Abstract
Background:
Restoration of proper anatomy and physiology is an integral part of cleft palate repair. The senior author has devised a new technique of radical release of greater palatine vessels, which helps in achieving tension-free closure of palatal cleft. In addition, release and transposition of palatal muscles is performed without the use of operative microscope, resulting in improved palatal function. This technique is applicable to all types of clefts of the palate and can be performed on adult patients as well.
Materials and Methods:
This is a retrospective case series of cleft palate repairs performed over a period of 3 years. Single-stage repair with modified Bardach’s technique for complete cleft palate and von Langenbeck’s technique for incomplete cleft palate with radical release of greater palatine vessels and levator complex retropositioning was performed. The outcome measures were closure of palatal defect and speech production. A follow-up of at least 6 months was completed in each patient.
Results:
A total of 1568 patients were included in the study. Their age ranged from 9 months to 54 years. The overall fistula rate was 6.1%. Improvement of speech was observed even in adult patients.
Conclusions:
Radical release of greater palatine artery and levator complex transposition can dramatically improve results of cleft palate repair. This technique helps in dynamic reconstruction of cleft palate and can be effectively applied in all age groups.
Repair of cleft palate abnormality has evolved considerably over the last 3 decades. The abnormal musculature of cleft palate has to be addressed properly for achieving a successful and functional soft palate. Fergusson1 tried to describe the anatomy of cleft palate musculature, whereas Veau and Ruppie2 described the abnormal tensor veli palatini (the tensor) attachment. The term “intravelar veloplasty” was coined by Kriens3 for correction of abnormal musculature. Boorman and Sommerlad4 described the detailed anatomy of normal velum and established the technique of muscle repositioning as the prime factor to revolutionize the cleft palate repair. Sommerlad5 emphasized the use of operative microscope for the muscle dissection and repair. The nonavailability of operative microscope, the time required, and the logistics may make it impractical universally.
The senior author has devised a technique of palate repair in which radical release of greater palatine artery is carried out without doing osteotomy at the greater palatine foramen or fracturing the pterygoid hamulus. Palatal muscles are identified, dissected radically, and then retropositioned posteriorly. This results in greater palatal flap mobilization, which helps in proper alignment of palatal musculature and repair of wide clefts of the palate as well. The entire procedure is carried out without the use of operative microscope. We believe that this technique achieves acceptable results in normal and wide clefts of the palate and is easily reproducible with minimal fistula rates.
MATERIALS AND METHODS
This study was carried out at the Clapp General Hospital, Lahore, Pakistan, over a period of 3 years (July 2012–June 2015). All patients presenting within this period were included in the study. Single-stage palate repair with modified Bardach’s technique (for complete cleft palate) and von Langenbeck’s repair (for incomplete cleft palate) with radical dissection of the greater palatine vessels and levator complex retroposition were carried out. Results were analyzed in terms of closure of the palatal defect and improvement of speech parameters. Complications such as fistula formation and dehiscence were also recorded. A follow-up of at least 6 months was completed in each patient. Regarding the assessment of speech, patients aged 6 years and above were assessed subjectively by Mary Burger method, preoperatively, postoperatively, and postspeech therapy. Hyponasality, hypernasality, nasal air emission, and hypernasality in connected speech were assessed.
