Abstract
There are limited data regarding factors affecting outcomes among acute coronary syndrome (ACS) patients presenting with varying degrees of left ventricle (LV) dysfunction. We aimed to identify factors associated with mortality according to LV ejection fraction (LVEF) at 1st admission in ACS patients.
A total of 8983 ACS patients prospectively enrolled in the Acute Coronary Syndrome Israeli Survey (2000–2010) were categorized according to their LVEF at admission: severe LV dysfunction (LVEF < 30% [n = 845]), mild-moderate LV dysfunction (LVEF 30%–49% [n = 4470]); preserved LV function (LVEF ≥ 50% [n = 3659]). Multivariable Cox proportional hazards regression modeling was used to assess the risk factors for 1-year mortality according to LVEF on admission.
Over the past decade there was a gradual decline in the proportion of patients admitted with low LVEF. Mortality rates were highest among patients with severe LV dysfunction (36%), intermediate among those with mild-moderate LV dysfunction (10%), and lowest among those with preserved LV function (4%, P < 0.001). We recognized different risk factors for mortality according to LVEF at admission. Admission clinical features (syncope, anterior myocardial infarction, and ST elevation myocardial infarction [STEMI]) predicted mortality risk in patients with severe LV dysfunction (all P < 0.05), whereas the presence of comorbidities (hypertension, diabetes mellitus, chronic renal failure, and peripheral arterial disease) predicted mortality risk in patients with more preserved LV function. Age and admission Killip class ≥II were consistent predictors in all LVEF subsets.
LVEF at admission is a strong predictor of mortality in ACS, and prognostic factors differ according to LVEF during admission. In patients with severe LV dysfunction signs of clinical instability are related to 1-year mortality; in patients with a more preserved LV function the prognosis is related to the presence of co-morbidities.
Keywords: acute coronary syndrome, echocardiography, left ventricle ejection fraction, risk assessment
1. Introduction
Risk stratification in acute coronary syndrome (ACS) by classical score systems such as thrombolysis in myocardial infarction (TIMI)[1] and Global Registry of Acute Coronary Events[2] has been successfully incorporated into clinical practice leading to better and more efficient patient management. These scores and others, combine clinical evaluation such as Killip score, presence of angina and laboratory values such as creatinine levels, blood pressure, dyslipidemia, and cardiac biomarkers.[1–6]
Even though ejection fraction (EF), as determined by echocardiography, has not been included in the early risk stratification algorithms, it is a well-documented strong predictor of mortality in patients with coronary disease including ST elevation (STEMI) and non-ST elevation acute myocardial infarction (NSTEMI).[7–11] Furthermore, there are limited data regarding factors affecting outcomes among ACS patients presenting with varying degrees of left ventricular dysfunction.
The Acute Coronary Syndromes-Israel Survey (ACSIS) database is a prospectively bi-annual evaluation of all patients admitted with ACS to 26 coronary units in Israel during a 2-month period since February to March 2000. During the last decade, this evaluation includes a complete clinical, laboratory, and echocardiographic data during hospitalization for most patients. This long-term data collection facilitates the evaluation of demographic and clinical changes over time.
The objectives of this study are to evaluate: the trends for changes in EF at admission from 2000 to 2010 in ACSIS study population and prognostic consequences of these trends; the association between left ventricle ejection fraction (LVEF) and outcomes in a contemporary cohort of ACS patients; and to identify predictors for 1-year mortality in the different group of patients according to their EF at 1st admission.
2. Methods
ACSIS is a prospective cohort study and includes all patients with ACS (NSTEMI and STEMI) who were admitted to 26 intensive cardiac care units in Israel from February to March 2000 to 2010 every 2nd year. During this period 6 surveys were performed for 2-month period in each unit in Israel.
Prespecified forms for all patients were filled and diagnosis for acute myocardial infarction (MI) was made by attending physicians according to clinical, laboratory, and electrocardiography data. Demographic, clinical, laboratory, and echocardiographic data were obtained from medical charts.
All echocardiographic studies were performed during the 1st 2 days of the index hospitalization, and visually estimated EF was reported. Echocardiography examinations were performed according to the European and American performance guidelines by experienced technicians. All exams were interpreted by experienced cardiologists specialized in echocardiography and approved by senior staff members.[12]
All patients who underwent percutaneous coronary intervention (PCI) were treated conventionally with bare metal or drug eluting stents and received antithrombotic treatment.
All ACSIS surveys have been approved by participating hospitals institutional review board and the Israeli ministry of health.
Mortality rates at 1-year were derived from hospital charts and by matching patients’ identification number with the Israeli National Population Registry. For detailed description of study design refer to previously reported study.[13]
Study patients were divided into 3 prespecified groups according to their LVEF on admission. Patients with LVEF < 30% were defined as severe left ventricle (LV) dysfunction, patients with an LVEF between 30% and 49% were defined as mild-moderate LV dysfunction and patients with an LVEF ≥ 50% – preserved LV function according to the current cardiac chamber quantifications by echocardiography guidelines.[14] The primary end point of the present study was all-cause mortality assessed at 1-year.
