Abstract
Little is known about the effect of nonsteroidal anti-inflammatory drugs (NSAIDs), thiazolidinediones (TZDs), nifedipine and nondihydropyridine calcium channel blockers (CCBs) usage on the risk of all-cause hospitalization among seniors with heart failure (HF). We assessed the risk of all-cause hospitalization associated with exposure to each of these drug classes, in a population of seniors with HF.
Using the Quebec provincial databases, we conducted a nested case-control study in a population of individuals aged ≥65 with a first HF diagnosis between 2000 and 2009. Patients were considered users of a potentially inappropriate drug class if their date of hospital admission occurred in the interval between the date of the last drug claim and the end date of its days’ supply. The risks of hospitalization were estimated using multivariate conditional logistic regression.
Of the 128,853 individuals included in the study population, 101,273 (78.6%) were hospitalized. When compared to nonusers, users of NSAIDs (adjusted odds ratio: 1.16; 95% confidence interval: 1.13–1.20), TZD (1.09; 1.04–1.14), and CCBs (1.03; 1.01–1.05) had an increased risk of all-cause hospitalization, but not the users of nifedipine (1.00; 0.97–1.03).
Seniors with HF exposed to a potentially inappropriate drug class are at increased risk of worse health outcomes. Treatment alternatives should be considered, as they are available.
Keywords: heart failure, hospitalization, potentially inappropriate treatment, risk, seniors
1. Introduction
Heart failure (HF) is a major health issue, especially among seniors. In fact, annual incidence of HF after age 65 is around 10/1000 person-years.[1] Clinical guidelines [2–4] recommend that patients with HF be exposed to drugs that reduce mortality and the need for hospitalization (bisoprolol, carvedilol, or metoprolol and angiotensin converting enzyme inhibitors [ACEI] or angiotensin receptor antagonists [ARB]). However, patients should also not be exposed to drugs that can cause fluid retention or decrease cardiac function, that is, nonsteroidal anti-inflammatory drugs (NSAIDs), thiazolidinediones (TZD) nifedipine and nondihydropyridine calcium channel blockers (CCBs).
Among HF patients aged ≥ 65, almost 50% depicts ≥ 5 comorbidities,[5,6] up to 21% are clinically frail [7] and almost 45% take ≥ 9 drugs.[8] Thus, elderly HF patients are at great risk of exposure to potentially inappropriate treatment while also being at risk of worse health outcomes if exposed.
The risk of adverse outcomes such as hospitalization due to contraindicated drug exposure is not well defined among HF patients, particularly seniors. Indeed, the adverse effects of exposure to nondihydropyridine CCBs on hospitalization in patients with HF come from studies in patients who had a myocardial infarction.[9,10] Previous studies on the effects of potentially inappropriate drugs in patients with HF, took place either when the β-blockers and ACEI were not yet standard therapy for HF, with respect to exposure to nifedipine,[11] or as a secondary objective, in the case of the effect of TZDs on hospitalizations.[12] One study looked at the effect of exposure to NSAIDs on hospitalization and mortality among patients with HF.[13] The authors observed that an exposure to NSAIDs was associated with an increased risk of hospitalization for HF or myocardial infarction. However, the risk of hospitalization for any cause, among elderly patients with multiple comorbidities, was never assessed. To help clinicians to better weigh the risks associated with potentially inappropriate drugs in HF, we designed a study in a population of individuals aged ≥ 65, to determine the effect of exposure to NSAIDs, TZDs, nifedipine or nondihydropyridine CCBs on all-cause hospitalization after the first HF diagnosis.
2. Methods
2.1. Study design and data sources
We conducted a nested case-control study using the databases of the Quebec Health Insurance Board (RAMQ), the Institut de la statistique du Québec (death registry), and the Quebec Registry of Hospitalizations. These databases include information on patient demographics, vital status, in-hospital and outpatient medical diagnoses, and the medical services used by all permanent residents of Quebec province. The RAMQ drug plan database contains information on prescription drugs for Quebec residents not covered by a private drug insurance group plan, welfare recipients and all seniors, except those living in long-term care facilities.
