whereas it was negative in 83.3% in group D (control) (Table 4). However, this difference between the intervention and the control group was not statistically significant.
Table 4.
Patient global impression of change (PGIC) by patients at visit 5.
3.2. Safety
Adverse events were thoroughly investigated in all participants and analyzed in the intention to treat group (n = 30). During the 12-week study period, none of the participants exhibited a significant change in vital signs, physical examination, targeted neurologic sensory examination, and clinical laboratory tests. The proportion of patients with adverse drug reactions was 23.33% (7 of 30 participants) with 10 events. Even then, there was no serious adverse event. Mild to moderate adverse events, which might be expected because of the CMO being taken, were mild gastrointestinal discomfort (6/30, 20.0%, 2 events in group A, 0 in group B, 1 in group C, and 3 in the control group), dysmenorrhea (1/30, 3.33%), facial edema (1/30, 3.33%), and constipation (2/30, 6.67%). However, there were no statistically significant differences in adverse events among the groups, and no patients were excluded because of their adverse events.
4. Discussion
CMO may show antiarthritic properties in the hydrolyzed free fatty acid form (myristoleic acid) as an active pharmacologic agent with antiinflammatory effects by stabilizing cell membranes, inhibiting formation of inflammatory mediators, and protecting against oxidation,[26,27] as shown with other fatty acids, such as oleic acid or linoleic acid, in human studies.[28–30] Myristoleic acid induces the apoptosis of prostate tumor cells, presumably by inhibiting 5-lipoxygenase.[31] The metabolites produced in the pathway mediated by 5-lipoxygenase are involved in inflammatory or allergic reactions. Therefore, the antiarthritic effect of CMO might be due to the myristoleic acid inhibition of 5-lipoxygenase activity. Whether we have found a unique antiinflammatory agent or another hydrolyzed free fatty acid component with already proven antiinflammatory properties needs further study.
The recommended dose of CMO has been somewhat arbitrary, and the minimal effective dose is unclear. Since being introduced in 1994, CMO was shown to prevent arthritis in animal models in very high doses; 450 and 900 mg/kg intraperitoneally[16] or 20 mg/kg/day orally. It has been released in the market worldwide, mainly as an ingredient of over-the-counter health supplements. Only 4 studies have examined the efficacy of CMO in humans,[17–19,21] without any consideration of its effective dose. A recent clinical study that reported the effectiveness of CMO for managing knee join pain due to OA used a daily oral dose of 250 mg CMO.[18] CMO improved both pain and function using a visual analogue pain score and a modified knee society knee score by 69.2% and 3.8%, respectively. However, no reference was provided on how the dose was set in the study, thus it seems unwarranted and probably based on anecdotal evidence in human effectiveness in existing health supplements.
In our study, patients in group C (FAC 62.4%) showed significant pain reduction, which may suggest that the currently recommended dose of CMO (group A, FAC 100%) could be reduced to 62.4%. We also found a significant reduction in pain intensity after 12 months of ingesting 100% and 62.4% FAC with 12.5% CMO, although the improvement in function was not statistically significant. The efficacy was more remarkable in reducing pain than improving function, which was consistent with the previous study.
The small sample size is a major limitation in our study. Apparently, we observed a tendency of improvement in all 3 parameters – NRS pain score, WOMAC index, and PGIC scale – in all 3 CMO groups. Interestingly, in group B, the NRS pain score reduction was not statistically significant (P = 0.058), contrary to the significant pain reduction in group C (P = 0.012), which contained less dose of CMO than that in group B. We presume this statistical insignificance, attributed to the small sample size, both in total and in group B, which were 6 instead of 7 as in groups A and C. In addition, although there was no statistical significance in demographics among groups, the slightly higher ratio of individuals with signs of advanced disease, regarding the knee X-ray findings, such as doubtful or minimal OA, was observed in group B (50%) than the other groups (group A = 42.9%; group C = 28.6%; and group D = 0%), which could have contributed to the results.
Besides a small sample size, there are other several flaws in the study. First, the subjects of the study were subhealthy persons, not patients. This was a practical limitation regarding the strict regulations of the Korean Food and Drug Administration, which restricts performing clinical trials regarding health supplements for managing real patients with NRS pain score >4 and any X-ray evidence of degeneration. Therefore, the results of our study need to extrapolate with care when applied to patients with more intense knee pain. Another limitation is the source of uncertainty, since we did not specify the disease condition in which CMO was effective. We aimed at knee joint pain, rather than a disease entity such as arthritis, for the same reason as the previous limitation of the Korean Food and Drug Administration regulation. Although OA of knee is the most common cause of knee pain and dysfunction, there are other less common causes, such as other inflammatory arthritis like rheumatoid arthritis, which have different mechanisms. Again, careful conclusion should be made from our study when considering each disease entities separately.
5. Conclusions
CMO, especially from beef-tallow-extracts, is effective in alleviating knee joint pain at an effective dose of 62.4%, compared to the currently recommended dose of 100% for knee arthritis. We may suggest that the minimal effective dose of CMO for managing knee pain could be less than the current dose of 100%. Further studies with larger numbers and more specific disease selection are needed to determine the minimal effective dose of this agent in arthritic pain and the exact mechanism of this agent in individuals with knee joint pain.
Footnotes
Abbreviations: CMO = cis-9-cetylmyristoleate, FAC = fatty acid complex, NRS = numerical rating scale, OA = osteoarthritis, PGIC = Patient Global Impression of Change, RCT = randomized controlled trial, WOMAC = Western Ontario and McMaster Universities Arthritis.
SCL, HSJ, and YJ contributed equally to this work.
Funding/support: The study was funded by SFC Bio, Inc., Seoul, Korea.
