Abstract
Walking speed is a measure of gait performance after a stroke and a predictor of community ambulatory competence. Although gait decrements during a cognitive or motor task after stroke are well-documented, the differential effects of motor and cognitive tasks on the comfortable and maximum walking speeds of individuals with chronic stroke have not been investigated. This study aimed to compare the effects of cognitive and motor tasks on the comfortable and maximum walking speeds of individuals with chronic stroke.
This is a cross-sectional study. Thirty community-dwelling chronic stroke individuals were included. Time taken to complete the 10-meter Walk Test under various conditions, including walking alone, walking while completing a cognitive task, and walking while completing a motor task, was recorded, with each condition performed at comfortable as well as maximum walking speeds. Accuracy in performing the cognitive tasks was also assessed.
The cognitive and motor tasks caused decrements in both comfortable and maximum walking speeds (P ≤ 0.001). The cognitive task had a greater influence than the motor task on maximum walking speed (P < 0.01).
Individuals with chronic stroke tend to prioritize task accuracy and completion over maintaining walking speed. This phenomenon was more evident during the cognitive task than the motor task and was especially evident at maximum walking speed.
Keywords: cognition, speed, stroke, walking
1. Introduction
Stroke is one of the major chronic illnesses worldwide that leads to severe long-term disability.[1] Approximately, 80% of stroke survivors initially find that they have lost their walking ability, although it gradually improves with rehabilitation.[2] However, some individuals are unable to walk safely in the community after a stroke and tend to have a higher incidence of falls.[2,3]
Community ambulation is often the primary goal of rehabilitation after a stroke, as it has important implications for overall health status and well-being.[4] It requires walking in a complex environment while focusing on multiple attention-demanding tasks, including walking faster when crossing the street, negotiating obstacles, and holding conversations in a busy environment. Walking speed is a strong predictor of community ambulatory competence, which correlates with walking ability. An improvement in walking speed may result in better functioning and quality of life.[3,5] Stroke survivors with walking dysfunction have been found to walk more slowly during the 10-meter Walk Test (10mWT) and cover less distance during the 6-minute Walk Test than healthy individuals.[6]
Community ambulation is considered much more demanding cognitively than walking in a controlled environment.[7] Cognitive motor interference, owing to a concurrent cognitive or motor task, is postulated to affect walking speed and the quality of walking, leading to poor participation in the community. Cognitive motor interference following a stroke could be because of a reduced capacity to perform cognitive and motor tasks simultaneously,[8] reduced cognitive function,[9] or decreased walking ability secondary to post-stroke motor deficits.[10] Evidence from several studies shows that gait performance is significantly affected when stroke survivors attempt walking and cognitive tasks simultaneously compared with walking or cognitive tasks alone.[11,12] Yang et al[13] then reported that different motor tasks could have different impacts on post-stroke gait performance.
Most stroke-specific studies have only explored the respective impacts of either cognitive or motor tasks on gait performance separately.[11–13] An exception is a study by Dennis et al that explored the effects of different cognitive tasks on preferred and fast walking speeds in chronic stroke populations.[11] Although gait decrements during a cognitive or motor task after stroke are well-documented, the differential effects of motor and cognitive tasks on the comfortable and maximum walking speeds of individuals with chronic stroke have not been investigated.
The objective of our study was to compare the effects of cognitive and motor tasks on the comfortable and maximum walking speeds of individuals with chronic stroke. We hypothesized that there would be significant differences in reduction of walking speed when performing cognitive or motor tasks while walking.
2. Methods
2.1. Participants
This was a cross-sectional study conducted in the rehabilitation center at The Hong Kong Polytechnic University. Owing to a lack of previous studies to refer to for effect size calculations, an F-test with an analysis of variance was used with a conventional effect size of 0.25 (medium effect size) for repeated measures within factors. This produced a sample size of 28 subjects. Statistical significance was set to 5% (alpha level 0.05) and the power to 80% (beta level at 0.2).