TECHNIQUE
Single-stage palate repair is carried out for repair of cleft palate starting at 9 months of age. There is no upper age limit for palate repair as we believe that even in adult patients with cleft palate, proper repair of cleft palate has a profound effect on speech production in addition to improvement of oral hygiene and deglutition. The cleft palate repair techniques for complete unilateral and bilateral cases are a 2-flap palatoplasty with radical release of greater palatine vessels and intravelar veloplasty (levator muscle dissection and retroposition as described by Brian Sommerlad). After positioning and preparation of the patient, a mixture of xylocaine and adrenaline (xylocaine 1% and adrenaline 1:100,000) is injected in both the hard and soft palate areas. In adult patients, we add 500 mg of tranexamic acid to the solution to control the bleeding. This helps in hydrodissection of the palatal tissues. We always wait for 7 to 10 minutes after infiltration so as to get the proper vasoconstriction. The medial incisions of the mucoperiosteal flaps are marked usually at 2 to 3 mm lateral to the medial margins of the cleft (Figs. 1A, B) and 4 to 6 mm in wide clefts so as to include more tissues and in turn transfer some oral mucosa to the nasal side, to get a tension-free closure of nasal mucosa. The lateral incision is made at the junction of the hard palate mucosa and gingiva. The lateral and medial incisions are joined at the anterior end to lift the mucoperiosteal flap from the palatal bone (Fig. 1C). Then using the periosteal elevators, a plane is created between the hard palate bone and the nasal lining in the hard palate area. This helps in easy mobilization of the nasal mucosa to achieve proper nasal layer closure. Once it is raised anteriorly, tip of the suction cannula is used to lift the remainder of the flap away from the bony part of the hard palate. Stay sutures placed at the anterior edge of the flaps help in retraction of flaps and prevent them from falling into the oral cavity. After the flap is raised completely away from the hard palate, attention is given to release of greater palatine vessels, which is located just anterolateral to the posterior end of the bony palate (Fig. 2A, B). Starting laterally, a number 15 blade is used to release the pedicle from the undersurface of the mucoperiosteal flap. This release may be 10 to 20 mm or sometimes even more (away from the exit of greater palatine vessels from the foramen) in adult palates. Depending upon the required mobilization of palatal flaps, the periosteal cuff can be dissected circumferentially to further release the pedicle. After the release of pedicle on both sides, the flaps are moved medially to check if both can be approximated without any tension over the suture line. Even after these maneuvers, if further mobilization is needed, soft tissues medial to pedicle are incised with surgical blade no. 11. This maneuver will completely release the pedicle, and hence the mucoperiosteal flaps can be moved further medially. We have never been compelled to break the bone at the greater palatine foramen in the last 12 years. However, special care is taken to avoid undue traction and injury to the pedicle. The next step is to separate the oral (glandular) mucosa of the soft palate from the palatal musculature. With the help of the scissors, a cut is made on medial side (just distal to the hard palate bone) between the oral part and nasal part of the soft palate. Then by using the tip of suction cannula, oral glandular part of soft palate is separated bluntly from the muscular part of soft palate (along with the nasal mucosal layer). The nasal layer is completely mobilized with the help of a periosteal elevator (Fig. 3A) so that it can be repaired with the opposite nasal layer or flap over the vomer bone. Before the dissection of the muscle complex, nasal layer is repaired with polyglactin 5/0 suture. As the nasal layer is completely mobilized in the region of the hard palate, a tension-free closure is achieved both in hard and soft palate areas (Fig. 3B). Repair of the nasal layer at this stage helps in identification and dissection of the palatal musculature. In the cleft palate anomaly, tensor and levator muscles are closely related, the tensor aponeurosis attaching to the posterior border of the hard palate and the levator inserting at the margins of