Abstract
This study was conducted to investigate the clinicopathological features of synchronous cancers and treatment options according to their locations.
Records of 8368 patients with colorectal cancer treated at our center between July 2003 and December 2010 were analyzed retrospectively. All synchronous colorectal cancer patients who underwent surgical treatment were included.
Synchronous cancers were identified in 217 patients (2.6%). Seventy-nine patients underwent either total colectomy, subtotal colectomy, or total proctocolectomy; 116 underwent 1 regional resection, including local excision; and 22 underwent 2 regional resections. The mean age was 62 years, slightly higher than that for the single-cancer patients. Synchronous cancers were more common in male patients, more frequently located in the left colon, had more microsatellite instability-high status, and showed more advanced stage than single cancer. Extensive resection was mainly performed for synchronous cancers located in both the right and left colon. Two regional resections were performed for cancers in the right colon and rectum. There were no differences in complication rates or the occurrence of metachronous cancer between the 2-region resection and extensive resection groups. Eight years postoperatively, the mean number of daily bowel movements for these 2 groups were 1.9 and 4.3, respectively.
We found that synchronous cancer was different from single cancer in terms of age, gender, location, and pathologic features. Synchronous colorectal cancer requires different treatment strategy according to the distribution of lesions. Comparison between the 2 regional resections and extensive resection approaches suggests that 2 regional resections are preferable.
Keywords: colorectal cancer, metachronous, surgery, synchronous
1. Introduction
Synchronous colorectal cancer refers to more than 1 primary colorectal cancer detected in a single patient simultaneously or within 6 months of the initial diagnosis. The reported incidence of synchronous colorectal cancers ranges from 2.3% to 12.4%.[1,2] Diagnosis of the presence of synchronous colorectal cancers is important because, if overlooked, they can develop into advanced-stage metachronous cancer and usually require re-operation. A preoperative diagnosis of synchronous colorectal cancer is also important because it may influence the treatment options with regard to the type and extent of surgical resection.[3]
Diagnosis of synchronous cancer has increased, primarily because of improvements in diagnostic modalities such as colonoscopy and computed tomography (CT) colonography.[4] However, there is much uncertainty about the most appropriate surgical treatment. Some authors have suggested that total or subtotal colectomy should be performed.[5,6] Passman et al[7] recommended a more extensive resection for lesions in adjacent segments. Some authors have recommended multiple resections aimed at retaining the normal colon.[8,9] Accordingly, there has been little agreement among surgeons regarding the appropriate surgical treatment for synchronous cancers located in separate segments.
There have been many studies on the clinicopathological features of synchronous cancer, and comparisons between synchronous and single cancers; however, few studies have investigated the treatment of synchronous cancer. The clinical implication of the present study lies in the investigation of the treatment options comparing patients who underwent 2 regional resections with those who underwent extensive resection. Accordingly, the aim of this study was to investigate the clinicopathological features of synchronous cancers and to evaluate surgical outcomes according to the extent of surgery by comparing 2 regional resections with extensive resection.
2. Material and methods
We retrospectively reviewed the medical records and prospectively managed colorectal cancer database of 8368 consecutive colorectal cancer patients who underwent surgery at Asan Medical Center between July 2003 and December 2010. Those with family history of familial adenomatous polyposis or hereditary nonpolyposis colorectal cancer were excluded from the study. Among the 8368 patients, 217 patients (2.6%) were identified with synchronous colorectal cancer. The present study protocol was approved by the Institutional Review Board of Asan Medical Center (registration number 2016-0996).
The Warren and Gates[10] criteria were used to define synchronous cancers. The criteria included the following key elements: each tumor must present a definite picture of malignancy; each had to be distinct; the probability of one being a metastasis of the other must be excluded; and the synchronous lesions must be diagnosed simultaneously or within 6 months of the initial diagnosis. Pathological diagnosis was based on the AJCC classification of malignant tumors, 7th edition. In cases of synchronous cancer, the lesion that was the most advanced pathologically was defined as the index lesion. When 2 or more lesions were in an identical pathological stage, the largest lesion was defined as the index lesion. Less-advanced lesions were considered to be concurrent lesions. Patients treated for intramucosal cancer were included, because colorectal cancer develops in the mucosa, even though it has no potential for lymph-node metastasis.