2.2. Source population
To assemble our source population many steps were done. First, RAMQ identify all Quebec residents covered by the public health insurance plan, who had at least 1 registered HF diagnosis in the outpatient medical visits database or the hospitalizations’ registry (ICD-9 code: 428; ICD-10 code: I50) from January 1, 2000, to December 31, 2009. From this group of people, RAMQ removed all those aged under 18 at their first HF diagnosis plus those not continuously eligible for Quebec's public drug insurance plan in the 365-day period before the first HF diagnosis and those who had a previous HF diagnosis during the same time period. The RAMQ provided all data registered in the databases until December 31, 2009, death or end of drug plan eligibility for each of them.
Second, we excluded all individuals who had only 1 HF diagnosis registered in the outpatient medical visits database.[14] In cases of more than 1 HF diagnosis, the date of the first HF diagnosis became the HF diagnosis baseline date. Next, we excluded everyone < 65 years at HF diagnosis, all individuals who died on the same date as their HF diagnosis or who died during the hospital stay in which HF was diagnosed, and all individuals without follow-up. For individuals whose HF was diagnosed in hospital, the discharge date became the diagnosis date. We followed individuals until study end (31 December 2009), loss of eligibility for the public drug plan, or death.
2.3. Cases and controls selection
We created a set of cases by identifying all individuals hospitalized during the follow-up. A hospitalization was defined as a stay longer than 1 day in an acute care hospital. The first day of the first hospitalization constitutes the event date. For each case, we randomly paired 4 to 10 controls using incidence density sampling. Incidence density sampling assures that the observation period (i.e. the time period between HF diagnosis [included] and event date [excluded]) is identical for a given case and its controls.[15] Controls and cases were matched according to age group, sex, calendar year at time of HF diagnosis, record of an ischemic heart disease, and record of a diabetes diagnosis in the 365 days prior the HF diagnosis. The event date for controls was the date when the matched case was hospitalized.
2.4. Independent variable: exposure to a potentially inappropriate drug
For each of the potentially inappropriate drug class examined (NSAIDs [COX-2 selective inhibitors: celecoxib and rofecoxib; nonselective NSAIDS: diclofenac, ibuprofen, naproxen, and other (diflunisal, indomethacin, ketoprofen, meloxicam, piroxicam, tenoxicam, and sulindac)], TZD [pioglitazone and rosiglitazone], nifedipine and nondihydropyridines CCB [verapamil and diltiazem]), we classified patients as user or nonuser. Among dihydropyridine CCBs, only nifedipine was considered as there is evidence of contra-indications in heart failure only for that drug.[16] We searched for a claim for a contra-indicated drug class in the 365 days before HF diagnosis and between HF diagnosis and the event date. We selected the claim closest to the event date and then determined the exposure time period by adding 1.5 times the number of days’ supply to this last claim date. If the event date occurred in the exposure time period, we deemed the patient as being a user. We repeated the process for each medication class.
2.5. Potentially confounding variables
We considered many variables as potential confounders. To determine each patient's frailty, we used an evaluation based on the accumulation of deficits model.[17,18] According to this model, frailty results from the additive effect of deficits and not just the presence of certain types of deficits.[17,18] We considered 47 deficits comprising 11 different anatomical systems.[19] To determine the number of deficits each patient had, we searched the outpatient medical services database and the hospitalizations’ registry for a diagnosis of any of the 47 selected deficits in the 365 days before HF diagnosis. To calculate the frailty index value, we divided the number of deficits found for a patient by the maximum number of possible deficits.
