Abstract
Background:
Breast augmentations are commonly performed aesthetic surgical procedures. As the breast is a changeable structure, the ideal location of an implant would be a plane that can adjust to the dynamic changes of the breast. We present a modified dual-plane technique for breast augmentation using the fascia for thin patients.
Methods:
Between June 2014 and June 2015, 27 patients with small breasts underwent breast augmentation using the modified dual-plane technique. The average age was 29.4 years (range, 20–41 y). The mean body mass index was 18.9 kg/m2 (range, 17.6–20.4 kg/m2).
Results:
The mean size of the implant was 288.9 ml (range, 255–360 ml) on the right side and 281.6 ml (range, 255–360 ml) on the left side. All the patients returned to daily-life activities within 1 week. There have been no complications during minimum follow-up periods of 18 months. The mean follow-up was 25.6 months (range, 18–36 mo).
Conclusions:
Visible, palpable implants and rippling after breast augmentation with implants are relatively common problems. Our modified dual-plane technique with the serratus anterior fascia can reduce visible rippling and yields a natural-looking breast, especially in thin patients.
Breast augmentations are commonly performed aesthetic surgical procedures with various indications, such as small breasts, asymmetric breast, and age-related changes. The most commonly used positions for breast implants are the subglandular, submuscular (including dual plane), and subfascial planes with their own strengths and weaknesses.1,2
As the breast is a changeable structure, its shape, size, and location change with age, pregnancy, and weight.2 These changes can affect the results of breast augmentation in the long term. Unfortunately, there are several long-term complications of breast augmentation with prosthetic implants, such as rippling, capsular contracture, implant malposition, bottoming out, implant exposure, and synmastia.3 Given such long-term complications, the optimal choice of surgical plane should be based on various factors considering not only the surgeon’s preference but also patient’s anatomy. The location and surgical plane of the implant control the amount of soft-tissue coverage, the pressure and tension over the implant, and the final position of the breast on the chest. Therefore, the ideal location of an implant would be a plane that can adjust to the dynamic changes of the breast.2,4
In this report, we introduce a modified dual-plane technique for breast augmentation and describe outcomes and advantages of this technique.
PATIENTS AND METHODS
Between June 2014 and June 2015, 27 patients with small breasts underwent breast augmentation using the modified dual-plane technique. All the cases were primary augmentations. The average age was 29.4 years (range, 20–41 y). The mean body mass index (BMI) was 18.9 kg/m2 (range, 17.6–20.4 kg/m2). The pinch test results were less than 2 cm in the upper pole and less than 1 cm in the lower pole and the lateral portion of the breast. The approach was through inframammary incision in all cases.
Surgical Technique
Under general anesthesia, the inframammary skin incision about 4 to 4.5 cm wide is made and dissection is carried out through skin incision down to the subcutaneous layer, through the Scarpa’s fascia, directly to the surface of the underlying muscle fascia (Fig. 1A). Underlying muscle can be identified by Bovie electrocautery stimulation. Depending on patient’s height and chest wall circumference, underlying muscle is differently identified. In the majority of cases, it is serratus anterior (SA) muscle and in rare cases, external oblique (EO) muscle. A fascial incision is made transversely over the exposed muscle (Fig. 1B). A subfascial dissection is initiated from the lower medial portion of the muscle and moved on superolaterally to the preoperatively designed lateral margin of the pocket. During the dissection, the lateral cutaneous branch of the fourth intercostal nerve is identified and preserved. After the SA and/or EO fascia is completely released and elevated in 1 sheet (Fig. 2), the dissection moves medially checking the lateral border of the pectoralis major muscle (Fig. 3). Once the PM muscle is exposed, the inferior costal origins of the PM muscle are divided. Dissection is continued medially until the point where the inframammary fold meets the sternum. Then, the subpectoral dissection proceeds superiorly, making the subpectoral pocket, while preserving all the medial origins of the PM along the sternum (Fig. 4). The pectoralis minor muscle and ribs are left intact as an underlying plane.
Fig. 1.