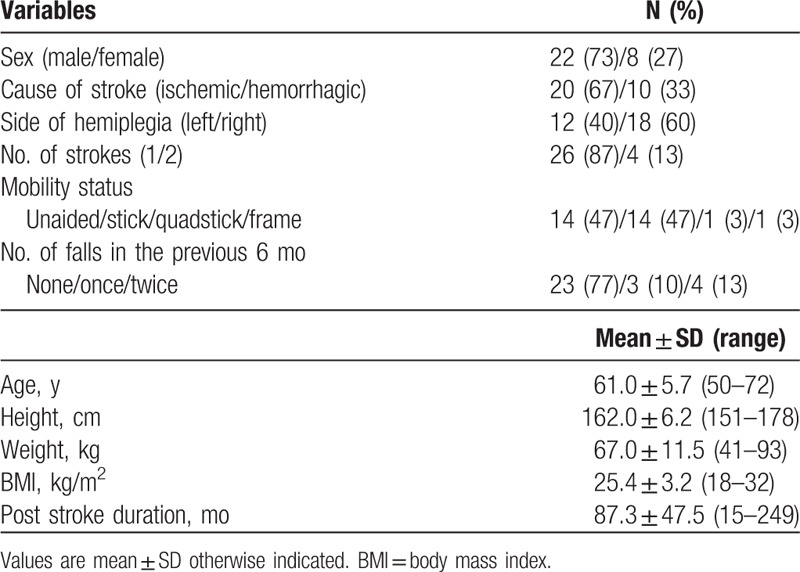
Thirty subjects (22 men, 8 women; mean age ± SD 61.0 ± 5.7 years) with a mean ± standard deviation (SD) post-stroke duration of 87.3 ± 47.5 months (Table 1) were recruited from self-help groups for stroke survivors in Hong Kong. The inclusion criteria were age ≥55 years; at least 9 months since the most recent stroke; able to walk 14 m independently without an assistive device. Subjects were excluded if they had an Abbreviated Mental Test (AMT) score[14] of <7; had a preexisting co-morbidity (other neurological or musculoskeletal disorders) that would hinder proper assessment; had communication problems, such as receptive aphasia; or were unable to give informed consent.
Table 1.
Descriptive characteristics of the subjects (n = 30).
The study was approved by the ethics committee of The Hong Kong Polytechnic University and conducted according to the Declaration of Helsinki for human experiments. The study's objectives and procedures were explained to all of the subjects, and written consent was obtained from each before the study.
3. Outcome measurements
3.1. Walking task (10mWT)
A 10-m walkway was marked out in a straight corridor using colored tape with an additional 2 m at each end for acceleration and deceleration. The time a subject took to walk through the 10-m section was recorded using a stopwatch. The 10mWT has shown excellent test–retest, intra-rater, and inter-rater reliability for patients with stroke, with intraclass correlation coefficient of 0.8 to 0.98 at both the comfortable and fast walking speeds.[15,16]
Each subject performed the walk test under 6 task conditions in a randomized order determined by drawing lots to avoid subject bias. One practice trial and 2 timed trials were performed for each task condition, with 1 minute of rest in between trials and after each task condition to minimize fatigue and learning effects. The mean value of the 2 timed trials was used in the data analysis. The 6 test conditions were
-
(1)
Condition 1: 10mWT at a comfortable walking speed only.
-
(2)
Condition 2: 10mWT at the maximum walking speed only.
-
(3)
Condition 3: 10mWT combined with a cognitive task at comfortable walking speed.
-
(4)
Condition 4: 10mWT combined with a cognitive task at maximum walking speed.
-
(5)
Condition 5: 10mWT combined with a motor task at comfortable walking speed.
-
(6)
Condition 6: 10mWT combined with a motor task at maximum walking speed.
The subjects were instructed to maintain the desired speed while performing the assigned cognitive or motor task to the best of their ability without prioritizing either walking or the task. Standardized instructions were given during assessment, “When I say go, walk ‘at your most comfortable speed’ or ‘as fast and as safely as you can’ to the end of the walkway” for the comfortable and maximum speed trials, respectively.
Two assessors (GLY, YL) were involved in the current study. Assessor A (GLY) gave the instructions, counted the steps, and recorded the time using a stopwatch. Assessor B (YL) recorded the numbers verbalized during the cognitive trials, as well as any stops or water spillages during the motor trials throughout the testing.
3.2. Combined cognitive and walking task
Subjects were instructed to perform serial subtraction in multiples of 3.[11,12] The additional instruction for the combined cognitive and walking trials was, “Count backward by multiples of three from X,” where X was a random number between 70 and 99 assigned by computer software to avoid any bias in the distribution of numbers. The subject started counting aloud before walking in response to an instruction by the assessor, but only the numbers verbalized in the middle 10 m of the 14-m walkway were recorded for data analysis. The total number of attempts and the number of correct attempts were used to quantify the subject's accuracy in the cognitive task.