We checked claims for an echocardiograph or for a pacemaker installation in the 365 days before HF diagnosis. We also looked for any recorded diagnosis for chronic kidney disease, high blood pressure, atrial fibrillation, chronic obstructive pulmonary disease/asthma, stroke, and peripheral atherosclerotic disease in the same time period. We also explored medical services used in the 365-day period up to the day of HF diagnosis, examining both the number of days of hospitalization and the number of outpatient medical consultations (<median or ≥ median). If the HF diagnosis was made during a hospitalization, we excluded this period from the calculation. At the time of HF diagnosis, we checked for the specialty of the diagnosing physician and the number of days of hospitalization (or 0 days if the patient was not hospitalized at that time). During the observation period, we looked for: (1) installation of a pacemaker, (2) an internal cardiac defibrillator or cardiac resynchronization device, (3) presence of medical consultation with a cardiologist, general practitioner, or other medical consultations, and (4) exposure to other cardiovascular medications (amiodarone, class I antiarrythmic, diuretics, spironolactone, digitalis, β-blocker, ACEI/ARBs, antiplatelets, warfarin, and nitrates).
2.6. Statistical analysis
Means, medians, and proportions described the characteristics of cases and controls. To assess the association between exposure to potentially inappropriate treatment and hospitalization, we performed a multivariate conditional logistic regression that included variables of exposure at time of event date for each medication part of the potentially inappropriate treatment (nonusers being the reference) and all the potential confounding variables. Since NSAIDs, as a class, include a large number of medications with different mechanisms of action,[13,20] we conducted another analysis to look at the effect of exposure to COX-2 selective inhibitors (celecoxib and rofecoxib), ibuprofen, diclofenac, and other NSAIDs on the risk of hospitalization. To test the sensitivity of the results to the length of the grace period used in defining users, we repeated the analysis using on one hand, no grace period, and on the other hand, a grace period equals to the number of days’ medication supply. Analyses were done using S.A.S. version 9.3 (SAS Institute Inc., Cary, NC).
2.7. Ethical considerations
The Commission d’accès à l’information du Québec and the Research Ethics Committee of the CHU de Québec—Université Laval approved this study.
3. Results
RAMQ identified 291,033 people who had a first HF diagnosis in the medical services database or the hospitalizations’ registry in our recruitment period, who were aged ≥ 18 and who were continuously eligible for Quebec's public drug insurance plan in the 365-day period before the first HF diagnosis. Among them, we excluded 21,402 people aged < 65 years, 124,023 who had only 1 outpatient HF diagnosis, 10,651 who died the same date their HF was diagnosed or died during the hospital stay in which HF was diagnosed and 6104 who had no follow-up. As a consequence, our source population comprised 128,853 individuals. Among them, 101,412 (78.7%) were hospitalized at least once during follow-up. The main cause of hospitalization for 16,070 patients (15.9%) was HF. For 22,267 (22.0%), it was cardiovascular causes other than HF and 63,075 (62.2%) patients were hospitalized for other causes. Median time between HF diagnosis and hospitalization was 105 days (16–422 days).
Table 1 shows the characteristics of cases and their controls. Mean length of exposure ranged from 2.5 months for NSAIDs to 8.7 months for nondihydropyridine CCBs. Users of NSAIDs, TZD, and nondihydropyridine CCBs had a statistically significant increased risk of hospitalization, whereas the risk among nifedipine users was not increased (Figs. 1 and 2). Also, among NSAIDs users, 53% were using COX-2 selective inhibitors, 17% diclofenac, 13% naproxen, 3% ibuprofen, and 14% other NSAIDs. Increased risk of all-cause hospitalization was similar among all type of NSAIDs, except for ibuprofen, which was associated with a nonstatistically significant increased risk in multivariate analysis (Fig. 1). Results were not sensitive to changes in the definition for users of drugs (data not shown).
Table 1.
Characteristics of the study population.