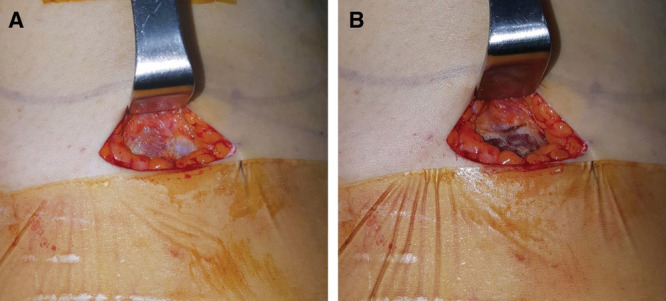
Inframammary incision on the right side of the breast. A, Muscle fascia is exposed; (B) transverse incision is made on its lower border.
Fig. 2.
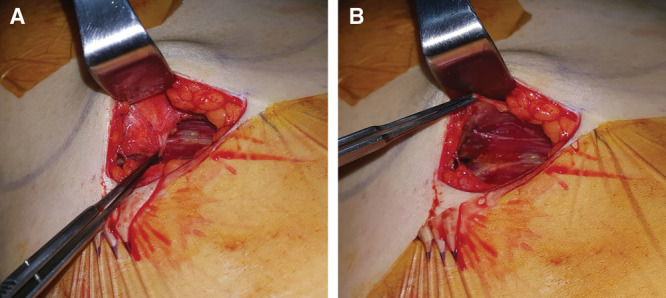
A, The fascia is elevated in 1 sheet. B, The muscle is exposed with its fascia fully elevated.
Fig. 3.
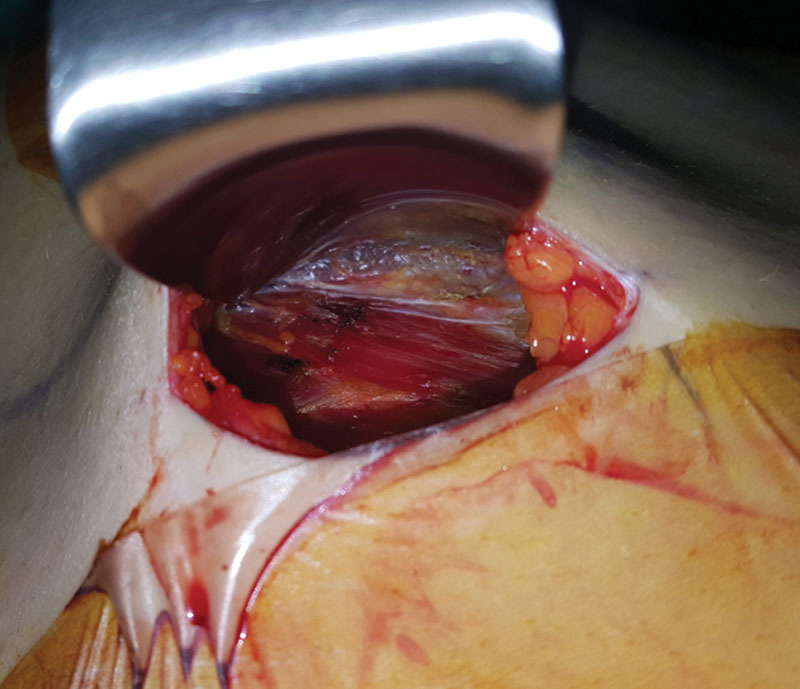
After fascia elevation, lateral border of PM muscle and underlying loose areolar tissue are encountered.
Fig. 4.