3.3. Combined motor and walking task
During the combined motor and walking trials, the subject was required to carry a cup of water without a handle with the surface of the water 3 cm from the top edge of the cup.[13,17] The additional instruction for those trials was, “while carrying this cup of water without spilling it.” The number of spillages and stops during the middle 10 m, if any, were recorded.
3.4. Single cognitive task
The subjects were also required to perform the serial subtraction task while seated[11,12] for the same length of time that the same subject took for conditions 3 and 4, respectively. The total number of attempts and the number of correct attempts were recorded.
3.5. Statistical analyses
Descriptive statistics were compiled to describe the demographic characteristics of the subjects. The differences in 2 walking speeds (comfortable and maximum walking speeds) between the 3 task conditions (walking task alone, combined cognitive and walking task, combined motor and walking task) were analyzed using 1-way repeated measures of analysis of variance. If an overall significant main effect was found, contrast analysis (pair-wise comparison) between conditions was explored applying the Bonferroni correction. The significance level was set to 5%.
The decrements in walking speed during the combined tasks were expressed as a percentage using the following formula:
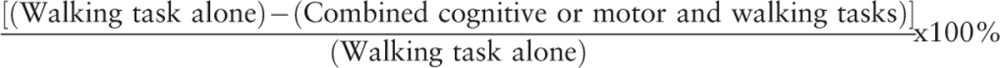 |
A paired t test was performed on this percentage change to evaluate the effect of cognitive and motor tasks on walking speed. The differences in accuracy of serial subtraction between the single cognitive task and the combined cognitive and walking tasks were analyzed using a paired t test. All the statistical analyses were conducted using the Statistical Package for the Social Sciences (version 21.0; SPSS Inc, Chicago, IL).
4. Results
The mean walking speeds under the 6 conditions ranged from 0.82 to 1.0 m/s (Table 2).
Table 2.
Comfortable and maximum walking speeds in three task conditions (n = 30).
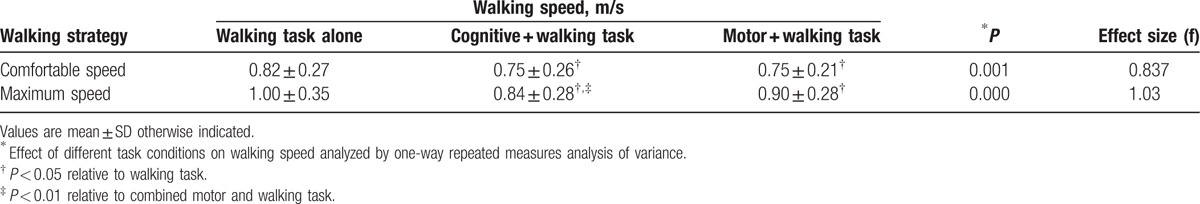
Significant differences in comfortable walking speed (P = 0.001) and maximum walking speed (P < 0.001) were observed among the 6 task conditions. Table 2 shows that there was a significant reduction in comfortable and maximum walking speeds during both the cognitive and motor tasks.
When comparing the combined cognitive and walking tasks to the combined motor and walking tasks, there was only a significant difference (P < 0.001) in maximum walking speed, not in comfortable walking speed (Fig. 1), with the cognitive task resulting in a more significant decrement in maximum walking speed. The combined cognitive tasks of serial subtraction and walking led to an 8.3% slower comfortable walking speed and a 15.6% slower maximum speed, which were significantly different (P < 0.001). No significant difference was observed in the subjects’ accuracy between subtracting while walking and subtracting while sitting at either the comfortable or maximum walking speed (Fig. 2).
Figure 1.