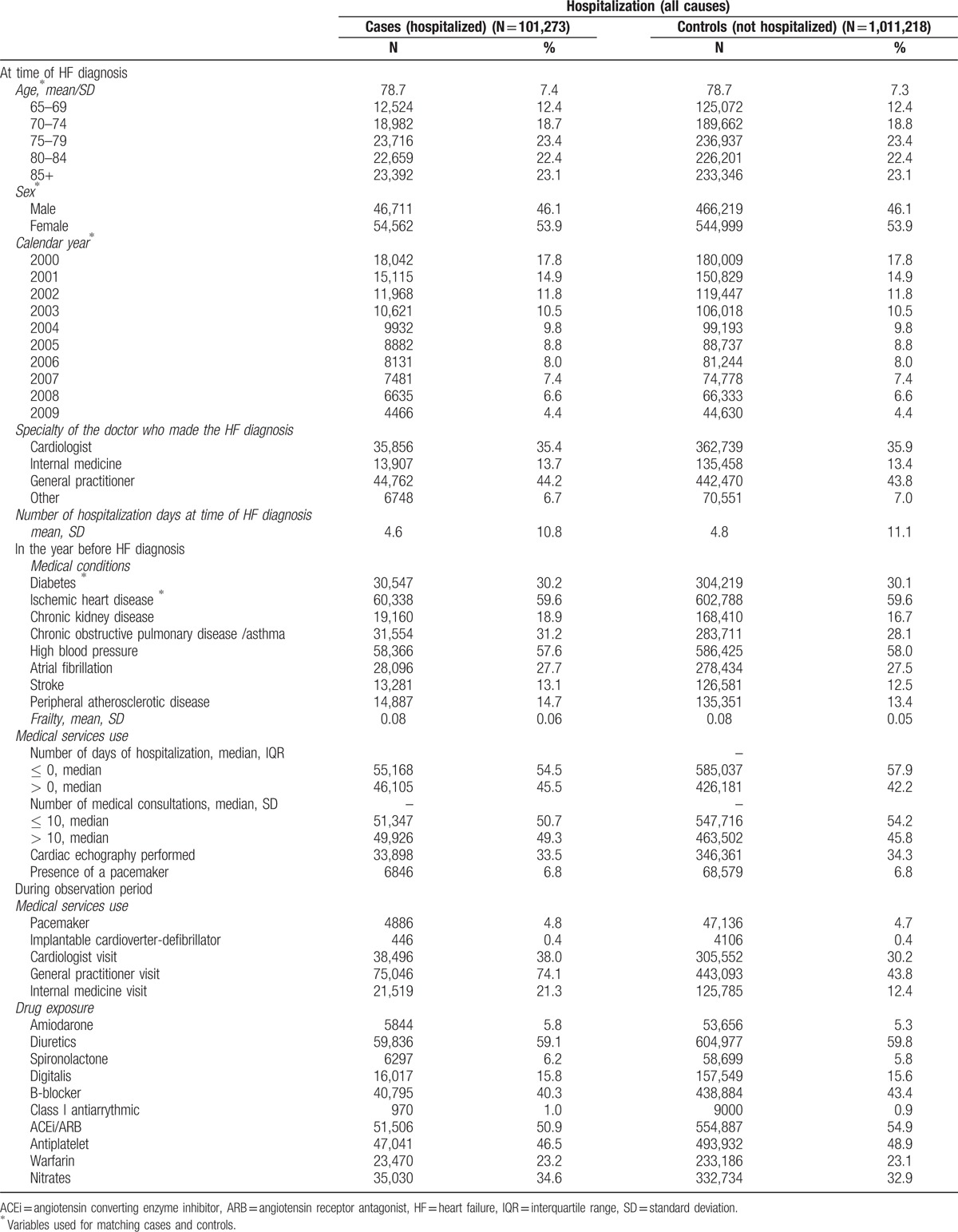
Figure 1.
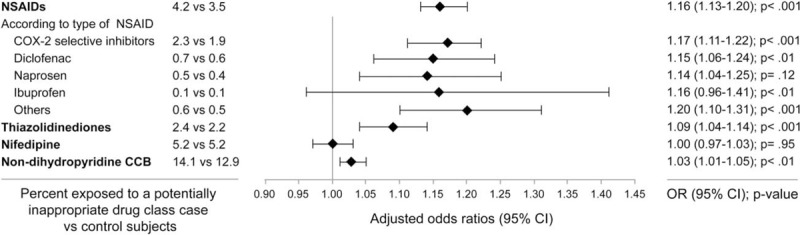
Adjusted associations between current exposure to nonsteroidal anti-inflammatory drugs (NSAIDs), thiazolidinediones, nifedipine and nondihydropyridines calcium channels blockers (CCB), and all-cause hospitalization. CCB = calcium channels blockers, NSAIDs = nonsteroidal anti-inflammatory drugs.