the cleft in the anterior half of the velum. The abnormal attachment of the tensor can be directly visualized at the posterior shelf of the hard palate as obliquely oriented fibers. These fibers are sharply released from the edge of the hard palate, and the tensor tendon is divided medial to the hamulus (Fig. 4A). Muscles are then separated from the nasal lining in the area, posterior to the posterior nasal spine. Fibers of tensor palati are cut sharply by scissors or sometimes by surgical blade no. 15. We use a sharp, long, and curved tenotomy scissors to cut the muscles away from the nasal mucosa. Once the plane between the muscles and nasal mucosa is found, gentle, blunt, as well as sharp dissection can separate the nasal mucosa from the muscles on the medial side and then along the lateral side as well. After the separation of nasal mucosa from the muscles is complete, long-handled tenotomy scissors are used to cut the muscles from the medial margin of the soft palate. The distal end of the muscles is also cut for a centimeter or so from medial to lateral side. By doing this, the whole muscle complex gets separated (anteriorly, medially, and posteriorly) from the nasal mucosa and is ready to be transposed medially and posteriorly (Fig. 4B). For proper retropositioning of the levator muscle complex, a polyglactin 4/0 suture is passed from the right-sided muscle. The needle is then passed through right-sided nasal mucosa near the uvula, and then left-sided nasal mucosa near the uvula and then left-sided muscle complex. A couple of mattress sutures (1 anterior and 1 posterior to the first suture) are placed so as to further strengthen the muscle repair. After the levators are stitched together, closure of the oral layer is started from posterior to the anterior direction (Fig. 5A). The anterior edge of the palatal flaps is sutured snugly to the mucosa of the incisor teeth anteriorly so as not to leave any gap in the hard palate. At the anterior-most edge of the palatal flaps, a special modification is used to avoid any fistula in the anterior palate (Figs. 5B, C). The needle of the suture is passed from gingival mucosa in the buccal sulcus on the right side going inside the oral cavity to the right palatal flap coming out on the dorsal surface of the flap. It is then passed from the dorsal surface of the left-sided palatal flap and then comes out into the buccal sulcus on the left-sided gingival sulcus and tied snuggly. The anterior edges of both the flaps are further secured to the maxilla anteriorly with a couple of sutures. If the gap between the 2 maxillary segments is wide, then the anterior-most part of the combined flaps is sutured to the anterior-most portion of the sutured nasal layer. Gel foam is placed into the lateral defect, and sutures are applied between the gingival margins and lateral side of the palatal flaps. Figure 6 shows the postoperative result 2 months later. We do not use any microscope to release the pedicle and dissect the levators as both procedures can be comfortably done with naked eye. However, use of magnifying loupes is quite helpful in dissection. In case of a patient with cleft lip and palate presenting at 3 to 8 months of age, our protocol is to repair the lip first with construction of the nasal floor. At the time of palate repair, the incision is U-shaped starting from one uvula anteriorly into the buccal sulcus and then curving again to the other side. While closing the nasal layer, the labionasal communication is closed so that there is no chance of labionasal fistula. In patients with cleft lip and palate presenting after 9 months of age, we do palate repair first. At this stage, we construct the nasal floor, by extending the incision in the buccal sulcus and repairing the nasal floor. In this way, there is no chance of labionasal fistula after lip repair.
Fig. 1.
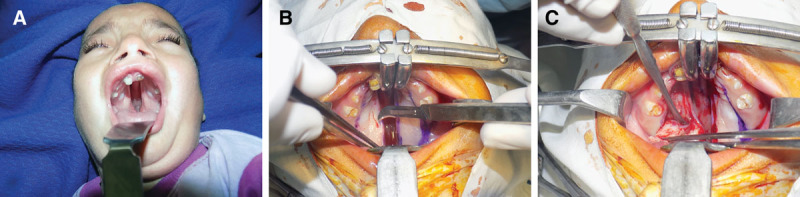
A, Preoperative photograph of a patient with cleft palate showing complete bilateral cleft palate. B, Marking of the incisions. Note the incision line on the cleft margin is 2 to 3 mm lateral to the margins of the cleft. C, Elevation of mucoperiosteal flap.
Fig. 2.
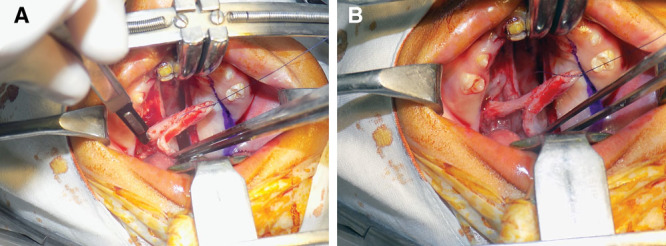
A and B, Release of pedicle from the undersurface of mucoperiosteal flap.
Fig. 3.
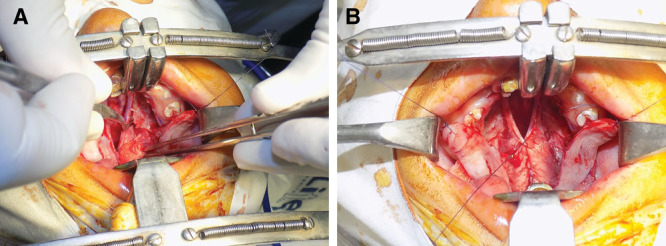
A, Mobilization of nasal mucosa. B, Repair of the nasal layer.
Fig. 4.