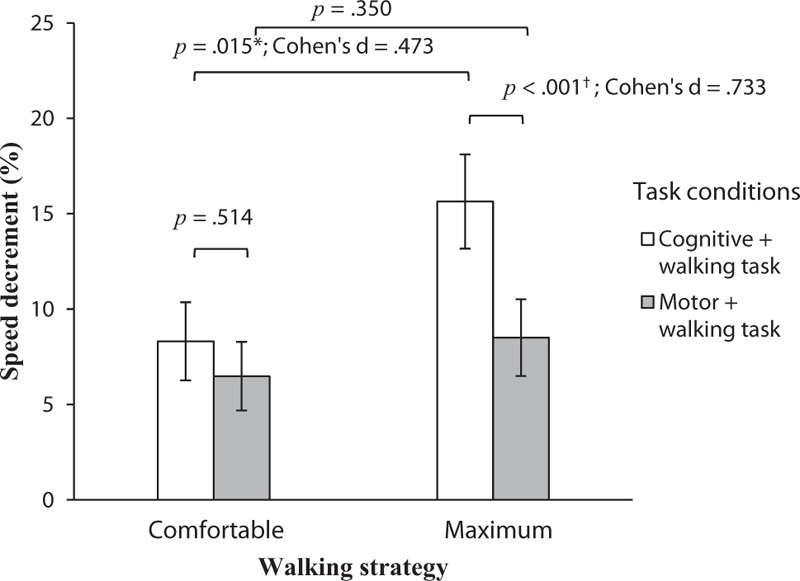
Walking speed decrements (%) in the combined tasks at comfortable and maximum speeds. ∗Statistically significant difference between comfortable and maximum walking speed (P < 0.05). †Statistically significant difference between combined cognitive and walking tasks and combined motor and walking tasks (P < 0.001).
Figure 2.
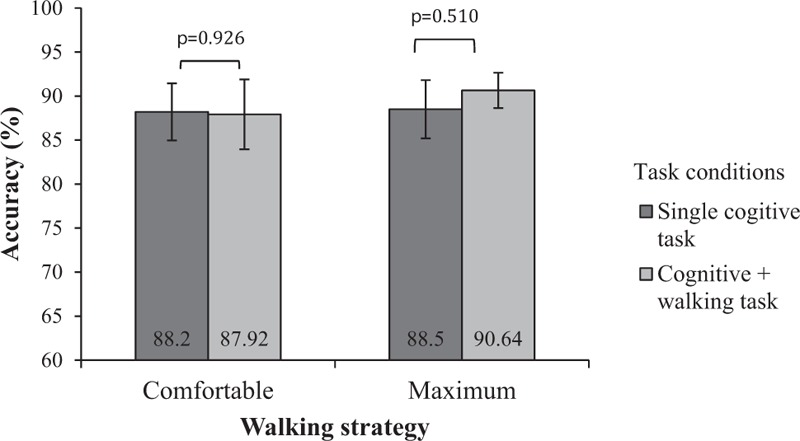
Accuracy (%) of serial subtraction tasks.
5. Discussion
5.1. Walking performance
The mean comfortable and maximum walking speeds using a 10-m walkway were comparable to the speeds observed in previous studies.[15,18] A plausible rationale for the lower range of the maximum walking speeds observed compared with other studies could be inherent differences in the inclusion criteria. Flansbjer et al[15] recruited subjects with better walking capacity who could walk at least 300 m with or without an assistive device, whereas our study accepted subjects who could walk at least 14 m independent of an assistive device. Flansbjer et al's subjects may have had better baseline walking performance and faster walking speeds.
5.2. Cognitive task reduced walking speed
The results showed significant decrements in comfortable and maximum walking speeds during the combined cognitive and walking trials compared to the walking task alone. Previous studies have shown that the walking performances of individuals with chronic stroke, healthy older adults,[19] and frail older adults[20] with recurrent falls were poorer, albeit to varying degrees, when they are simultaneously performing a cognitive task.[12,21] These results were consistent with those reported by Patel and Bhatt,[12] whose stroke group tended to walk slower during serial subtraction.
Cognitive decline in subjects with stroke could explain the reduction in walking speed while performing the serial subtraction task. It has been postulated that higher-level cognitive processes such as executive functions and attention play vital roles in complex walking performance.[22,23] Individuals with stroke often have some degree of subclinical cognitive impairment unrelated to dementia[24] and a significant deficit in working memory.[25] Stroke-related brain lesions are closely associated with working memory performance.[12] Although the nature, regions, and side of the brain lesions in these subjects were not examined extensively, previous studies have shown that unilateral lesions of the prefrontal cortex strongly predict deficits in cognition, especially in working memory tasks such as mental arithmetic.[12,26] As gait control requires attention, it seems that any additional cognitive task will lead to increased competing demands on the brain's executive resources.[27]
Although these subjects all shown intact cognition in their cognitive assessments, studies have shown substantial declines in working memory, information processing, and attention in subjects with chronic stroke compared to age-matched controls.[12,25] Such declines may not be apparent in simple situations.[28] The subjects’ poorer performance with the concurrent cognitive task and at faster speeds may reveal a gradual decline in cognitive functioning. Thus, it is postulated that a decline in working memory performance owing to stroke resulted in a higher cognitive requirement during combined cognitive and walking tasks, leading to slower comfortable and maximum walking speeds.