Figure 2.
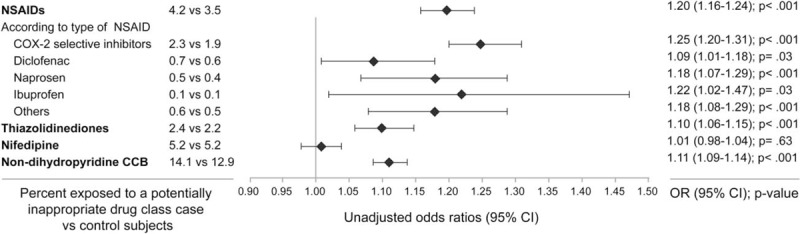
Crude associations between current exposure to nonsteroidal anti-inflammatory drugs (NSAIDs), thiazolidinediones, nifedipine and nondihydropyridines calcium channels blockers (CCB), and all-cause hospitalization. CCB = calcium channels blockers, NSAIDs = nonsteroidal anti-inflammatory drugs.
4. Discussion
In this large population-based study of seniors with HF, we found an increased risk of hospitalization among those exposed to contraindicated NSAIDs, TZD, and nondihydropyridines CCBs.
Individuals exposed to NSAIDs had a 16% greater risk of hospitalization than those who were not exposed to these drugs. In addition to their anti-inflammatory effects, NSAIDs are known to increase gastrointestinal bleeding risk,[21] increase risk of cardiovascular event,[22,23] and increase risk of developing HF. However, only Gislason et al[13] evaluated the risks of hospitalization associated with the use of NSAIDs among patients suffering from HF. In their cohort of patients aged ≥ 30, the risks of hospitalizations for HF and acute myocardial infarction ranged from 1.16 to 1.40 and 1.30 to 1.52, respectively. These risks of hospitalization are comparable to the risk of all-cause hospitalization observed in our cohort. Also, in accordance with our data, they found increased risk to be consistent among all NSAIDs. This result suggests that patients with HF who need an NSAID should be switched to an alternative treatment. For example, patients suffering from osteoarthritis can benefit from physiotherapy [24] and when a pharmacological treatment is needed, the American Geriatric Society recommends using opioids among seniors instead of NSAIDs, given the increased risk of adverse effects from this class of molecules.[25]
Secondly, we found an increased risk of hospitalization among patients exposed to TZD. Since studies among patients with diabetes have reported evidence of early plasma volume expansion after TZD initiation (8 to 12 weeks),[26,27] it is likely that patients with HF also display signs of early stage volume overload and must be hospitalized. Some studies have looked at the risk of developing HF among patients with diabetes.[28,29] Only Masoudi et al[12] looked at the risk of exposure to TZD among patients with diabetes with HF. They observed a nonstatistically significant increase in HF and all-cause mortality among patients exposed to a TZD after a hospitalization for HF. Since hospitalization was a secondary outcome, they may have lacked the power to detect any significant increase in the risk of hospitalization.
Next, we found no statistically significant increase in the risk of all-cause hospitalization among individuals exposed to nifedipine. Nifedipine reduces systemic vascular resistance and has a neurohumoral effect and a negative inotropic and chronotropic effect.[16,30] Nifedipine, contrary to NSAID and TZD, may be beneficial to HF patients with preserved ejection fraction (HFpEF). In fact, guidelines recommend that blood pressure has to be treated optimally among patients with HFpEF to help relax the ventricle.[31] Since our population included both HF individuals with decreased and preserved left ventricular ejection fraction, it is possible that the benefit of exposure to nifedipine among HFpEF masked the risk of exposure among those individuals with decreased left ventricular ejection fraction.