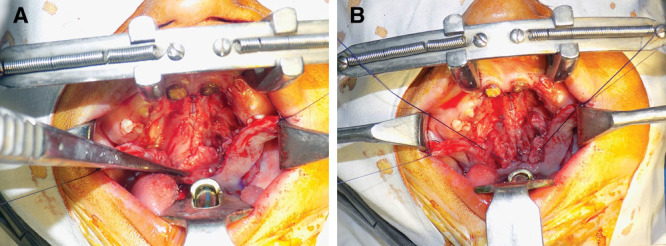
A, Dissection of abnormal obliquely oriented levator muscle fibers. B, Complete release of the levator fibers, division of tensor tendon, and retropositioning of the muscle complex.
Fig. 5.
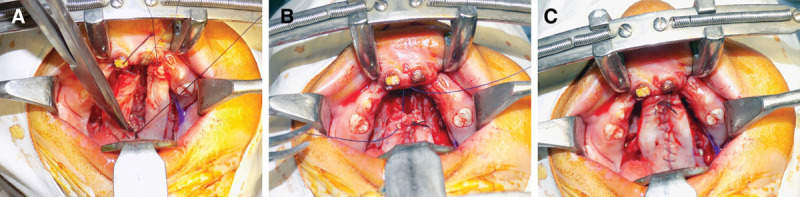
A, Closure of the oral layer. B and C, Fixing of the anterior edge of the palatal flaps to the anterior hard palate.
Fig. 6.
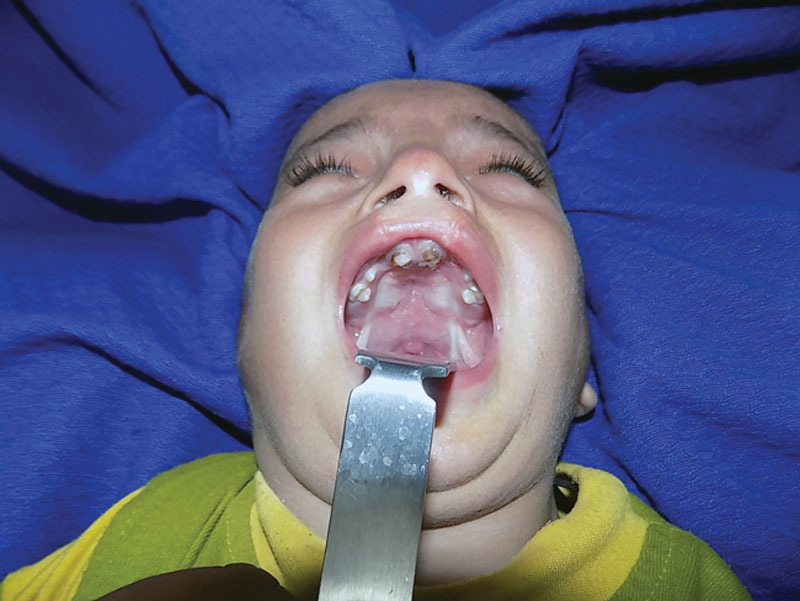
Postoperative picture at 2-month follow-up.
RESULTS
This is a retrospective analysis of 1,568 patients carried out over a period of 3 years. There were 676 females and 892 males (1:1.32). Their age ranged from 9 months to 54 years with mean age of 5.6 years. Age-wise distribution of the patients is shown in Table 1. A variety of different types of soft and hard palate clefts as shown in Table 2 were operated upon. Figures 7, 8 show different types of cleft palates with their postoperative results. The overall fistula rate was 6.1%. The results of the speech production are summarized in Table 3. Because of the problem of compliance at follow-up, only 232 patients could be assessed for speech. There is marked improvement in hypernasality, hyponasality, and nasal air emission in 6 to 10 years of age group patients. Even in patients above the age of 20 years, there is improvement of hypernasality and other speech parameters.
Table 1.