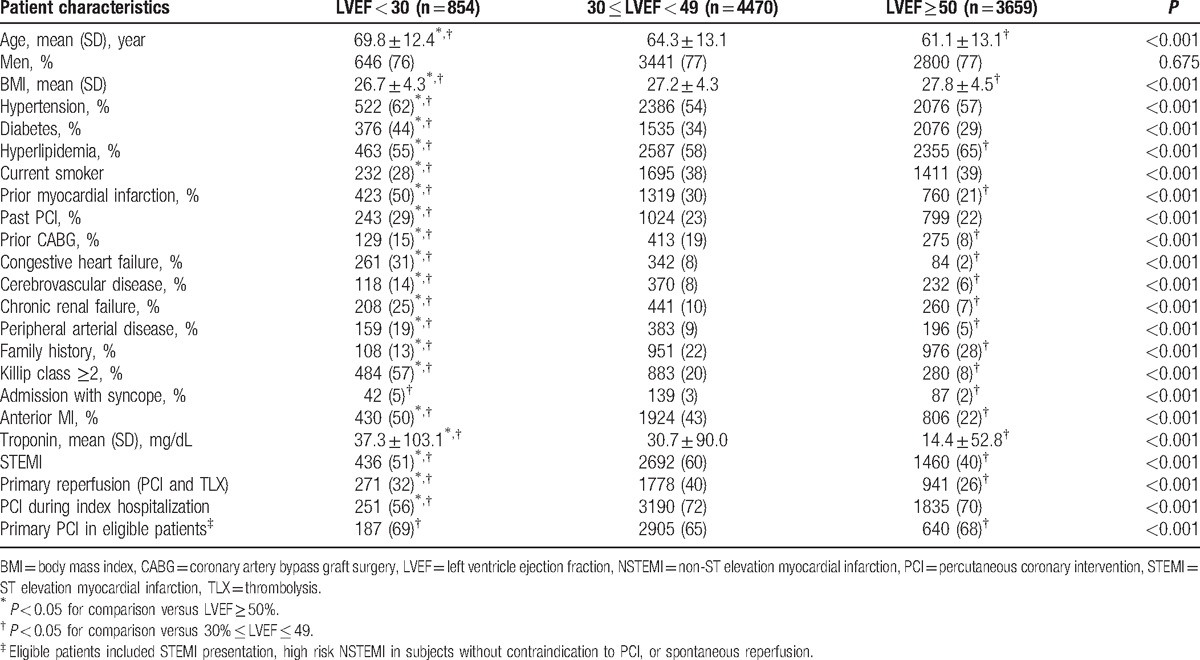
Baseline clinical characteristics were compared among the 3 LVEF groups, using the χ2-test or Fisher exact test for categorical variables with Z-test and Bonferroni correction for column proportion comparison. Continuous data were compared using the Kruskal–Wallis test followed by post hoc pairwise Wilcoxon rank-sum test, with Bonferroni correction. Categorical data are presented as frequencies and percentages and continuous variables as mean ± standard deviation.
The 1-year survival estimates are displayed according to the Kaplan–Meier method according to the 3 baseline LVEF groups, with comparisons of cumulative event rates by the log-rank test. We further similarly explored the survival estimates in subjects with and without diabetes and with or without STEMI at presentation.
In order to assess the independent risk associated with LVEF categories, we used Cox proportional hazard modeling. We assessed the independent risk associated with LVEF <30% and LVEF 30% to 49% against the group of LVEF ≥ 50% serving as reference. This model was further adjusted for the following covariates: age, prior MI, past PCI, hypertension (HTN), diabetes mellitus (DM), dyslipidemia, peripheral arterial disease (PAD), chronic renal failure, ST segment elevation on admission, Killip class ≥2, admission with syncope, and anterior MI.
We further used multivariable Cox proportional hazards regression models to identify predictors for 1-year mortality in each LVEF group, using best subset method (candidate covariates are listed in Table 1).
Table 1.
Characteristics of study population.
All statistical tests were 2-sided, a P-value of <0.05 was considered statistically significant. Analyses were carried out with SAS software (version 9.4, SAS institute, Cary, NC).
3. Results
A total of 11,536 patients were prospectively enrolled in the ACSIS surveys between 2000 and 2010, of whom 8983 (77.8%) had an echocardiogram performed within 48 hours of admission and comprised the present study population. The clinical characteristics of patients with and without a baseline echocardiogram were not significantly different (data not shown).
Among the 8983 study patients, 4588 (51%) had STEMI, and the remaining had NSTEMI. A total of 4470 patients (49.7%) were in the mild-moderate LV dysfunction group, 3659 (40.7%) patients had preserved LV function, and 854 (9.6%) patients had an LVEF < 30% during admission. The clinical characteristics of study patients according to their LVEF on admission are shown in Table 1.