The diagnosis of synchronous cancer was based upon colonoscopy and CT. In cases of partial obstruction that prevented the passage of the colonoscope, CT colonography was performed. Our standard postoperative surveillance to detect metachronous colorectal cancer was to perform a 1st colonoscopic examination approximately 6 to 12 months after surgery; in patients who did not undergo a preoperative complete colonoscopy, the 1st examination was within 3 months of surgery. Thereafter, colonoscopy was performed 2 to 3 times a year. All polyps were removed during surveillance.
The patients’ demographic data, the clinicopathological features, and locations of the synchronous cancers were reviewed. Synchronous cancers were compared with single cancers. Surgical treatments were reviewed with respect to the location of the treated cancers, and a comparison was made between 2 regional resections and extensive resection. Tumor locations were divided into 3 groups: the right colon, which included the appendix, cecum, ascending colon, hepatic flexure colon, and transverse colon; the left colon, which included the splenic flexure colon, descending colon, sigmoid colon, and rectosigmoid colon; and the rectum. “Standard resection” was defined as radical resection, “2 regional resections” as 2 radical resections with 2 anastomoses, and “extensive resection” as total colectomy, subtotal colectomy, or total proctocolectomy.
To compare surgical outcomes, we investigated functional outcome, incidence of metachronous cancer, and any complication that developed after surgery during the hospital stay. At a median of 94 months after the surgery, we surveyed each patient's daily number of bowel movements by telephone interview. The response rate was 65.1%, with 53 respondents (2 regional resections group: 63.2%, 12 respondents; and extensive resection group: 65.9% with 41 respondents).
Student t test and the chi-squared test were used to compare factors between groups. A P value of <0.05 was considered statistically significant, and SPSS software version 21 (IBM Inc., Armonk, NY) was used for the statistical calculations.
3. Results
3.1. Characteristics of synchronous cancers
Of the 8368 patients reviewed, 217 (2.6%) were identified as having developed synchronous colorectal cancer. Table 1 presents a comparison between the patients with synchronous cancer and those with a single cancer. The mean age at diagnosis was slightly higher for the synchronous cancers than for the single colorectal cancers (62.3 ± 10.4 vs 59.8 ± 11.1, P = 0.001), and synchronous colorectal cancer was more common in male patients (71.9% vs 61.2%, P = 0.001). In the patients with synchronous cancers, tumors were most frequently located in the left colon, whereas in patients with single-cancer tumors were most frequently located in the rectum (41.9% vs 44.1%, P < 0.001). Patients with synchronous colorectal cancer showed a more advanced stage than those with single cancer (P = 0.007). Patients with synchronous cancers had a higher proportion of microsatellite instability-high cancers than patients with single cancer (12.8% vs 6.6%, P = 0.001).
Table 1.
Characteristics of synchronous colorectal cancer patients.