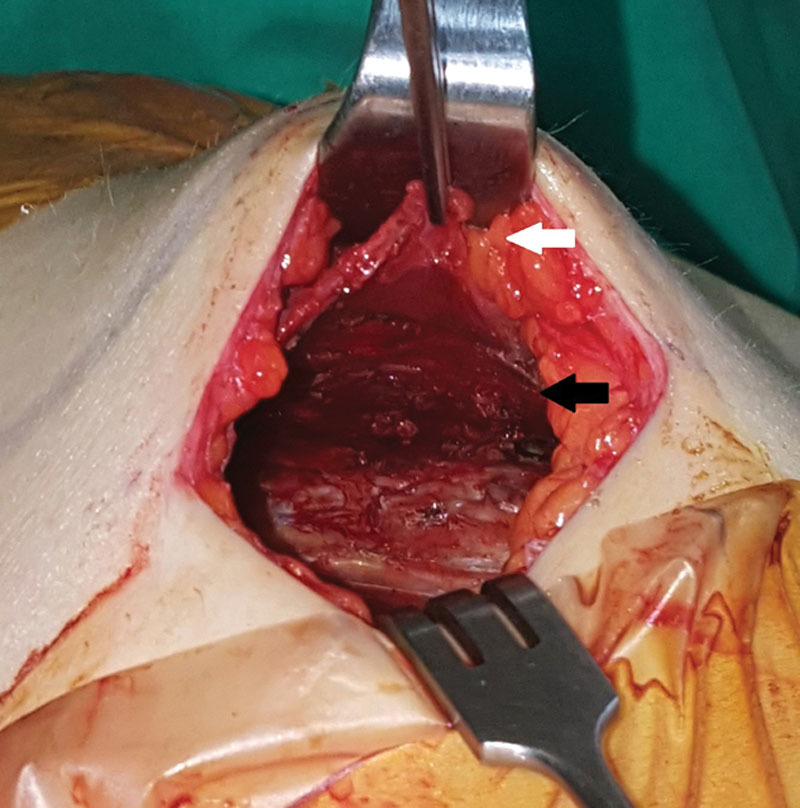
After creating a pocket, the elevated fascia (white arrow) is connected to the pectoralis major muscle (black arrow).
The pocket is irrigated with normal saline mixed with cefazolin and gentamicin, and meticulous hemostasis is achieved. The implant is put in place, covered with PM muscle at the superomedial portion and the SA and/or EO fascia at the inferolateral portion.
The incision is closed layer by layers, Scarpa’s fascia is approximated using a 2-0 absorbable suture, and the subcutaneous layer is closed with a 5-0 absorbable suture. Finally, the skin layer is closed with topical skin adhesive (Dermabond, Ethicon, Somerville, N.J.). No drain was used.
RESULTS
Twenty-seven anatomical and 3 round textured cohesive gel implants were used. The mean size of the implant was 288.9 ml (range, 255–360 ml) on the right side and 281.6 ml (range, 255–360 ml) on the left side. All the patients returned to daily-life activities within 1 week.
There were no complications such as hematoma, seroma, or displacement of implants during follow-up. The patients maintained sensitivity around nipple-areolar area. During a minimum follow-up period of 18 months, implants were not palpable or visible (Figs. 5–7). The mean follow-up was 25.6 months (range, 18–36 mo).
Fig. 5.
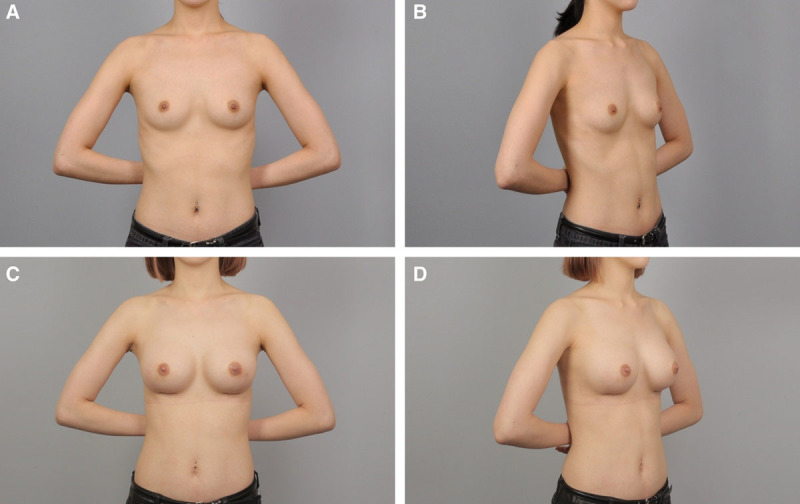
A and B, Preoperative view of a 24-year-old patient. C and D, Postoperative view approximately 4 months after breast augmentation using anatomical cohesive gel implants, 295 ml on the right side and 255 ml on the left side.