Patients with severe LV dysfunction were older, and had a significantly higher prevalence of comorbidities, including HTN, DM, congestive heart failure, chronic renal failure, PAD, cerebrovascular disease, and prior IM. The prevalence of ST-segment elevation on admission was highest among patients with mild-moderate LV dysfunction (60%), compared to 51% and 40% among patients with severely reduced and preserved LV function, respectively (P < 0.001). There was no significant gender difference among the 3 LVEF groups (Table 1).
In patients with LVEF < 30%, there was a lower rate of primary PCI (32%) and reperfusion therapy than in patients with 30 ≤ LVEF < 49 (40%), but higher than in patients with LVEF > 50% (26%). However, there was not a clinically significant difference in primary PCI in eligible patients (patients without contraindication to primary PCI, no spontaneous reperfusion or other conditions that made primary PCI no longer indicated) among the 3 groups (about 65% to 69%). The decision of primary PCI was a clinical decision made by the cardiologist in each medical center.
PCI during hospitalization are much lower in LVEF < 30% group compared to the other groups. In this group, the patients were much older, had higher rate of renal failure, peripheral artery disease, prior congestive heart failure, DM, and prior CVA, and therefore were more prone to receive conservative therapy.
The frequency of patients admitted with preserved, mild to moderate, and severe LV dysfunction from 2000 to 2010 is shown in Fig. 1, demonstrating a gradual decline in the proportion of patients with decreased LVEF in more recent years. Accordingly, significantly more patients were admitted with preserved LV function in 2010 as compared to a decade ago, and a significantly lower frequency of patients with LV dysfunction.
Figure 1.
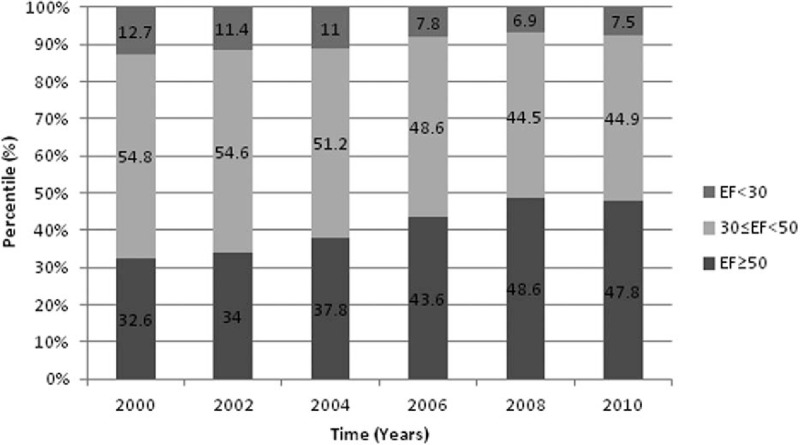
Left ventricle ejection fraction trend over the years of the study in total study population.
Kaplan–Meier analysis showed that mortality rates at 1-year were significantly correlated to LVEF; 36%, 10%, and 4% for patients with severe LV dysfunction, mild-moderate LV dysfunction, and preserved LV function, respectively (log rank P < 0.001 for the overall difference during follow-up [Fig. 2]). Notably, the association between reduced LVEF and increased mortality rates was consistent throughout all survey years (data not shown).
Figure 2.
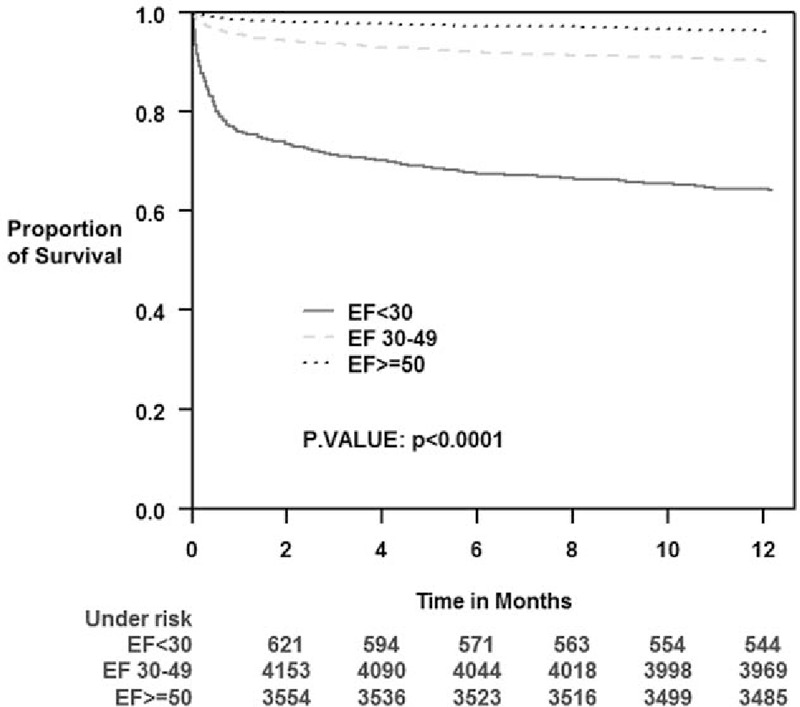
Kaplan–Meier survival estimates according to left ventricle ejection fraction groups during 1-year follow-up period.