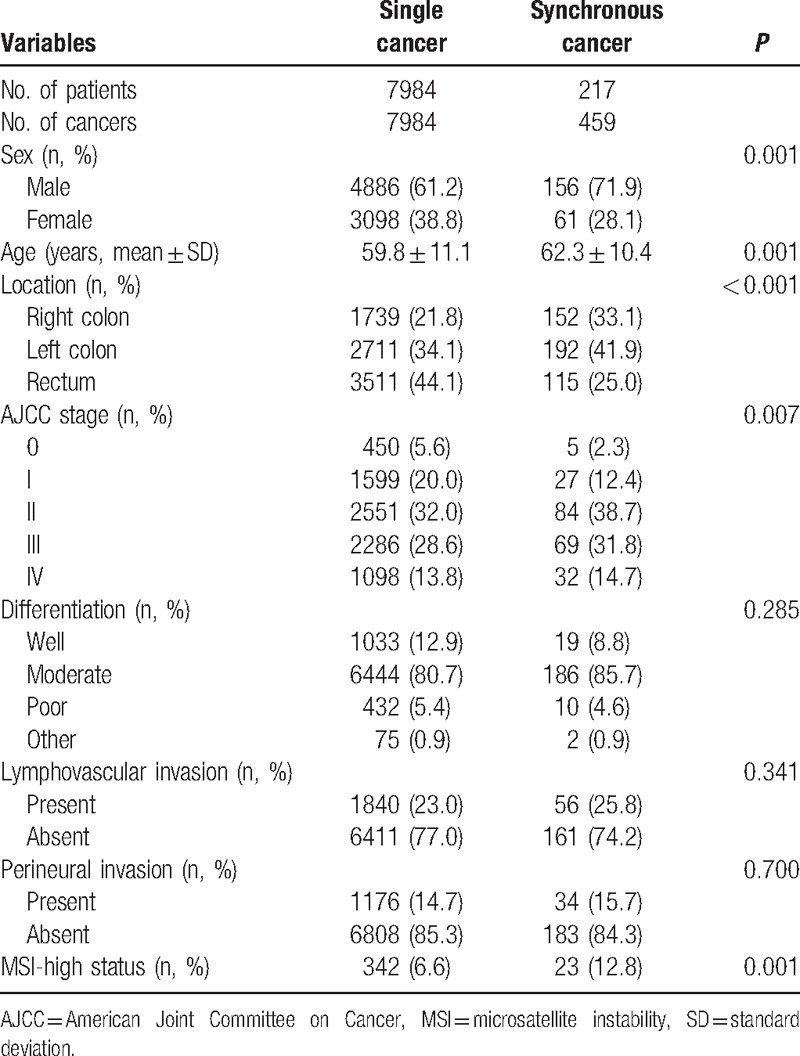
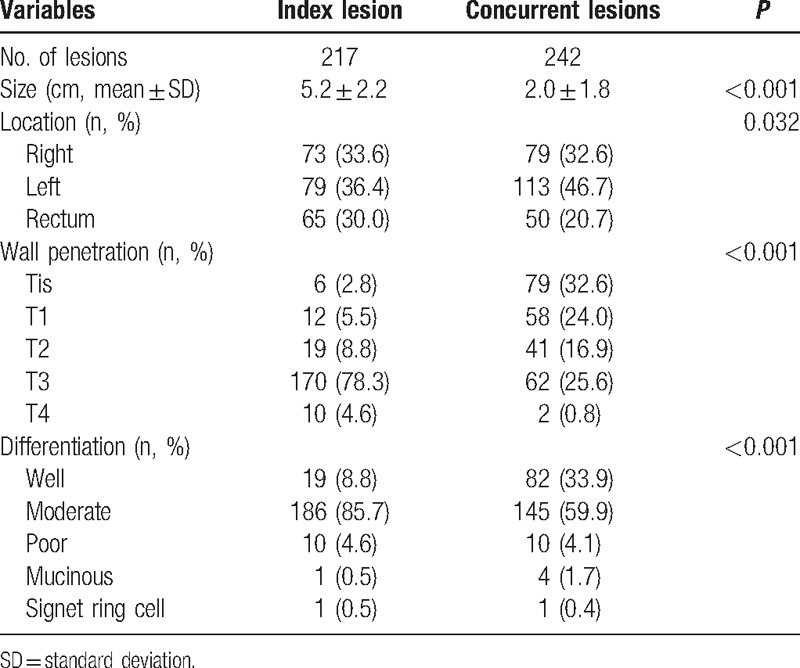
Table 2 shows a comparison of concurrent and index lesions in the patients with synchronous cancer. The concurrent lesions were smaller than the index lesions, more of them were well differentiated, and the wall penetration was generally less deep. Concurrent lesions were most frequently in the left colon, whereas the index lesions were evenly distributed in the right colon, left colon, and rectum.
Table 2.
Differences between index and concurrent tumors in synchronous cancers.
3.2. Distribution of synchronous cancers and surgical treatment
Several types of operation were performed according to the distribution. The cancers were more often located in multiple segments (69.6%) than in one segment (30.4%). When the cancers were located in 1 segment, the most frequent treatment approach (81.8%) was a regional resection that included local excision, such as endoscopic mucosal resection, endoscopic surgical dissection, and transanal excision. When the cancers were located in multiple segments, a variety of surgical resections were performed. Two regional resections were performed for 22 patients (14.6%) and extensive resection for 67 patients (44.4%). When the cancers were located in the right and left colon, the most common procedure was extensive resection (65.7%), and for cancers located in the right colon and rectum the most frequent treatment was 2 regional resections (63.6%) (Fig. 1A).