Fig. 7.
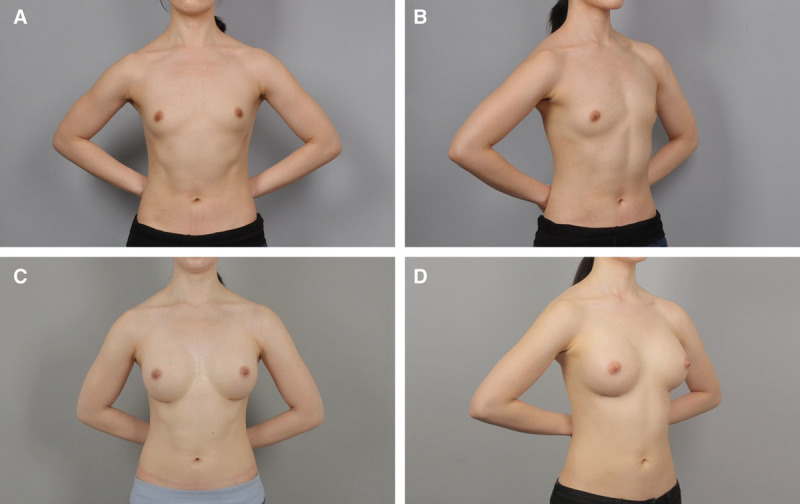
A and B, Preoperative view of a 27-year-old patient. C and D, Postoperative view approximately 12 months after breast augmentation using 275 ml anatomical cohesive gel implants.
DISCUSSION
Breast augmentation is a commonly performed surgical procedure. Despite the advances in surgical technique and implant, using a wide range of shapes and sizes, complication rates still remain high.3 In studies by major breast implant companies, the rates of complications for primary breast augmentation requiring reoperation were 15% and 28% within the first 3 and 6 years, respectively.3,5–7 Each reoperation is associated with surgical risks as well as imposing a financial burden, the need for recovery time and time off work.8 Because complications related to breast implants are very difficult to manage, high recurrence frequencies and low patient satisfaction are common. The risk of further reoperation after the first revision is high, and the interval between revisions becomes shorter.8
The complications of breast augmentation using implants include rippling, capsular contracture, implant malposition, implant exposure, and synmastia. These complications are due to thinning of the overlying skin, periprosthetic tissue change, formation of a capsule, and inadequate filling of a large breast pocket. The most common complication is capsular contracture, and the rippling is the second most common complication.3,8 Therefore, the most frequent reoperation procedures are capsulectomy and capsulotomy for capsular contracture. Other reoperation procedures are due to rippling, implant rupture, infection, scarring, size change, asymmetry, and mastopexy.3
Rippling is the appearance of vertical folds on the skin over the implant. Increased risk of rippling was reported with the use of saline, textured implants, and subglandular pocket.3,5,9 Also rippling occurs frequently in patients with inadequate breast tissue or who are thin, with a BMI less than 18.5.3 As thin patients often have less soft tissue, evaluating BMI and performing a pinch test to ensure adequate soft-tissue coverage before surgery may prevent this complication.