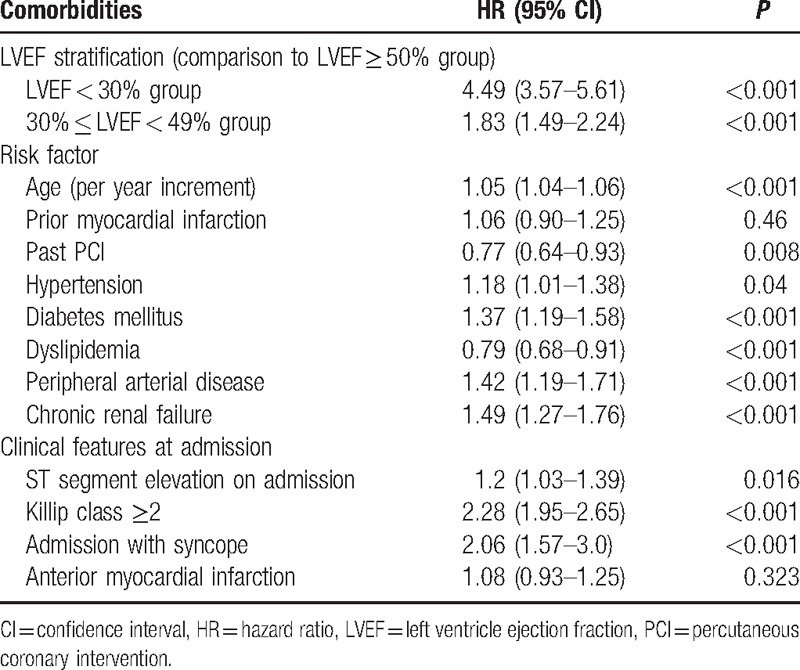
Consistently, Cox multivariable analysis demonstrated that subjects with LVEF < 30% and LVEF 30% to 49% had 4.49- and 1.83-fold greater mortality risk compared to the LVEF 50% group serving as the reference group (HR 4.49; [95% CI 3.57–5.61 and HR 1.83; [1.49–2.24], respectively). This independent association was adjusted for other important predictors of 1-year outcomes (Table 2). Interaction-term analysis was performed to assess the consistency of our findings by the type of MI. This analysis showed that the association between LVEF and 1-year mortality was evident among both STEMI and NSTEMI patients (all P-values for interaction >0.10).
Table 2.
Multivariate analysis: independent predictors of 1-year mortality.
In order to identify independent predictors for 1-year mortality within each LVEF subgroup (Table 3), we performed multivariable analysis within each LVEF group using the same best subset covariates. This analysis showed that age and admission Killip class were associated with increased risk for 1-year mortality in all LVEF subsets. However, other prognostic factors were different according to admission LVEF.
Table 3.
Adjusted hazard ratio (95% CI) for 1-year all-cause mortality outcome.
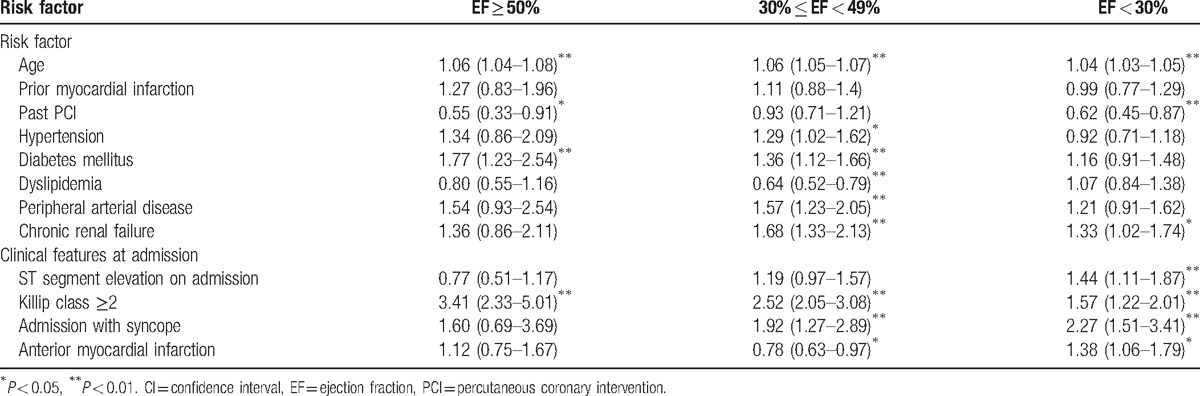
Within the group of patients with severe LV dysfunction the clinical features at admission were the most significant mortality predictors. Patients admitted with syncope experienced a significant >2-fold increased risk for 1-year mortality, patients with anterior MI experienced a significant 38% risk-increase, and those with STEMI versus NSTEMI experienced a corresponding 44% risk-increase (all P-values <0.05 [Table 3]).