Finally, exposure to nondihydropyridine CCBs was associated with an increased risk of hospitalization. Although small in magnitude, this increased risk could be of clinical importance. As nondihydropyridine CCBs have negative chronotropic and inotropic activity, it might explain their effect on increased risk of hospitalization. A study looking at the effect of CCBs after myocardial infarction[9] found that patients with signs/symptoms of HF who were exposed to CCBs were at increased risk of a cardiovascular event. In addition, since patients exposed to nondihydropyridine CCBs may not have been properly exposed to β-blockers due to the increased risk of severe bradycardia, patients exposed to nondihydropyridine CCBs are at increased risk of adverse outcomes since they cannot benefit from the complete protection conferred by β-blockers.
This study has strengths and limitations that should be considered when interpreting our results. One of the limitations is that we may have classified some patients as unexposed when they were, in fact, exposed to over-the-counter NSAIDs. We have no information about exposure to those over-the-counter drugs. However, only ibuprofen in small dosages is available over-the-counter in the province of Quebec. This misclassification is likely to be nondifferential and so would result in an underestimation of the risk of hospitalization observed in our study. A further limitation is that, since clinical guidelines [31] indicate that these drugs are to be used with caution in patients with HF and therefore physicians are aware of the risks for patients in being exposed to these drugs, it is likely that a higher proportion of patients with less severe disease and considered at low risk of hospitalization are being exposed than others.[32] Another limitation also came from our data source. Since the database does not include echocardiographic data, it is impossible to distinguish between HF patients with decreased and preserved left ventricular ejection fraction. Since nifedipine and nondihydropyridine CCBs might be of benefit to HFpEF,[2] the risk of exposure among patients with decreased ejection fraction may have been underestimated.
The study's greatest strength is that it includes all elderly Quebecers with a first HF diagnosis during the study period. These individuals typify “real-world” patients with HF. In addition to this feature, we could access extensive information about medication use.
5. Conclusion
Many seniors with HF have multiple comorbidities and are exposed to numerous medications, some of which being potentially harmful. Clinicians should care about their patient's quality of life. One way to maximize their quality of life is to keep them out of the hospital. We observed that using NSAIDs, TZD and nondihydropyridine CCBs might increase the risk of all-cause hospitalization among HF seniors. Since alternatives do exist for each of these drug classes, potentially inappropriate treatment should not be used.
Acknowledgments
The authors thank Eric Demers, a statistician in our research center, for statistical support, Joanne Vidal, a freelance editor, for editing the manuscript, and Norma Perez Herrera, for research assistance.
Footnotes
Abbreviations: ACEI = angiotensin converting enzyme inhibitors, ARB = angiotensin receptor antagonists, CCBs = calcium channel blockers, COX = cyclooxygenase, HF = heart failure, HFpEF = heart failure patients with preserved ejection fraction, ICD-9 = International Classification of Diseases, Ninth Revision, NSAIDs = nonsteroidal anti-inflammatory drugs, RAMQ = Quebec Health Insurance Board, TZDs = thiazolidinediones.