The authors have no conflicts of interest to disclose.
References
- [1].Thiem U, Lamsfuss R, Gunther S, et al. Prevalence of self-reported pain, joint complaints and knee or hip complaints in adults aged >/=40 years: a cross-sectional survey in Herne, Germany. PLoS One 2013;8:e60753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nguyen US, Zhang Y, Zhu Y, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med 2011;155:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pereira D, Peleteiro B, Araujo J, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19:1270–85. [DOI] [PubMed] [Google Scholar]
- [4].Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005;64:669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007;15:981–1000. [DOI] [PubMed] [Google Scholar]
- [6].Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137–62. [DOI] [PubMed] [Google Scholar]
- [7].Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476–99. [DOI] [PubMed] [Google Scholar]
- [8].Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. [DOI] [PubMed] [Google Scholar]
- [9].McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- [10].Hauk L. Treatment of knee osteoarthritis: a clinical practice guideline from the AAOS. Am Fam Physician 2014;89:918–20. [PubMed] [Google Scholar]
- [11].National clinical guideline center (UK). Osteoarthritis: Care and management in adults. NICE clinical guideline CG177; 2014. Available from: https://www.nice.org.uk/guidance/cg177 Accessed February 12, 2014. [Google Scholar]
- [12].Van Vijven JP, Luijsterburg PA, Verhagen AP, et al. Symptomatic and chondroprotective treatment with collagen derivatives in osteoarthritis: a systematic review. Osteoarthritis Cartilage 2012;20:809–21. [DOI] [PubMed] [Google Scholar]
- [13].Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum 2001;44:2531–8. [DOI] [PubMed] [Google Scholar]
- [14].Lee SY, Kwon HK, Lee SM, et al. a new herbal medicine with multifunctional mechanism for joint disease: first therapeutic application for the treatment of osteoarthritis. Arch Pharm Res 2011;34:1774–7. [DOI] [PubMed] [Google Scholar]
- [15].Diehl HW, May EL. Cetyl myristoleate isolated from Swiss albino mice: an apparent protective agent against adjuvant arthritis in rats. J Pharm Sci 1994;83:296–9. [DOI] [PubMed] [Google Scholar]
- [16].Hunter KW, Jr, Gault RA, Stehouwer JS, et al. Synthesis of cetyl myristoleate and evaluation of its therapeutic efficacy in a murine model of collagen-induced arthritis. Pharmacol Res 2003;47:43–7. [DOI] [PubMed] [Google Scholar]
- [17].Xie Q, Shi R, Xu G, et al. Effects of AR7 Joint Complex on arthralgia for patients with osteoarthritis: results of a three-month study in Shanghai, China. Nutr J 2008;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee SH, Kim JH, Cho WS, et al. The efficacy and safety of beef tallow extract including cis-9-cetylmyristoleate in patients with osteoarthritis: as an adjuvant pharmacological treatment. J Food Sci Nutr 2005;10:285–9. [Google Scholar]
- [19].Ha TK, Kang JH, Lee IB, et al. Effect of Cis-9-cetylmyristoleate containing fatty acids complex extracted from vegetable oils on knee pain in patients with osteoarthritis. Korean J Fam Med 2010;31:16–23. [Google Scholar]
- [20].Morelli V, Naquin C, Weaver V. Alternative therapies for traditional disease states: osteoarthritis. Am Fam Physician 2003;67:339–44. [PubMed] [Google Scholar]
- [21].Edwards AM. CMO (cerasomo-cis-9-cetyl myristoleate) in the treatment of fibromyalgia: and open pilot study. J Nutr Environ Med 2001;11:105–11. [Google Scholar]
- [22].Bae SC, Lee HS, Yun HR, et al. Cross-cultural adaptation and validation of Korean Western Ontario and McMaster Universities (WOMAC) and Lequesne osteoarthritis indices for clinical research. Osteoarthritis Cartilage 2001;9:746–50. [DOI] [PubMed] [Google Scholar]
- [23].Quintana JM, Escobar A, Arostegui I, et al. Health-related quality of life and appropriateness of knee or hip joint replacement. Arch Intern Med 2006;166:220–6. [DOI] [PubMed] [Google Scholar]
- [24].Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- [25].Petersen SG, Beyer N, Hansen M, et al. Nonsteroidal anti-inflammatory drug or glucosamine reduced pain and improved muscle strength with resistance training in a randomized controlled trial of knee osteoarthritis patients. Arch Phys Med Rehabil 2011;92:1185–93. [DOI] [PubMed] [Google Scholar]
- [26].Grimble RF. Modification of inflammatory aspects of immune function by nutrients. Nutr Res 1998;18:1297–317. [Google Scholar]
- [27].Grimm H, Mayer K, Mayser P, et al. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br J Nutr 2002;87(Suppl 1):S59–67. [DOI] [PubMed] [Google Scholar]
- [28].Martinez-Dominguez E, de la Puerta R, Ruiz-Gutierrez V. Protective effects upon experimental inflammation models of a polyphenol-supplemented virgin olive oil diet. Inflamm Res 2001;50:102–6. [DOI] [PubMed] [Google Scholar]
- [29].Ariza-Ariza R, Mestanza-Peralta M, Cardiel MH. Omega-3 fatty acids in rheumatoid arthritis: an overview. Semin Arthritis Rheum 1998;27:366–70. [DOI] [PubMed] [Google Scholar]
- [30].Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr 2000;71:349S–51S. [DOI] [PubMed] [Google Scholar]
- [31].Iguchi K, Okumura N, Usui S, et al. Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate 2001;47:59–65. [DOI] [PubMed] [Google Scholar]