Among patients with mild to moderate LV dysfunction there was a prominent association between the presence of comorbidities and increased 1-year mortality (Table 3): HTN, DM, PAD, and chronic renal failure were respectively associated with significant 29%, 36%, 57%, and 68% increased risk for 1-year mortality (P < 0.05 for all).
Similar to patients with mild to moderate LV dysfunction, among patients admitted with preserved systolic function (ie, EF ≥ 50 DM [HR 1.77, CI 1.23–2.54]) was a significant prognostic risk factor (Table 3).
Consistent with predictors identified in multivariable analysis, Kaplan–Meier survival analysis showed that the association between the presence or absence of ST-segment elevation on admission and the increased 1-year mortality was most prominent among patients with severe LV dysfunction on admission and less prominent among those with mild to moderate and preserved LV function (Fig. 3A and B). In Fig. 4, we can see that low EF was associated with worse prognosis in both DM and non-DM patients (Fig. 4A and B, respectively).
Figure 3.
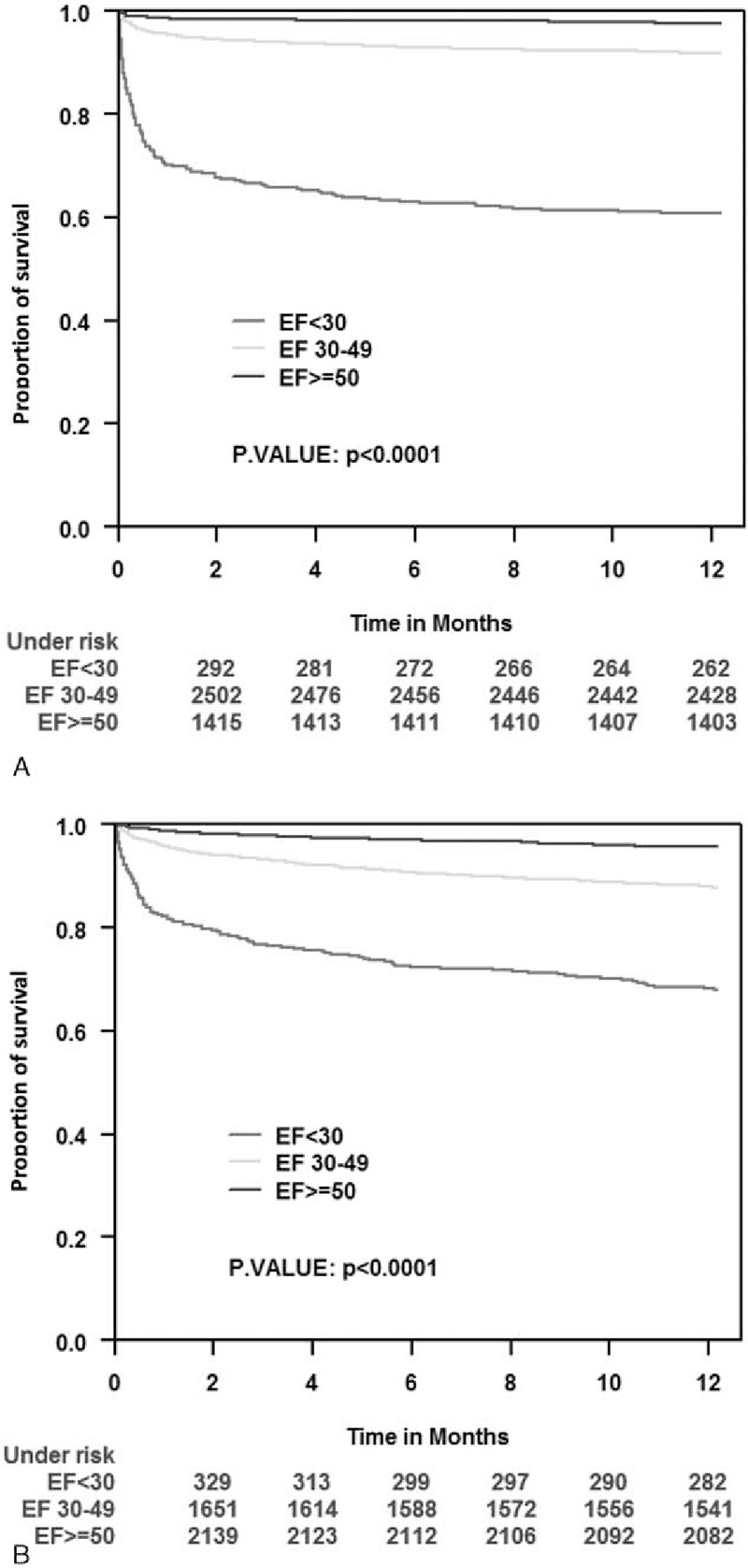
Kaplan–Meier survival estimates according to admission ST elevation and non-ST elevation during 1-year follow-up period by left ventricle ejection fraction groups. (A) STEMI population. (B) NSTEMI population. NSTEMI = non-ST elevation myocardial infarction, STEMI = ST elevation myocardial infarction.