Funding: This work was supported by an unrestricted grant from Servier Canada and by the Chaire sur l’adhésion aux traitements de l’Université Laval. The Chair was supported by nonrestricted grants from AstraZeneca Canada, Merck Canada, Pfizer Canada, Sanofi Canada and the Prends soin de toi Program. Catherine Girouard received a scholarship from the Chaire de gériatrie de l’Université Laval and from Fonds de recherche du Québec –Santé (FRQS). The sponsors had no role or influence in matters relating to research design, methods or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012;125:188–97. [DOI] [PubMed] [Google Scholar]
- [2].Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977–2016. [DOI] [PubMed] [Google Scholar]
- [3].Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- [4].McKelvie RS, Moe GW, Ezekowitz JA, et al. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol 2013;29:168–81. [DOI] [PubMed] [Google Scholar]
- [5].Saczynski JS, Go AS, Magid DJ, et al. Patterns of comorbidity in older adults with heart failure: the Cardiovascular Research Network PRESERVE study. J Am Geriatr Soc 2013;61:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wong CY, Chaudhry SI, Desai MM, et al. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011;124:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McNallan SM, Chamberlain AM, Gerber Y, et al. Measuring frailty in heart failure: a community perspective. Am Heart J 2013;166:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marcum ZA, Pugh MJ, Amuan ME, et al. Prevalence of potentially preventable unplanned hospitalizations caused by therapeutic failures and adverse drug withdrawal events among older veterans. J Gerontol A Biol Sci Med Sci 2012;67:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].The effect of diltiazem on mortality and reinfarction after myocardial infarction.. The Multicenter Diltiazem Postinfarction Trial Research Group. N Engl J Med 1988;319:385–92. [DOI] [PubMed] [Google Scholar]
- [10].Goldstein RE, Boccuzzi SJ, Cruess D, et al. Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation 1991;83:52–60. [DOI] [PubMed] [Google Scholar]
- [11].Elkayam U, Amin J, Mehra A, et al. A prospective, randomized, double-blind, crossover study to compare the efficacy and safety of chronic nifedipine therapy with that of isosorbide dinitrate and their combination in the treatment of chronic congestive heart failure. Circulation 1990;82:1954–61. [DOI] [PubMed] [Google Scholar]
- [12].Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005;111:583–90. [DOI] [PubMed] [Google Scholar]
- [13].Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med 2009;169:141–9. [DOI] [PubMed] [Google Scholar]
- [14].Schultz SE, Rothwell DM, Chen Z, et al. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can 2013;33:160–6. [PubMed] [Google Scholar]
- [15].Rothman K, Greenland S, Lash T. Modern Epidemiology. 3rd edn.Philadelphia: Lippincott, Williams and Wilkins; 2008. [Google Scholar]
- [16].Amabile CM, Spencer AP. Keeping your patient with heart failure safe: a review of potentially dangerous medications. Arch Intern Med 2004;164:709–20. [DOI] [PubMed] [Google Scholar]
- [17].Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001;1:323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci 2004;59:1310–7. [DOI] [PubMed] [Google Scholar]
- [19].Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation 2005;112:759–70. [DOI] [PubMed] [Google Scholar]
- [21].Koda-Kimble MA, Young LY, Kradjan WA, et al. Applied Therapeutics - The Clinical Use of Drugs 8th edition Lippincott Williams & Wilkins, 2005. p.43–4. [Google Scholar]
- [22].Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006;332:1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006;296:1633–44. [DOI] [PubMed] [Google Scholar]
- [24].Jevsevar DS, Brown GA, Jones DL, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am 2013;95:1885–6. [DOI] [PubMed] [Google Scholar]
- [25].American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009;57:1331–46. [DOI] [PubMed] [Google Scholar]
- [26].Aronoff S, Rosenblatt S, Braithwaite S, et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000;23:1605–11. [DOI] [PubMed] [Google Scholar]
- [27].Lebovitz HE, Dole JF, Patwardhan R, et al. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab 2001;86:280–8. [DOI] [PubMed] [Google Scholar]
- [28].Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298:1180–8. [DOI] [PubMed] [Google Scholar]
- [29].Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 2007;298:1189–95. [DOI] [PubMed] [Google Scholar]
- [30].Mahe I, Chassany O, Grenard AS, et al. Defining the role of calcium channel antagonists in heart failure due to systolic dysfunction. Am J Cardiovasc Drugs 2003;3:33–41. [DOI] [PubMed] [Google Scholar]
- [31].Jessup M, Abraham WT, Casey DE, et al. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009;119:1977–2016. [DOI] [PubMed] [Google Scholar]
- [32].Csizmadi I, Collet JP. Strom BL, Kimmel SE. Bias and confounding in pharmacoepidemiology. Textbook of Pharmacoepidemiology. West Sussex: Wiley; 2006. 261–76. [Google Scholar]


