Abstract
CXCR5+ T follicular helper (Tfh) cells are associated with aberrant autoantibody production in patients with antibody-mediated autoimmune diseases including lupus. Follicular regulatory T (Tfr) cells expressing CXCR5 and Bcl6 have been recently identified as a specialized subset of Foxp3+ regulatory T (Treg) cells that control germinal center reactions. In this study, we show that retroviral transduction of CXCR5 gene in Foxp3+ Treg cells induced a stable expression of functional CXCR5 on their surface. The Cxcr5-transduced Treg cells maintained the expression of Treg cell signature genes and the suppressive activity. The expression of CXCR5 as well as Foxp3 in the transduced Treg cells appeared to be stable in vivo in an adoptive transfer experiment. Moreover, Cxcr5-transduced Treg cells preferentially migrated toward the CXCL13 gradient, leading to an effective suppression of antibody production from B cells stimulated with Tfh cells. Therefore, our results demonstrate that enforced expression of CXCR5 onto Treg cells efficiently induces Tfr cell-like properties, which might be a promising cellular therapeutic approach for the treatment of antibody-mediated autoimmune diseases.
Keywords: CXCR5, Retroviral transduction, Treg cell, Tfh cell, Tfr cell, Germinal center reactions
INTRODUCTION
Effective B cell responses are essential for host defense against pathogens, particularly in case of a viral infection, via the generation of neutralizing or opsonizing antibodies; however, excessive B cell responses can also be detrimental in case of autoimmunity. For instance, elevated autoantibodies in circulation have been associated with various systemic autoimmune diseases such as systemic lupus erythematosus, Sjögren’s syndrome and rheumatoid arthritis (Wahren-Herlenius and Dorner, 2013; Suurmond and Diamond, 2015). The existence of autoantibodies indicates insufficient immune tolerance to self-reactive B cells in the patients. Given that a large fraction of autoantibodies are IgG isotypes, it is evident that auto-reactive T helper cells are also involved in the development of autoantibodies. Among diverse helper T cell subsets, follicular helper T (Tfh) cells are tightly associated with autoantibodies in humans with systemic autoimmune diseases (Crotty, 2014; Ueno et al., 2015).
Tfh cells play crucial roles in germinal center reactions to exogenous antigens as well as to self-antigens in autoimmunity (Crotty, 2014; Ueno et al., 2015; Vinuesa et al., 2016). Tfh cells induce clonal expansion of B cells, affinity maturation and isotype class switching of antibodies and subsequent differentiation of B cells into long-lived plasma cells or memory B cells. Tfh cells are characterized by high expression of CXCR5, which enables primed T cells to emigrate from T cell zone to B cell follicles when it is accompanied by simultaneous downregulation of CCR7 (Ansel et al., 1999; Hardtke et al., 2005; Haynes et al., 2007; Chang and Chung, 2014). The generation of Tfh cells requires interaction with B cells as well as ICOS, CD28, and CD40L signals (Haynes et al., 2007; Nurieva et al., 2008; Crotty, 2011; Wang et al., 2015). In addition, IL-6, IL-21 and IL-27 are known to drive Tfh cell differentiation from naïve precursors via the activation of STAT3 (Nurieva et al., 2008; Eddahri et al., 2009; Batten et al., 2010). These signal 2 and signal 3 likely drive the expression of two transcription factors Bcl-6 and Ascl2 to complete the Tfh cell commitment (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009; Liu et al., 2014).
CD4+ T cells expressing Foxp3 are essential for maintaining immune tolerance to self-antigens (Lutz, 2016). Mutation in Foxp3 causes detrimental systemic autoimmune responses as shown in scurfy mice as well as IPEX syndrome in humans (Sakaguchi et al., 2008). Recent studies by independent researchers confirm that there are at least four functional subsets in Foxp3+ T regulatory (Treg) cells that specifically inhibit Th1, Th2, Th17, and Tfh cell responses, respectively (Campbell and Koch, 2011). Among them, Treg cells expressing CXCR5 and Bcl6, named T follicular regulatory (Tfr) cells, appear to be located in the B cell follicles to control germinal center reactions. Tfr cells are originated from thymic Treg cells, and their development depends on CD28, CD40L as well as ICOS costimulation in addition to Bcl6 (Chung et al., 2011; Linterman et al., 2011; Wollenberg et al., 2011; Sage and Sharpe, 2015). Of note, decreased Tfr cells are associated with increased autoantibodies in animal models of lupus. For instance, BXD2 mice that spontaneously develop antibodies to dsDNA and histone exhibit reduced ratio of Tfr cells to Tfh cell or to germinal center B cells (Kim et al., 2015). More importantly, adoptive transfer of Tfr cells significantly ameliorates the development of lupus in BXD2 mice (Ding et al., 2014), indicating that malfunction of Tfr cells can trigger autoimmune B cell responses in vivo. In addition, a recent human study found that contraction of Tfr cells was inversely correlated with expansion of Tfh cells in the peripheral blood of IPEX patients (Charbonnier et al., 2015). Therefore, these studies collectively propose that the use of Tfr cells might be a promising therapeutic approach for the treatment of autoimmunity associated with increased autoantibodies.