Figure 1.
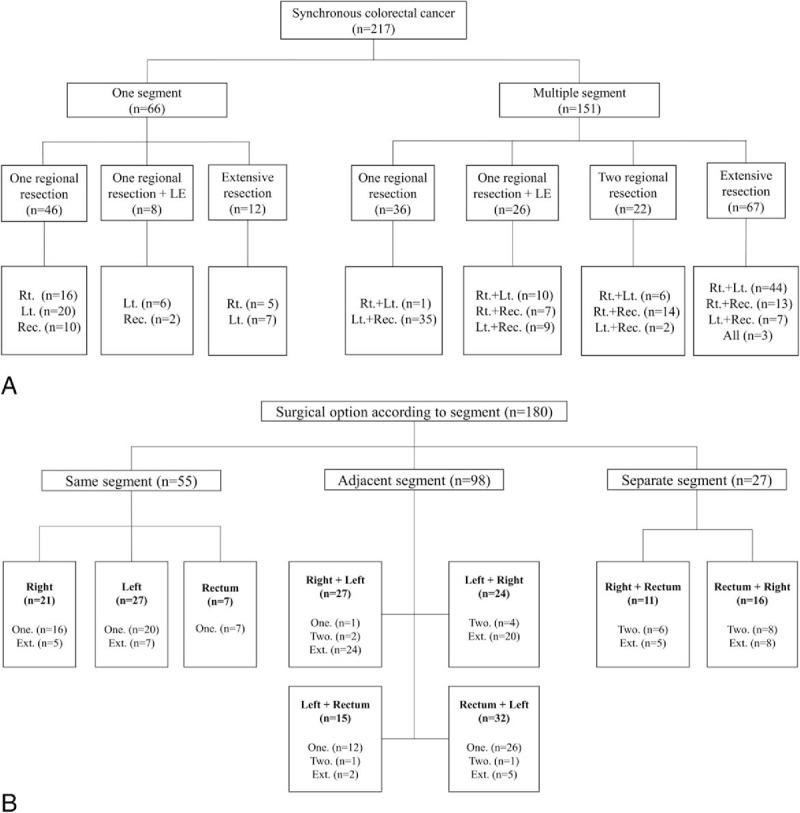
Surgical options. (A) Distribution of synchronous cancers and surgical options. (B) Surgical option according to segment and location of index/concurrent lesions. Several types of operation was performed according to the distribution. Ext. = extensive resection, LE = local excision, Lt. = left colon, One. = one regional resection, Rec. = rectum, Index + concurrent lesion, Rt. = right colon, Two. = two regional resection.
Surgical options according to the location of index and concurrent lesions for 180 patients who underwent surgical resections are shown in Fig. 1B. There was no difference in surgical options according to the location of index and concurrent lesions.