The serial subtraction task involved not simply mental arithmetic but also a personally generated answer sequence.[11,12,29] Some subjects were observed to resort to talking and walking with identical rhythms to cope with the overall demands of the combined cognitive and walking tasks.[11,30] The culmination of these various demands could likely exhaust the subject's attention resources.
Attention deficits are among the most common stroke sequelae, resulting in greater difficulties in dividing attention between motor and cognitive tasks.[31] While subtracting at maximum walking speed, a subject with an attention deficit was required to tax further the motor-attention resources applied to maintaining walking speed and gait control. Thus, the cognitive and motor processing conflict also increased, resulting in slower speeds.[11,32] Serial subtraction taxes the working memory component of cognition.[12,33] The subjects were instructed to prioritize neither the walking nor the additional cognitive or motor task. However, the results show no significant differences in the accuracy of the serial subtractions at the comfortable and maximum walking speeds (Fig. 2). Dennis et al[11] reported that when walking at maximum speed, their subjects prioritized successful completion of the cognitive task over maintaining maximum walking speed. They would revert to their “default” speed to minimize attention cost and maximize safety.[11] This observation may explain the greater decrease in walking speed when our subjects combined the serial subtraction task with maximum walking speed as opposed to comfortable walking speed.
5.3. Motor task reduced walking speed
The combined motor task of carrying a cup of water and walking resulted in a significant decrease in both comfortable and maximum walking speeds when compared with the walking task alone (see Table 2). These results were consistent with the findings of a study by Yang et al,[13] who found a significant reduction in comfortable walking speed when their full community ambulatory subjects were carrying a tray of glasses bimanually.
Performing a motor task in addition to walking will often exceed the available attention resources of stroke survivors.[11,13] As the difficulty of the task increases, the increased attention demanded competes for the limited attention resources available.[11,12,30] In this study, the attention needed to carry a cup of water without spilling had to compete with the attention needed to control the gait during walking.[11,34] This attentional competition was exacerbated at maximum walking speed, resulting in greater speed decrements at maximum walking speed as subjects prioritized not spilling the water over walking.
Visual attention was vital for successful completion of the combined motor and walking task for stroke survivors.[34] The subjects were required not only to judge visually the distance for their walking task but also to visually monitor the water in the cup they were carrying to avoid spillage.
Although both the cognitive and motor tasks resulted in reduced walking speed, it is interesting to note that the cognitive task caused a greater reduction in maximum walking speed than the motor task. The variability in speed reduction depended on the nature of the additional cognitive or motor task. A more difficult task would demand greater attention, resulting in greater cognitive-motor interference, resulting in poorer walking performance.[12,30] Serial subtraction utilizes working memory, and when compared with spatial and executive functioning, is considered more complex and demanding.[11,12] However, the results showed that the subjects’ serial subtraction task accuracy was better when they were trying to walk at their maximum speed rather than their comfortable walking speed (Fig. 2). Evidently, these subjects prioritized the cognitive task over the walking task despite being instructed not to do so, and even more so when they tried to walk at maximum speed, thus resulting in a greater speed reduction.
Serial subtractions are not, of course, a realistic voluntary task while walking, especially when compared to walking while holding a cup of water. This difference may have encouraged the subjects to be partial to the additional task that was more “natural” and consistent with their activities of daily living, which in this case, was the motor task rather than the cognitive task.
5.4. Study limitations
The subjects recruited in our study were active members of local self-help groups. They had reasonably good cognitive and motor abilities and were motivated to participate. Cognitive functions and rehabilitation and health are dependent on social income, environment, medical care, and physical activities.[35] These factors were not fully examined during this study to determine confounding factors; this should be examined in further studies. Our subjects may also be physically active, which studies have shown to have a positive influence on cognitive function by stimulating neurogenesis and benefit brain plasticity in older populations.[36–38] However, the complexities and mechanism of neurobiological rehabilitation may be better understood in further studies.[39,40] Our results may not apply to individuals with poorer cognitive or motor functioning. The population studied here could have been more evenly distributed to ensure that there was no sex bias. Moreover, the study was conducted in a controlled environment, and hence, the results may not be readily transferable to a community setting.