Figure 4.
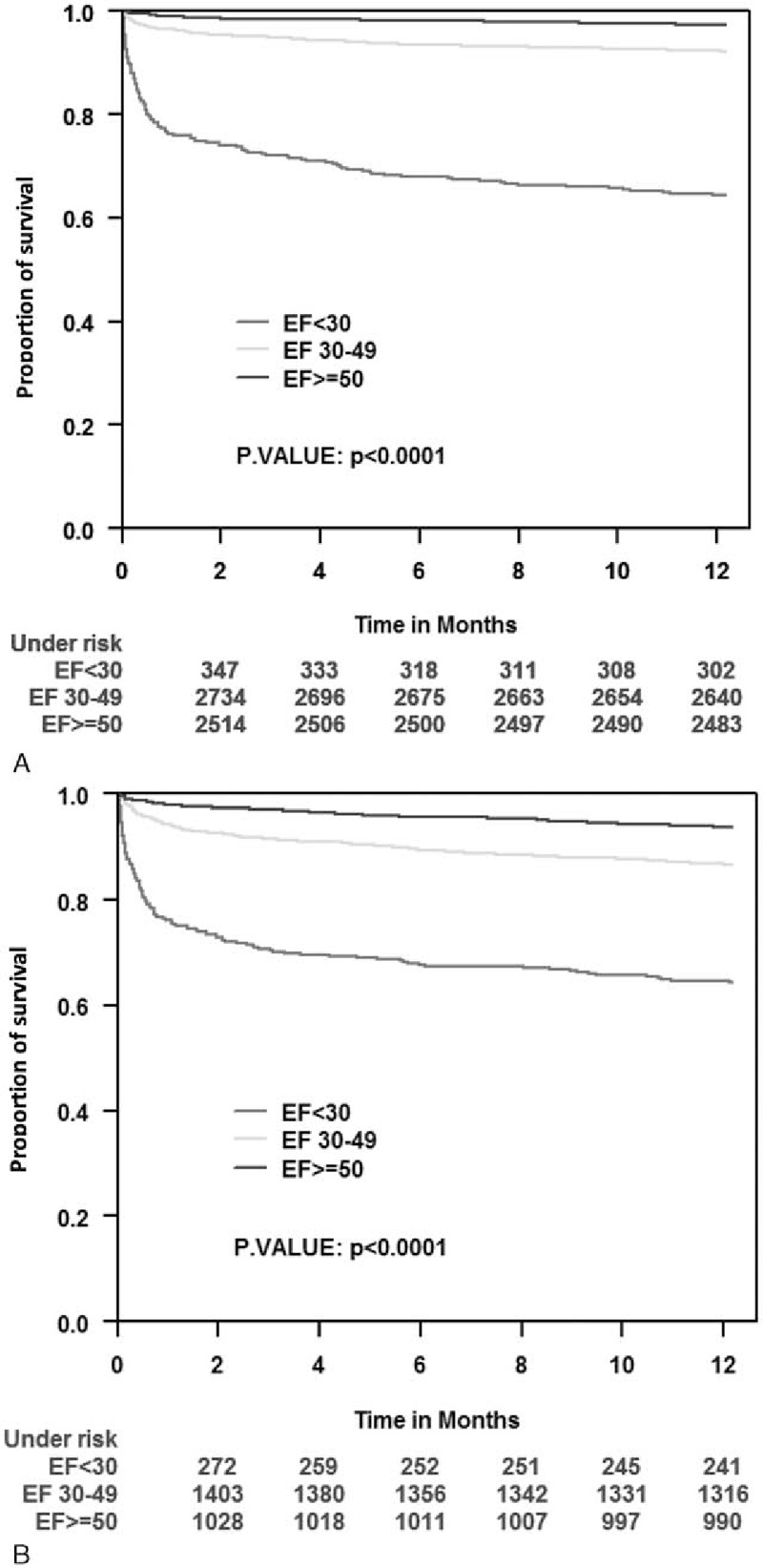
Kaplan–Meier survival estimates according to diabetes status during 1-year follow-up period by left ventricle ejection fraction groups. (A) Diabetes mellitus population. (B) Nondiabetes mellitus population.