3.3. Comparison between 2 regional resections and extensive resection
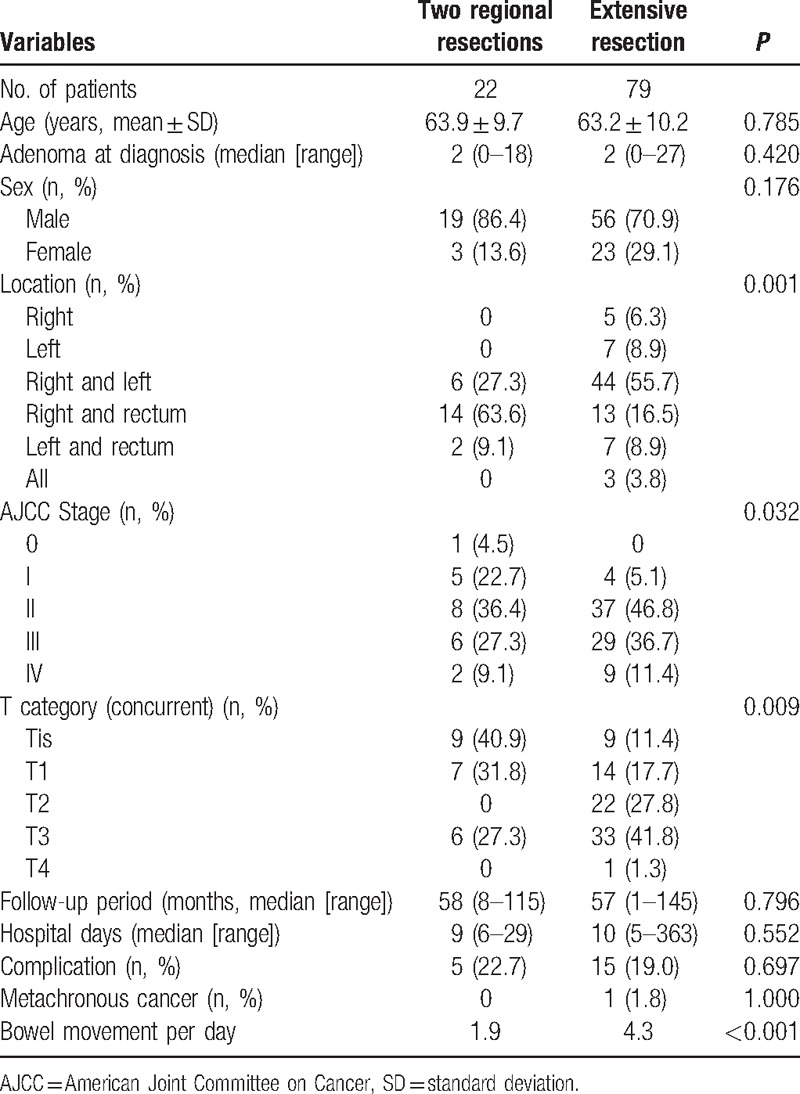
Table 3 compares the patients who underwent 2 regional resections with those who received an extensive resection. Twenty-two patients underwent 2 regional resections with 2 anastomoses, and 79 patients underwent extensive resection. There were no statistically significant differences between these 2 groups in age, gender, hospital stay, median follow-up period, the incidence rate of metachronous colorectal cancer, and the rate of complications. Four of the patients who underwent 2 regional resections and 26 of the patients who underwent extensive resection did not receive preoperative colonoscopy or CT colonography because of obstruction; this did not differ significantly between the 2 groups (P = 0.181). Patients who underwent 2 regional resections experienced the following complications: ileus (2), stricture, pneumonia, and pulmonary thromboembolism. Patients who underwent extensive resection had more severe complications: ileus (9), leakage (2), intraabdominal fluid collection (not an abscess, 2), a wound problem, and superior mesenteric vein thrombosis with ischemia. Functional outcomes differed significantly between the 2 regional resections group and the extensive resection group. The mean number of bowel movements per day 94 months after surgery (median) was 1.9 and 4.3 in the 2 regional resections group and the extensive resection group, respectively (P < 0.001). In the extensive resection group, 1 patient developed metachronous cancer after subtotal colectomy and was treated with endoscopic resection.
Table 3.
Comparison between 2 regional resections and extensive resection for synchronous colorectal cancer.