This study examined only the subjects’ walking speeds, not their gaits quality. Further studies could apply computerized gait analysis to measure the temporal and spatial parameters objectively. Although the cognitive task employed in this study was derived from those used in previous studies, it probably did not realistically represent the distractions affecting those with chronic stroke. Further studies with more realistic cognitive tasks and in a community setting are warranted.
The serial subtraction task used here is related to the working memory domain of cognitive functioning. Performing working memory tasks has been reported to discriminate effectively between fallers and nonfallers better than performance in other domains of cognitive functioning.[41] Further studies incorporating working memory tasks with walking might be able to define an optimum protocol as an intervention for individuals with chronic stroke.
6. Conclusions
Both cognitive and motor tasks resulted in significant decrements in both the comfortable and the maximum walking speeds of individuals with chronic stroke. The cognitive task produced a greater reduction than the motor task in walking speeds, especially at maximum speed.
Acknowledgments
The authors acknowledge Dr. Raymong Chung of the Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, for invaluable input for statistical consultation, and Mr. Patrick Kwong of the Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, for enabling the recruitment of study participants.
Footnotes
Abbreviations: 10mWT = 10-meter Walk Test, AMT = abbreviated mental test, BMI = body mass index, SD = standard deviation.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
No specific funding support exists for this study.
Second revision submission to Medicine (January 14, 2017).
References
- [1].Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil 2002;83:165–70. [DOI] [PubMed] [Google Scholar]
- [3].Jørgensen HS, Nakayama H, Raaschou HO, et al. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 1995;76:27–32. [DOI] [PubMed] [Google Scholar]
- [4].Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res 1988;11:181–4. [Google Scholar]
- [5].Perry J, Garrett M, Gronley JK, et al. Classification of walking handicap in the stroke population. Stroke 1995;26:982–9. [DOI] [PubMed] [Google Scholar]
- [6].Ng S, Hui-Chan C. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil 2005;86:1641–7. [DOI] [PubMed] [Google Scholar]
- [7].Lundin-Olsson L, Nyberg L, Gustafson Y. Stops walking when talking as a predictor of falls in elderly people. Lancet 1997;349:617. [DOI] [PubMed] [Google Scholar]
- [8].Regnaux J, David D, Daniel O, et al. Evidence for cognitive processes involved in the control of steady state of walking in healthy subjects and after cerebral damage. Neurorehabil Neural Re 2005;19:125–32. [DOI] [PubMed] [Google Scholar]
- [9].Sheridan PL, Solomont J, Kowall N, et al. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer's disease. J Am Geriatr Soc 2003;51:1633–7. [DOI] [PubMed] [Google Scholar]
- [10].Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture 2009;29:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dennis A, Dawes H, Elsworth C, et al. Fast walking under cognitive-motor interference conditions in chronic stroke. Brain Res 2009;1287:104–10. [DOI] [PubMed] [Google Scholar]
- [12].Patel P, Bhatt T. Task matters: influence of different cognitive tasks on cognitive-motor interference during dual-task walking in chronic stroke survivors. Top Stroke Rehabil 2014;21:347–57. [DOI] [PubMed] [Google Scholar]
- [13].Yang Y-R, Chen Y-C, Lee C-S, et al. Dual-task-related gait changes in individuals with stroke. Gait Posture 2007;25:185–90. [DOI] [PubMed] [Google Scholar]
- [14].Jitapunkul S, Pillay I, Ebrahim S. The abbreviated mental test: its use and validity. Age Ageing 1991;20:332–6. [DOI] [PubMed] [Google Scholar]
- [15].Flansbjer U-B, Holmbäck AM, Downham D, et al. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med 2005;37:75–82. [DOI] [PubMed] [Google Scholar]
- [16].Fulk GD, Echternach JL. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J Neurol Phys Ther 2008;32:8–13. [DOI] [PubMed] [Google Scholar]