Age-wise Distribution of the Patients
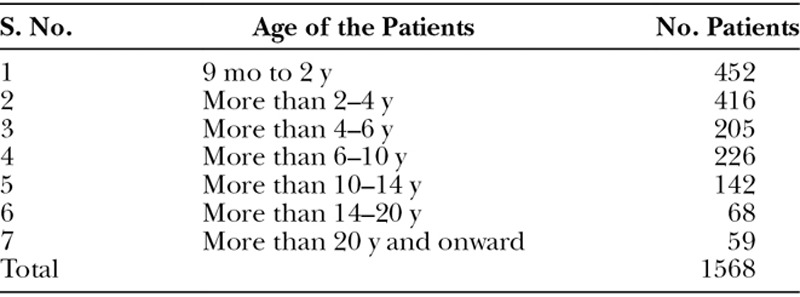
Table 2.
Types of Cleft Palates Included in the Study
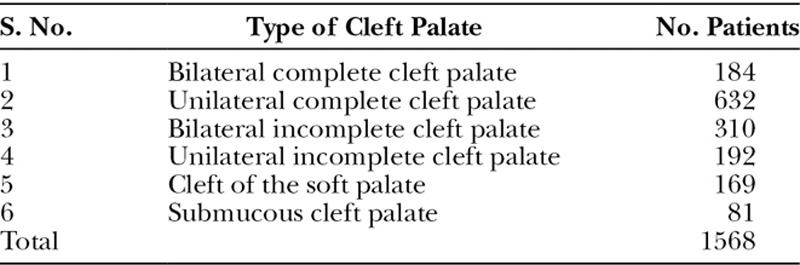
Fig. 7.
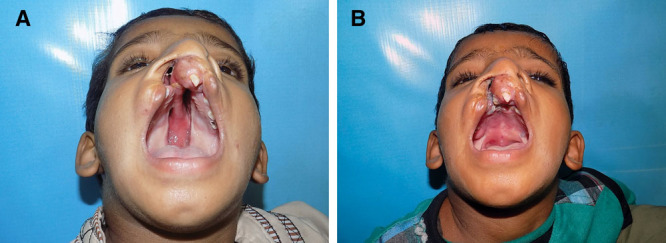
A, Bilateral cleft palate, incomplete on the right side and complete on the left side. B, Postoperative at 6 months.
Table 3.
Speech Therapy Results
Fig. 8.
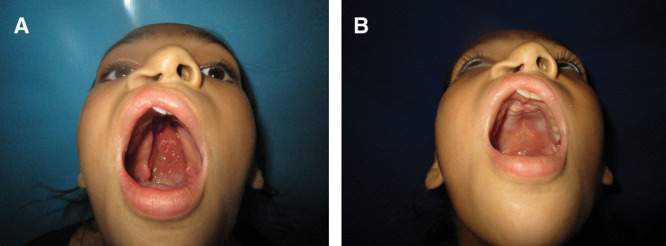
A, Preoperative picture of a girl with wide cleft palate. B, Postoperative picture at 1-year follow-up.
DISCUSSION
A variety of techniques have been used for the repair of cleft palate.6 Over the last few decades, much emphasis has shifted on the repair of muscles as speech improvement has gained prime importance as an outcome measure of palate repair.7 Sommerlad5 popularized the radical release of palate musculature with the help of operating microscope and its repair in retroposition, claiming excellent results in term of speech production and fistula formation. The use of microscope for palate repair as recommended by Sommerlad is not practical universally. Moreover, there are many countries in the world where presurgical orthopedics and nasoalveolar molding are not available readily,8 as a result of which wide cleft palate is a common occurrence in the underdeveloped and developing countries. The main burden of cleft lip and palate is present in such countries and that is why repair of palate becomes more challenging.8
Another problem that can make the palate repair a nightmare is higher age of the patient. As the age of the cleft patient reporting for surgery in most of the developed countries is usually within the first 18 months,9 most of the surgeons have not experienced the problems like variability of pedicle location, increased chances of bleeding, the amount of dissection required to achieve tension-free closure, and presence of spicules and irregularities on the bony surface of the hard palate, which are usually present in adult patients.10 For this reason, many cleft organizations who conduct medical missions in the underdeveloped and developing countries have a strict protocol of advising obturator device to the adult patients with cleft palate rather than surgically correcting the deformity.11 Because of the radical release of the pedicle as recommended in our technique, wide clefts even in adult patients can be easily repaired without any tension.