Using Treg cells for the prevention and/or treatment of immune disorders has been investigated in experimental animals as well as in clinical setting in humans (Riley et al., 2009). A number of clinical studies conducted with Treg cells are currently awaiting FDA approval for clinical use in graft-versus-host disease (GVHD) after bone marrow transplantation, as well as autoimmune diseases such as type 1 diabetes (T1D), Crohn’s disease and multiple sclerosis (Trzonkowski et al., 2015). Despite their strong immune suppressive activity, there are a few limitations that need to be addressed for the optimal therapeutic efficacy of Treg cell-based immunotherapy. Firstly, the antigen specificity of Treg cells is likely important for the therapeutic efficacy; however, it is difficult to obtain antigen-specific Treg cells in case of autoimmunity, although there has been some progress in expanding allo-reactive Treg cells in case of GVHD. Secondly, the functional specificity of Treg cell subsets should be considered in the preparation of therapeutic Treg cells. For instance, use of Tfr cells is likely far more effective than pooled Treg cells consisting of diverse Treg cell subsets in suppressing an autoreactive germinal center reaction. This is because Tfr cells efficiently migrate into B cell follicles while the other Treg cell subsets fail to do so due to lack of CXCR5 on their surface. Thirdly, the stability of Treg cells should be fully explored since Treg cells are known to be converted into non-Treg cells, especially in response to inflammatory cytokines in vivo (Zhou et al., 2009; Miyao et al., 2012; Sakaguchi et al., 2013; Komatsu et al., 2014). Hence, the application of stable and functionally relevant Treg cell subsets with known antigen-specificity would be ideal for the development of efficacious immunotherapy with minimal adverse effects.
Although the use of Tfr cells can be efficacious for the treatment of antibody-mediated immune disorders, obtaining sufficient number of Tfr cells is an obvious obstacle for the development of such Treg-cell therapy. The reason for that is because it only consists of ~5–10% of Treg cells which is only ~10% of CD4+ T cells (Chung et al., 2011; Linterman et al., 2011; Wollenberg et al., 2011). In the present study, we aimed to investigate whether CXCR5 transduction onto Treg cells endows Tfr cell-like properties. We found that Cxcr5-transduction mediated stable expression of CXCR5 on Treg cells in vitro as well as in vivo. These Cxcr5-transduced Treg cells retained potent immunosuppressive activity against conventional T cells. In addition, Cxcr5-transduced Treg cells pre ferentially migrated in response to the CXCL13 gradient to suppress the production of antibodies from B cells in vitro.
MATERIALS AND METHODS
Mice
C57BL/6, Foxp3-IRES-mRFP (Foxp3RFP), B6.SJL (CD45.1+), and Tcrb−/ − (TCRβ KO) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All mice were maintained in the specific pathogen-free facilities at the vivarium of the Institute of Molecular Medicine, the University of Texas Health Science Center at Houston (TX, USA). All animal experiments were performed using protocols approved by Institutional Animal Care and Use Committee of the University of Texas at Houston.
Antibodies and flow cytometry
For cell sorting, lymphoid cells isolated from mouse spleens or draining lymph nodes, were obtained and stained with PerCp-Cy5.5-conjugated anti-CD4 (clone GK1.5, BioLegend, San Diego, CA, USA), Alexa488-conjugated anti-CD62L (clone MEL-14, BioLegend), PE-conjugated anti-CD25 (clone PC61, BioLegend), Alexa647-conjugated anti-CD44 (clone IM7, BioLegend), APC-conjugated anti-CD45R/B220 (clone RA3-6B2, BioLegend), Alexa488 anti-GL7 (clone GL7, BD Pharmingen, San Jose, CA, USA), PE-conjugated anti-IgD (clone 11-26c.2a, BioLegend), FITC-conjugated anti-CD279 (PD-1, clone J43, eBioscience, San Diego, CA, USA), Biotinconjugated anti-CXCR5 (clone L138D7, BioLegend), and APC-conjugated Streptavidin (BioLegend).