4. Discussion
The incidence of synchronous colorectal cancers in this study was 2.6%, which was similar to the report in other studies.[1,2] When comparing between synchronous and single cancer, several studies have reported a predominance of male patients in synchronous cancer.[4,11,12] Indeed, in the present study we found more synchronous cancers in men than in women (2.56:1), whereas the ratio was 1.58:1 for single cancers. Synchronous cancers were more advanced pathologically than single cancers. This may be influenced by a downstaging effect of preoperative chemoradiotherapy for rectal cancer (the rate of preoperative chemoradiotherapy: 7.4% in synchronous cancer and 12.9% in single cancer). Most studies have reported that the most frequent location for synchronous cancers is the right colon.[7,8,13] However, we found synchronous cancers located predominantly in the left colon (41.8%); this finding was also reported by some authors.[12,14] With regard to the distribution, some studies have reported that the majority of patients had lesions distributed in different segments of the colon.[7] We also found more cases of synchronous cancers distributed in different segments (68.2%). In contrast, others have reported that most patients had lesions in the same segment.[4,11]
When synchronous cancers are distributed in different segments or preoperative inspection is insufficient because of occlusive distal cancer, the treatment strategy becomes more complicated. In this situation, surgeons must take into consideration factors such as the patients’ age, comorbidity, functional outcome, multiple anastomoses, and postoperative complications. When synchronous cancers were distributed in the right and left colon, extensive resection was often performed, but it was more common for 2 regional resections to be performed when distributed in the right colon and rectum. It might be of concern to most surgeons that postoperative functional outcomes after total proctocolectomy would be poor. For synchronous cancers, preoperative detection is important. In our study, 4 patients who received 2 regional resections and 26 patients who received extensive resection did not undergo preoperative colonoscopy because of an obstruction. However, this did not differ significantly between the 2 groups (P = 0.181), indicating that insufficient preoperative inspection may not influence the surgical options.
With regard to the extent of the resection, controversies remain about synchronous cancers in multiple segments. Some authors have suggested that total or subtotal colectomy should be performed.[5,6,15,16] This is because if synchronous lesions are overlooked at the time of surgery the patient may have to undergo repeated surgeries for metachronous cancer.[12] In addition, Muto et al[17] argued that colectomized patients with ileorectal anastomosis develop near-normal bowel habits, and their mortality rate is comparable to those who have had a conventional hemicolectomy. Some authors have suggested the utility of extensive procedures such as proctocolectomy with ileal pouch–anal anastomosis.[18] Conversely, some other authors have suggested multiple resections with the aim of retaining the normal colon.[8,9,19] The reasons for such an approach are that the treatment of 2 regional resections does not appear to be associated with an increased risk of complication and extensive colectomy may increase bowel movement. Our study results showed that there were no differences in complication rate and hospital stay between the 2 regional resection groups and the extensive resection group. The complications were even milder in the 2 regional resection groups.
There was a significant difference in bowel movements in the regards to functional outcome between the 2 groups. Several studies have investigated the functional outcome after various bowel resections by questionnaires, telephone interview, and outpatient records. The response rate in these studies was 50% to 71%.[20–22] The response rate in our study was 65.1% with 53 respondents. Functional outcome after total colectomy and total proctocolectomy has been reported and was better after total colectomy.[23–25] In comparison with total and subtotal colectomy, subtotal colectomy was better in functional outcome and preservation of part of the sigmoid colon was beneficial regarding frequency of defecation.[20,22] In these studies, bowel movements after total or subtotal colectomy were reported to be 3.6 ± 2.4[20] and 4 to 5[22] per day, respectively. Some authors reported a decrease of bowel movement after total colectomy as 3 and 2.9 per day, respectively.[26,27] These different bowel movements may reflect differences in bowel adaptation. In our study, patients who underwent extensive resection showed decreased bowel movements in adaptation over time, but even after a period of 94 months (median), they had a significantly greater number of bowel movements than the patients who underwent 2 regional resections (P < 0.001). Preservation of normal colon may be beneficial in terms of bowel movement. Another factor to affect bowel movements is the resection of rectum. In our study, among 22 patients who underwent 2 regional resections, 16 patients (73%) received low anterior resection. Among 79 patients who underwent extensive resection, 55 patients (70%) preserved their rectum. In this regard, preservation of rectum does not seem to greatly affect the bowel movements.
Extensive resection is recommended as a safe treatment option to prevent metachronous cancer.[28] However, the incidence of metachronous cancer has been reported to be 0.5% to 3.6%.[29,30] A total of 0.7% of colorectal cancer patients in a previous study by our center and 0.9% in this study developed metachronous cancer.[31] This indicates that the incidence is too low to perform prophylactic surgery. The incidence of benign polyps ranges from 12% to 62% in patients with single cancer and from 40% to 75% in patients with multiple cancers.[13,32] It is believed that the entire colorectal mucosa is unstable and that there is a high possibility of malignant change in patients with multiple primaries.[32] However, it takes several years for an adenoma to develop into a carcinoma,[17] and colonoscopic resection is possible during the follow-up period before this development takes place. During the follow-up period, the rate of occurrence of metachronous cancer did not differ between the patients who underwent 2 regional resections and those who received extensive resection. In this regard, if proper surveillance colonoscopy is performed, 2 regional resections, the procedure that retains the most normal colon in order to decrease stool frequency, could be considered as the optimal surgical option.