One of the major advantages of the radical release of the greater palatine vessels is that the palatal flaps can be moved medially or advanced anteriorly so that hard palatal defects even at the most anterior edge of the palate can be easily closed without causing any tension on the repair site. Because the palatal flaps are more mobile, the whole of the cleft can be closed in a single stage without the need for staging of the palate repair as was practiced earlier in wide clefts.10
The fistula rate in the present study is 6.1%, which seems to be higher when compared with some other studies carried out in internationally recognized cleft centers.5 In most of these studies, the patients included were mostly under 18 months, and these patients received presurgical nasoalveolar molding. Also, most of these were carried out by single surgeon. The patients included in this study did not receive any presurgical care, and a large number of patients were above the age of 18 months. Moreover, these patients were operated upon by different surgeons, though majority of the cases were performed by the senior author.
Speech production is a major determinant of the success of palate surgery. Speech assessment by our speech and language pathologist has shown marvelous improvement of speech in majority of the patients. This improvement is due to levator dissection and retroposition in posteromedial direction. Most of the present literature does not support any advantage of repairing cleft palate with increasing age or in adult patients.12 Operating upon many adult cleft palate patients over many years, we have concluded that there is certainly a marked improvement of speech production in adult patients as demonstrated by their speech assessment. Senior author has noted that the voice of many of our adult palate patients can be easily understood even when hearing them on cell phone. Therefore, we strongly recommend the levator complex dissection and its retroposition as one of the key factors in improving velopharyngeal mechanism for the cleft palate patients.
CONCLUSIONS
Repair of the cleft palate is a rewarding procedure at any age provided dynamic reconstruction is achieved in addition to closure of the cleft. It results in restoration of anatomy and improvement of speech at the same time.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by PRS Global Open at the discretion of the Editor-in-Chief.
REFERENCES
- 1.Fergusson W.Observations on cleft palate and on staphylorrhaphy. Med Tim Gaz. 1844;2:256. [Google Scholar]
- 2.Veau V, Ruppie C.Anatomie chirurgicale de la division palatine. J Chir (Paris) 1992;1:20. [Google Scholar]
- 3.Kriens OB.An anatomical approach to veloplasty. Plast Reconstr Surg. 1969;29:43. [DOI] [PubMed] [Google Scholar]
- 4.Boorman JG, Sommerlad BC.Levator palate and palatal dimples: their anatomy, relationship and clinical significance. Br J Plast Surg. 1985;38:326. [DOI] [PubMed] [Google Scholar]
- 5.Sommerlad BC.A technique for cleft palate repair. Plast Reconstr Surg. 2003;112:1542–1548. [DOI] [PubMed] [Google Scholar]
- 6.Leow AM, Lo LJ.Palatoplasty: evolution and controversies. Chang Gung Med J. 2008;31:335–345. [PubMed] [Google Scholar]
- 7.Pearson GD, Kirschner RE.Surgery for cleft palate and velopharyngeal dysfunction. Semin Speech Lang. 2011;32:179–190. [DOI] [PubMed] [Google Scholar]
- 8.Fayyaz GQ, Gill NA, Ishaq I, et al. A model humanitarian cleft mission: 312 cleft surgeries in 7 days. Plast Reconstr Surg Glob Open 2015;3:e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw WC, Semb G, Nelson P, et al. The Eurocleft project 1996-2000: overview. J Craniomaxillofac Surg. 2001;29:131–140; discussion 141. [DOI] [PubMed] [Google Scholar]
- 10.Morioka D, Yoshimoto S, Udagawa A, et al. Primary repair in adult patients with untreated cleft lip-cleft palate. Plast Reconstr Surg. 2007;120:1981–1988. [DOI] [PubMed] [Google Scholar]
- 11.Schneider WJ, Politis GD, Gosain AK, et al. Volunteers in plastic surgery guidelines for providing surgical care for children in the less developed world. Plast Reconstr Surg. 2011;127:2477–2486. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz-Monasterio F, Serrano A, Barrera G, et al. A study of untreated adult cleft palate patients. Plast Reconstr Surg. 1966;38:36–41. [DOI] [PubMed] [Google Scholar]