4. Discussion
The ACSIS reflects a broad, unselected ACS population studied in real life since 2000. We used this registry to evaluate the trends for changes in EF at admission from 2000 to 2010 and to evaluate prognostic predictors of morbidity and mortality in the different group of patients according to their LVEF during admission as well as the prognostic impact of these changes overtime.
The main findings of this study are: over the past decade there was a statistically significant trend to more improved cardiac function following ACS, possibly related to a corresponding improvement in medical and procedural management strategies in this population; admission LVEF is still a powerful independent predictor of long-term prognosis in this contemporary cohort of real world ACS patients; and predictors of 1-year mortality following ACS appear to be related to LV function on admission. Although in patients admitted with severe LV dysfunction, the prognosis is mainly related to clinical factors and their clinical instability on admission, in patients with mild-moderate LV dysfunction or with preserved LV function the prognosis relates to classical risk factors such as HTN, dyslipidemia, and the presence of severe comorbidities such as DM and PAD. These findings suggest that LV function-specific risk factors should be employed in the risk stratification of ACS patients and are in complete accordance with the current guidelines, which advocate for an early invasive strategy in patients at high risk as well as an aggressive approach on the treatment of risk factors.[4–5,15]
LVEF is well known risk factor for prognosis in patients with ischemic heart disease, but to our knowledge, the present study is the first to evaluate cardiac function-specific risk factors for long-term mortality in ACS patients. Our data reinforces the current knowledge and provides a new insight into the risk stratification of this complex patient population. It demonstrates that the predictors of survival are different according the degree of LV dysfunction at admission.
Interestingly, the use of echocardiography for the early assessment of LV function in ACS patients with non-ST elevation ACS has a class I indication at the 2015 ESC guidelines while in the extensive AHA/ACC guidelines for ST and for NSTEMI, the evaluation of LV function is indicated only before discharge (class I indication).[4–6]
Patients with ACSs are a heterogeneous population with varying risks of death and recurrent cardiac events, in long-term as well as short-term follow-up. In these patients, early risk stratification plays a central role, as the benefit of newer and more aggressive and a costly treatment strategy seems to be proportional to the risk of adverse clinical events. The TIMI score was developed with the database from a large clinical trial of NSTE-ACS.[1] The Global Registry of Acute Coronary Events score was developed from the registry, with a population of patients across the entire spectrum of ACS.[2] Even though the prognostic impact of both scores have been confirmed after longer follow-up periods both these scores were initially developed for short-term assessment of risk and to define risk-oriented therapies in emergency departments and chest pain units.[16]
LVEF has long been recognized as one of the most important predictors of mortality in patients with an MI and in patients with coronary artery disease in general.
The added predictive effect of early evaluation of LVEF in patients admitted with NSTEMI/ACS was described by Bosch et al[7] who evaluated 1104 patients from the PRISM-plus trial registry whose LVEF was obtained during admission. Adding LVEF to the TIMI score model improved mortality prediction. The odds for in hospital death significantly increased for each 1% decrease in LVEF. The mortality rate was 3.3 times higher within each TIMI score stratum in patients with an LVEF < 48%.
Palmerini et al[17] evaluated the predictive values of 6 different risk scores in 2094 patients with NSTEMI who had an early PCI in the ACUITY trial. Scores that incorporated clinical data and EF to the angiographic data such as the clinical syntax score[18] and the new risk stratification score[19] had a higher discriminative effect for ischemic end points and mortality that strictly angiographic scores.[17] They validated a new prognostic score (ACUITY-PCI score) in 846 patients, with LVEF of 54% ± 11%, admitted due to ACS and treated invasively. In this study, in addition to the electrocardiographic and angiographic variables, the presence of insulin-treated DM and renal insufficiency were strong prognostic predictors.[20]
The prognostic impact of associated risk factors and comorbidities states in patients with ACS and the need for treatment is well known. In this study, we demonstrated that the significance of this prognostic impact is variable according to LV function at baseline.
5. Limitations
ACSIS is an observational study of the current clinical practice in Israel, and each patient is treated according to local policies in each participating center. A core-lab was not utilized as ACSIS is a real-world national survey representing all cardiology departments in Israel. Even though measurements of LVEFs were subjectively obtained in different clinical centers and the timing of LVEF assessment during admission was not strictly controlled; previous studies by our group have shown a clear correlation between subjectively assessed LVEF and prognosis.[12] In addition, we do not have any data about EF prior to discharge or a month later.
There is a selection bias. As this study enrolled only patients admitted to the cardiac care unit/cardiology wards, patients who were admitted to internal medicine departments or died before admission to cardiology departments are not represented in this study. For the same reason a higher than expected proportion of STEMI is reported, so that this finding may not be generalized to ACS patients admitted to other wards. Finally, there is no information regarding the specific cause of death.