- [17].Silsupadol P, Shumway-Cook A, Lugade V, et al. Effects of single-task versus dual-task training on balance performance in older adults: a double-blind, randomized controlled trial. Arch Phys Med Rehabil 2009;90:381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maeda A, Yuasa T, Nakamura K, et al. Physical performance tests after stroke: reliability and validity. Am J Phys Med Rehabil 2000;79:519–25. [DOI] [PubMed] [Google Scholar]
- [19].Dubost V, Kressig RW, Gonthier R, et al. Relationships between dual-task related changes in stride velocity and stride time variability in healthy older adults. Hum Mov Sci 2006;25:372–82. [DOI] [PubMed] [Google Scholar]
- [20].Beauchet O, Annweiler C, Allali G, et al. Recurrent falls and dual task–related decrease in walking speed: is there a relationship? J Am Geriatr Soc 2008;56:1265–9. [DOI] [PubMed] [Google Scholar]
- [21].Hyndman D, Ashburn A, Yardley L, et al. Interference between balance, gait and cognitive task performance among people with stroke living in the community. Disabil Rehabil 2006;28:849–56. [DOI] [PubMed] [Google Scholar]
- [22].Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing 2006;35:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Springer S, Giladi N, Peretz C, et al. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Movement Disord 2006;21:950–7. [DOI] [PubMed] [Google Scholar]
- [24].Jin Y-P, Di Legge S, Ostbye T, et al. The reciprocal risks of stroke and cognitive impairment in an elderly population. Alzheimers Dement 2006;2:171–8. [DOI] [PubMed] [Google Scholar]
- [25].Schaapsmeerders P, Maaijwee NA, van Dijk EJ, et al. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke 2013;44:1621–8. [DOI] [PubMed] [Google Scholar]
- [26].Baier B, Karnath H-O, Dieterich M, et al. Keeping memory clear and stable—the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci 2010;30:9788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Beauchet O, Dubost V, Aminian K, et al. Dual-task-related gait changes in the elderly: does the type of cognitive task matter? Journal Motor Behav 2005;37:259. [PubMed] [Google Scholar]
- [28].Cockburn J, Haggard P, Cock J, et al. Changing patterns of cognitive-motor interference (CMI) over time during recovery from stroke. Clin Rehabil 2003;17:167–73. [DOI] [PubMed] [Google Scholar]
- [29].Coelho FG, Andrade LP, Pedroso RV, et al. Multimodal exercise intervention improves frontal cognitive functions and gait in Alzheimer's disease: a controlled trial. Geriatr Gerontol Int 2013;13:198–203. [DOI] [PubMed] [Google Scholar]
- [30].Plummer P, Eskes G, Wallace S, et al. Cognitive-motor interference during functional mobility after stroke: state of the science and implications for future research. Arch Phys Med Rehabil 2013;94:2565–74. e2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lincoln N, Majid M, Weyman N. Cognitive rehabilitation for attention deficits following stroke. Cochrane Db Syst Rev 2000;4. [DOI] [PubMed] [Google Scholar]
- [32].Warburton EC, Wilson M, Lynch M, et al. The cognitive benefits of movement reduction evidence from dance marking. Psychol Sci 2013;24:1732–9. [DOI] [PubMed] [Google Scholar]
- [33].Dubost V, Beauchet O, Herrmann François R, et al. Stride-to-stride variability while backward counting among healthy young adults. J Neuroeng and Rehabil 2005;2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther 2003;83:237–52. [PubMed] [Google Scholar]
- [35].Leischik R, Dworrak B, Strauss M, et al. Plasticity of health. German J Med 2016;1:1–7. [Google Scholar]
- [36].Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002;25:295–301. [DOI] [PubMed] [Google Scholar]
- [37].Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med 2009;43:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Strout KA, David DJ, Dyer EJ, et al. Behavioral interventions in six dimensions of wellness that protect the cognitive health of community-dwelling older adults: a systematic review. J Am Geriatr Soc 2016;64:944–58. [DOI] [PubMed] [Google Scholar]
- [39].Anthony K. Neurobiology: rise of resilience. Nature 2016;531:S18. [DOI] [PubMed] [Google Scholar]
- [40].McGilvray A. Ageing: restoration project. Nature 2016;531:S4. [DOI] [PubMed] [Google Scholar]
- [41].Baetens T, De Kegel A, Palmans T, et al. Gait analysis with cognitive-motor dual tasks to distinguish fallers from nonfallers among rehabilitating stroke patients. Arch Phys Med Rehabil 2013;94:680–6. [DOI] [PubMed] [Google Scholar]