For thin patients, a subglandular pocket possesses the possibility of visible and palpable implant and rippling. On the other hand, submuscular pocket compromises the expansion of the lower pole by limiting the descent of the implant and positioning the implant high, leading to unnatural appearance.2,10 The dual-plane technique offers the advantages of subglandular and subpectoral breast augmentation by positioning the implant partly behind the PM and partly in a subglandular location.4 This technique interferes the relationship between implant and parenchyma indirectly by controlling the parenchyma–muscle relationship due to division of the PM muscle from the costal origin.4 These procedures ensure soft-tissue coverage superiorly while permitting expansion of the lower pole, which makes more natural outcomes.2,4 However, the drawback of this technique is a risk of visibility and palpability of the implant in the lower lateral aspect of the breast, because the inferolateral portion of the implant is not covered with PM muscle.11
To prevent or to correct rippling, many surgeons use acellular dermal matrix (ADM).12 The use of ADM as a filler or a barrier has been commonplace for many years. ADM is put in place either by onlay or lower pole suspension like a hammock to reverse any rippling.13 However, there is a risk of infection, seroma formation, and increased cost. In addition, if the skin is too thin, the margin of the ADM may be visible.11,14 Another treatment for rippling is an autologous fat graft. This procedure is popular because of the ease of fat harvest and the abundance of donor sites.15 However, harvesting, processing, and grafting the fat increase operation times, and the survival of grafted fat differs from patient to patient, which makes for unpredictable results. Moreover, thin patients have thin subcutaneous tissue layers, which makes fat grafting technically difficult and often results in skin rumpling or damage to implants. The major drawbacks of fat grafting are cyst formation and benign calcification, which may obscure the diagnosis of future malignant lesions.13
In 1978, Jarrett et al.16 described total submuscular placement of implants. The implant can be placed beneath entirely muscular planes beneath the intact PM and upper part of the SA muscles.10,16 We, also, first began augmentation with total submuscular placement to avoid the problems related to the subglandular plane, such as rippling and visibility and palpability of the implant, especially in thin patients. On the other hand, successful breast augmentation should achieve the balance between implant and soft tissue. In thin patients with inadequate soft tissue, a subglandular position of the implant in the lower pole allows expansion of the lower pole and yields more natural outcomes. When we stopped using the total submuscular technique and adopted the dual-plane technique, we retained the SA and EO muscle fascia as the submuscular technique for the coverage of inferolateral aspect of the breast in thin patients.
The muscle fascia is a well-vascularized autologous tissue. It is quite thin and pliable; however, when harvested in a sheet, it can reliably support the implants. Besides, as the material is from own tissue, it has very low risk of infection and no additional charge. We currently use this fascia flap to prevent the implant visibility and rippling of the dual-plane technique. The fascia covers and conceals the lateral edges of the implant. It provides strength and adds safety to the thin envelope. With the inframammary approach, the SA and EO fascia can be easily located and elevated together, and dissection continued medially for the PM muscle dissection, just as the conventional dual-plane technique. Then the PM muscle, SA fascia, and EO fascia are easily elevated en bloc. The fascia dissection requires care to elevate it as in a continuous sheet without injury. The average increase in operation time due to harvest of the fascia was 5 to 10 minutes, which did not affect patient morbidity or operation-related complications.
The procedure is especially useful for small and nonptotic breasts in thin patients. The mean BMI of our patients was 18.9 kg/m2, whereas the average implant size was 285 ml. More patients are thin with low BMI in Asian than in Western countries, while demand for larger breast implants is increasing. To satisfy patients’ requests, we have sought a simple and safe procedure with less complications. Although our modification seems minimal, our technique is safe and effective for primary breast augmentation and can be used in revision operations to enhance the thin envelope. Patients returned to daily activities within 1 week; only exercise is restricted for 4 weeks. They did not notice any loss of muscle strength or function.10,17
In breast augmentation particularly in thin patients, it is possible to encounter visible or palpable implant edges and rippling. Our modified dual-plane technique is safe and effective for implant coverage.
CONCLUSIONs
Visible, palpable implants and rippling after breast augmentation with implants are relatively common problems, especially in thin patients. Our modified dual-plane technique with the fascia can reduce visible rippling and improve the overall aesthetic result.
Fig. 6.