The present study had some limitations. First, it was retrospective and there may have been bias in the selection of patients, which is clinically unavoidable. Second, the number of patients who underwent 2 regional resections was small, leaving the results less persuasive. Despite these limitations, our study was met an important need in that there have been few studies on the treatment of synchronous cancer to date. Accordingly, we compared perioperative outcome, occurrence of metachronous cancer, and functional outcomes between the 2 regional resections and extensive resection treatments.
5. Conclusion
We have shown that synchronous cancer occurred in 2.6% of patients with colorectal cancer. Synchronous cancer was more common in male and older patients and located on left colon, and showed more advanced stage than single cancers.
Synchronous colorectal cancer requires different treatment strategy from those for single colorectal cancer. Different treatment options were chosen according to the locations of the synchronous cancers. Our comparison between the 2 regional resections and extensive resection approaches suggests that 2 regional resections is preferable.
Footnotes
Abbreviation: CT = computed tomography.
The authors have no conflicts of interest to disclose.
References
- [1].Cunliffe WJ, Hasleton PS, Tweedle DE, et al. Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg 1984;71:941–3. [DOI] [PubMed] [Google Scholar]
- [2].Welch JP. Multiple colorectal tumors: an appraisal of natural history and therapeutic options. Am J Surg 1981;142:274–80. [DOI] [PubMed] [Google Scholar]
- [3].van Leersum NJ, Aalbers AG, Snijders HS, et al. Synchronous colorectal carcinoma: a risk factor in colorectal cancer surgery. Dis Colon Rectum 2014;57:460–6. [DOI] [PubMed] [Google Scholar]
- [4].Latournerie M, Jooste V, Cottet V, et al. Epidemiology and prognosis of synchronous colorectal cancers. Br J Surg 2008;95:1528–33. [DOI] [PubMed] [Google Scholar]
- [5].Enker WE. Multiple carcinomas of the large bowel: a natural experiment in etiology and pathogenesis [proceedings]. Proc Inst Med Chic 1977;31:178–9. [PubMed] [Google Scholar]
- [6].Wang HZ, Huang XF, Wang Y, et al. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol 2004;10:2136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Passman MA, Pommier RF, Vetto JT. Synchronous colon primaries have the same prognosis as solitary colon cancers. Dis Colon Rectum 1996;39:329–34. [DOI] [PubMed] [Google Scholar]
- [8].Adloff M, Arnaud JP, Bergamaschi R, et al. Synchronous carcinoma of the colon and rectum: prognostic and therapeutic implications. Am J Surg 1989;157:299–302. [DOI] [PubMed] [Google Scholar]
- [9].Holubar SD, Wolff BG, Poola VP, et al. Multiple synchronous colonic anastomoses: are they safe? Colorectal Dis 2010;12:135–40. [DOI] [PubMed] [Google Scholar]
- [10].Warren S, Gates O. Carcinoma of ceruminous gland. Am J Pathol 1941;17:821–6. [PMC free article] [PubMed] [Google Scholar]
- [11].Kaibara N, Koga S, Jinnai D. Synchronous and metachronous malignancies of the colon and rectum in Japan with special reference to a coexisting early cancer. Cancer 1984;54:1870–4. [DOI] [PubMed] [Google Scholar]
- [12].Oya M, Takahashi S, Okuyama T, et al. Synchronous colorectal carcinoma: clinico-pathological features and prognosis. Jpn J Clin Oncol 2003;33:38–43. [DOI] [PubMed] [Google Scholar]
- [13].Chen HS, Sheen-Chen SM. Synchronous and “early” metachronous colorectal adenocarcinoma: analysis of prognosis and current trends. Dis Colon Rectum 2000;43:1093–9. [DOI] [PubMed] [Google Scholar]