In addition due to the very small number of patients with Killip class IV, we included all patients with clinical cardiac failure on admission (Killip ≥II) rather than Killip class IV as a covariate in the multivariable models.
6. Conclusions
We have shown that the proportion of ACS patients admitted with more preserved LV function increased over the past decade and that admission LVEF still remains is a powerful predictor of 1-year mortality, and should therefore be a part of routine evaluation and risk stratification in these patients. Our findings indicate that prognostic factors following ACS differ according to admission LVEF, suggesting a possible role for cardiac function-specific risk assessment in this population.
Footnotes
Abbreviations: ACS = acute coronary syndrome, ACSIS = Acute Coronary Syndromes-Israel Survey, DM = diabetes mellitus, EF = ejection fraction, HTN = hypertension, LV = left ventricle, LVEF = left ventricle ejection fraction, MI = myocardial infarction, NSTEMI = non-ST elevation myocardial infarction, PAD = peripheral arterial disease, PCI = percutaneous coronary intervention, STEMI = ST elevation myocardial infarction, TIMI = thrombolysis in myocardial infarction.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–42. [DOI] [PubMed] [Google Scholar]
- [2].Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163:2345–53. [DOI] [PubMed] [Google Scholar]
- [3].de Araujo Goncalves P, Ferreira J, Aguiar C, et al. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J 2005;26:865–72. [DOI] [PubMed] [Google Scholar]
- [4].Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–228. [DOI] [PubMed] [Google Scholar]
- [5].O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78–140. [DOI] [PubMed] [Google Scholar]
- [6].Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- [7].Bosch X, Theroux P. Left ventricular ejection fraction to predict early mortality in patients with non-ST-segment elevation acute coronary syndromes. Am Heart J 2005;150:215–20. [DOI] [PubMed] [Google Scholar]
- [8].Bedetti G, Gargani L, Sicari R, et al. Comparison of prognostic value of echographic [corrected] risk score with the Thrombolysis in Myocardial Infarction (TIMI) and Global Registry in Acute Coronary Events (GRACE) risk scores in acute coronary syndrome. Am J Cardiol 2010;106:1709–16. [DOI] [PubMed] [Google Scholar]
- [9].Morici N, Savonitto S, Murena E, et al. Causes of death in patients >/=75 years of age with non-ST-segment elevation acute coronary syndrome. Am J Cardiol 2013;112:1–7. [DOI] [PubMed] [Google Scholar]
- [10].Burns RJ, Gibbons RJ, Yi Q, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 2002;39:30–6. [DOI] [PubMed] [Google Scholar]
- [11].Lansky AJ, Goto K, Cristea E, et al. Clinical and angiographic predictors of short- and long-term ischemic events in acute coronary syndromes: results from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial. Circ Cardiovasc Interv 2010;3:308–16. [DOI] [PubMed] [Google Scholar]
- [12].Carasso S, Sandach A, Beinart R, et al. Usefulness of four echocardiographic risk assessments in predicting 30-day outcome in acute myocardial infarction. Am J Cardiol 2005;96:25–30. [DOI] [PubMed] [Google Scholar]
- [13].Zahger D, Hod H, Gottlieb S, et al. Influence of the new definition of acute myocardial infarction on coronary care unit admission, discharge diagnosis, management and outcome in patients with non-ST elevation acute coronary syndromes: a national survey. Int J Cardiol 2006;106:164–9. [DOI] [PubMed] [Google Scholar]
- [14].Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1.e14–39.e14. [DOI] [PubMed] [Google Scholar]
- [15].Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- [16].Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J 2007;153:29–35. [DOI] [PubMed] [Google Scholar]
- [17].Palmerini T, Caixeta A, Genereux P, et al. Comparison of clinical and angiographic prognostic risk scores in patients with acute coronary syndromes: analysis from the Acute Catheterization and Urgent Intervention Triage StrategY (ACUITY) trial. Am Heart J 2012;163:383–91. 391.e381-385. [DOI] [PubMed] [Google Scholar]
- [18].Garg S, Sarno G, Garcia-Garcia HM, et al. A new tool for the risk stratification of patients with complex coronary artery disease: the Clinical SYNTAX Score. Circ Cardiovasc Interv 2010;3:317–26. [DOI] [PubMed] [Google Scholar]
- [19].Chen SL, Chen JP, Mintz G, et al. Comparison between the NERS (New Risk Stratification) score and the SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) score in outcome prediction for unprotected left main stenting. JACC Cardiovasc Interv 2010;3:632–41. [DOI] [PubMed] [Google Scholar]
- [20].Palmerini T, Genereux P, Caixeta A, et al. A new score for risk stratification of patients with acute coronary syndromes undergoing percutaneous coronary intervention: the ACUITY-PCI (Acute Catheterization and Urgent Intervention Triage Strategy-Percutaneous Coronary Intervention) risk score. JACC Cardiovasc Interv 2012;5:1108–16. [DOI] [PubMed] [Google Scholar]