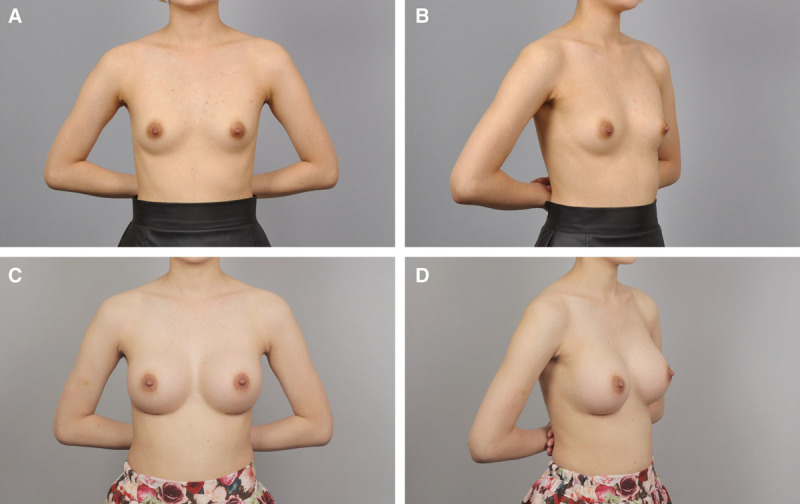
A and B, Preoperative view of a 27-year-old patient. C and D, Postoperative view approximately 8 months after breast augmentation using 255 ml anatomical cohesive gel implants.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Lang Stümpfle R, Figueras Pereira-Lima L, Alves Valiati A, et al. Transaxillary muscle-splitting breast augmentation: experience with 160 cases. Aesthetic Plast Surg. 2012;36:343–348. [DOI] [PubMed] [Google Scholar]
- 2.Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117:757–767; discussion 768. [DOI] [PubMed] [Google Scholar]
- 3.Codner MA, Mejia JD, Locke MB, et al. A 15-year experience with primary breast augmentation. Plast Reconstr Surg. 2011;127:1300–1310. [DOI] [PubMed] [Google Scholar]
- 4.Tebbetts JB.Dual plane breast augmentation: optimizing implant-soft-tissue relationships in a wide range of breast types. Plast Reconstr Surg. 2001;107:1255–1272. [DOI] [PubMed] [Google Scholar]
- 5.Khan UD.Muscle-splitting breast augmentation: a new pocket in a different plane. Aesthetic Plast Surg. 2007;31:553–558. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham B, McCue J.Safety and effectiveness of Mentor’s MemoryGel implants at 6 years. Aesthetic Plast Surg. 2009;33:440–444. [DOI] [PubMed] [Google Scholar]
- 7.Spear SL, Murphy DK, Slicton A, et al. Inamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):8S–16S; discussion 17S. [DOI] [PubMed] [Google Scholar]
- 8.Forster NA, Künzi W, Giovanoli P.The reoperation cascade after breast augmentation with implants: what the patient needs to know. J Plast Reconstr Aesthet Surg. 2013;66:313–322. [DOI] [PubMed] [Google Scholar]
- 9.Spear SL, Bulan EJ, Venturi ML.Breast augmentation. Plast Reconstr Surg. 2004;114:73E–81E. [PubMed] [Google Scholar]
- 10.Hendricks H.Complete submuscular breast augmentation: 650 cases managed using an alternative surgical technique. Aesthetic Plast Surg. 2007;31:147–153. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Lee PK, Oh DY, et al. Subpectoral-subfascial breast augmentation for thin-skinned patients. Aesthetic Plast Surg. 2012;36:115–121. [DOI] [PubMed] [Google Scholar]
- 12.Breuing KH, Colwell AS.Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg. 2007;59:250–255. [DOI] [PubMed] [Google Scholar]
- 13.Busse B, Orbay H, Sahar DE.Sterile acellular dermal collagen as a treatment for rippling deformity of breast. Case Rep Surg. 2014;2014:876254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614. [DOI] [PubMed] [Google Scholar]
- 15.15Chiu CH. Autologous fat grafting for breast augmentation in underweight women. Aesthet Surg J. 2014;34:1066–1082. [DOI] [PubMed] [Google Scholar]
- 16.Jarrett JR, Cutler RG, Teal DF.Subcutaneous mastectomy in small, large, or ptotic breasts with immediate submuscular placement of implants. Plast Reconstr Surg. 1978;62:702–705. [DOI] [PubMed] [Google Scholar]
- 17.Beals SP, Golden KA, Basten M, et al. Strength performance of the pectoralis major muscle after subpectoral breast augmentation surgery. Aesthet Surg J. 2003;23:92–97. [DOI] [PubMed] [Google Scholar]