- [14].Finan PJ, Ritchie JK, Hawley PR. Synchronous and ‘early’ metachronous carcinomas of the colon and rectum. Br J Surg 1987;74:945–7. [DOI] [PubMed] [Google Scholar]
- [15].Fogler R, Weiner E. Multiple foci of colorectal carcinoma; argument for subtotal colectomy. N Y State J Med 1980;80:47–51. [PubMed] [Google Scholar]
- [16].Lillehei RC, Wangensteen OH. Bowel function after colectomy for cancer, polyps, and diverticulitis. J Am Med Assoc 1955;159:163–70. [DOI] [PubMed] [Google Scholar]
- [17].Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975;36:2251–70. [DOI] [PubMed] [Google Scholar]
- [18].Arenas RB, Fichera A, Mhoon D, et al. Incidence and therapeutic implications of synchronous colonic pathology in colorectal adenocarcinoma. Surgery 1997;122:706–9. discussion 709–710. [DOI] [PubMed] [Google Scholar]
- [19].Heald RJ. Synchronous and metachronous carcinoma of the colon and rectum. Ann R Coll Surg Engl 1990;72:172–4. [PMC free article] [PubMed] [Google Scholar]
- [20].Duclos J, Lefevre JH, Lefrancois M, et al. Immediate outcome, long-term function and quality of life after extended colectomy with ileorectal or ileosigmoid anastomosis. Colorectal Dis 2014;16:O288–296. [DOI] [PubMed] [Google Scholar]
- [21].Haanstra JF, de Vos Tot Nederveen Cappel WH, Gopie JP, et al. Quality of life after surgery for colon cancer in patients with Lynch syndrome: partial versus subtotal colectomy. Dis Colon Rectum 2012;55:653–9. [DOI] [PubMed] [Google Scholar]
- [22].You YN, Chua HK, Nelson H, et al. Segmental vs. extended colectomy: measurable differences in morbidity, function, and quality of life. Dis Colon Rectum 2008;51:1036–43. [DOI] [PubMed] [Google Scholar]
- [23].Aziz O, Athanasiou T, Fazio VW, et al. Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg 2006;93:407–17. [DOI] [PubMed] [Google Scholar]
- [24].Soravia C, Klein L, Berk T, et al. Comparison of ileal pouch-anal anastomosis and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum 1999;42:1028–33. discussion 1033–1024. [DOI] [PubMed] [Google Scholar]
- [25].van Duijvendijk P, Slors JF, Taat CW, et al. Functional outcome after colectomy and ileorectal anastomosis compared with proctocolectomy and ileal pouch-anal anastomosis in familial adenomatous polyposis. Ann Surg 1999;230:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elton C, Makin G, Hitos K, et al. Mortality, morbidity and functional outcome after ileorectal anastomosis. Br J Surg 2003;90:59–65. [DOI] [PubMed] [Google Scholar]
- [27].Eu KW, Lim SL, Seow-Choen F, et al. Clinical outcome and bowel function following total abdominal colectomy and ileorectal anastomosis in the Oriental population. Dis Colon Rectum 1998;41:215–8. [DOI] [PubMed] [Google Scholar]
- [28].Diaconu C, Dogaru C, Scripcariu V, et al. Synchronous colonic cancers. Chirurgia (Bucur) 2002;97:351–5. [PubMed] [Google Scholar]
- [29].Agrez MV, Ready R, Ilstrup D, et al. Metachronous colorectal malignancies. Dis Colon Rectum 1982;25:569–74. [DOI] [PubMed] [Google Scholar]
- [30].Kiefer PJ, Thorson AG, Christensen MA. Metachronous colorectal cancer. Time interval to presentation of a metachronous cancer. Dis Colon Rectum 1986;29:378–82. [DOI] [PubMed] [Google Scholar]
- [31].Park IJ, Yu CS, Kim HC, et al. Metachronous colorectal cancer. Colorectal Dis 2006;8:323–7. [DOI] [PubMed] [Google Scholar]
- [32].Miller BJ, Cohen JR. Gastrointestinal: synchronous and metachronous colorectal cancers. J Gastroenterol Hepatol 2003;18:457. [DOI] [PubMed] [Google Scholar]