The stained cells were analyzed by FACSAria II (BD Bioscience, San Jose, CA, USA), and the data were analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
Cell isolation and culture
CD4+ T cells and B220+ B cells were isolated by anti-CD4 and anti-CD45R microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), respectively. B220+GL7–IgD+ naïve B cells, and CD4+CD25–CD44–CD62L+ naïve T cells were isolated from pooled spleen and peripheral lymph nodes of naïve C57BL/6 mice. CD4+PD-1+CXCR5+ Tfh cells were isolated from the draining lymph nodes of mice immunized with KLH by FACSAria II. Treg cells isolated from Foxp3RFP mice using Treg isolation kit (Miltenyi Biotec) were stimulated using Treg expansion kits (Miltenyi Biotec), according to the manufacturer’s protocols with a small modification (50 U/ml of mIL-2, instead of 1000 U/ml).
Cells were cultured in RPMI 1640 medium (Lonza, Houston, TX, USA) supplemented with 10% FBS, 55 μM 2-mercaptoethanol, 2 mM L-glutamine, 100 units penicillin-streptomycin (all from Gibco, Carlsbad, CA, USA), and 10 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO, USA). 293T cells were cultured in DMEM medium (Lonza) supplemented with 10% FBS 4.5g/l glucose, 2 mM L-glutamine, and 100 units penicillin-streptomycin.
CXCR5 cloning and retroviral transduction
Mouse Cxcr5 cDNA PCR fragment was prepared using iProof High-Fidelity DNA polymerase (BIORAD, Hercules, CA, USA), with Cxcr5 cloning primer sets (Forward 5′-ATCGAGATCTATGAACTACCCACTAACCCTGGAC-3′ and Reverse 5′-ATCGCTCGAGCTAGAAGGTGGTGAGGGAAGTAGC-3′). After Bgl II and Xho I (all from New England Biolabs, Beverly, MA, USA) enzyme digestion, the mCXCR5 fragment was ligated into the unique BglII and XhoI site of RVKM-IRES-Gfp vector (RV) using T4 ligase (Invitrogen, Carlsbad, CA, USA). 10 μg of pCL-Eco packaging vector with 10 μg of RV-empty vector or RV-Cxcr5 were co-transfected into the 293T cells using calcium phosphate/chloroquine (100 μM, Sigma, St. Louis, MO, USA) method. Twenty four hours later, stimulated Treg cells were transduced with RV-empty vector or RV-Cxcr5 in the presence of 8 μg/ml of polybrene (Sigma). Four days after the transduction, GFP and RFP double positive cells were sorted by FACSAria II (BD Bioscience, San Jose, CA, USA) for further approaches.
In vitro treg suppression assay
Cell proliferation dye eFluor670 (eBioscience, 5 μM) labeled conventional CD4+ T cells (Tconv, 1.0×105) isolated from congenic B6. SJL mice were co-cultured with indicated number of FACS-sorted GFP+RFP+ retrovirally transduced Treg cells in a round-bottomed 96-well plate in the presence of 0.5 μg/ml of anti-CD3 and irradiated (3000 cGy) T cell-depleted splenocytes (1.0×105) for 3 days. The proliferation of the Tconv cells was measured based on eFluor670 dilution by the CD4+CD45.1+ cell population by flow cytometry.
In vitro cell migration assay
FACS-sorted GFP+RFP+ retrovirally transduced Treg cells (3.0×105) were rested at 37°C for 2 hours in complete RPMI media. Cells were placed in the upper chamber [(Corning, Corning, NY, USA), Polycarbonate, 6.5 mm diameter, 5 μm pore size] containing 100 μl of complete RPMI media. The lower chamber was filled with 600 μl complete RPMI media containing various concentrations of CXCL13 (PeproTech, Rocky Hill, NJ, USA). After 4 hours of incubation, cells from the lower chamber were collected and the cell count was determined by running samples at a fixed flow rate (60 μl/min) for 1 min by FACS Calibur (BD Bioscience, San Jose, CA, USA). Migration index was calculated as follows: ((number of migrated cells/number of input cells)*100).
In vitro co-culture assay
RV-empty vector or RV-Cxcr5-transduced Treg cells (1.5×104) were placed in the upper chamber (Corning, Polycarbonate, 96 well, 3 μm pore size) containing 75 μl of complete RPMI media with the lower chamber containing 245 μl complete RPMI media with 1.5 μg/ml of CXCL13. After 1 hour of incubation, cells from the lower chamber were collected and co-cultured with B220+GL7–IgD+ (1.0×105) naïve B cells and CD4+PD-1+CXCR5+ Tfh cells (3.0×104) in the presence of 2.0 μg/ml soluble anti-CD3e (clone 145-2C11, BioXcell, West Lebanon, NH, USA) and anti-IgM (AffiniPure F(ab’)2 Fragment Goat anti-IgM, μ chain specific, Jackson ImmunoResearch, West Grove, PA, USA) for 6 days. The levels of murine IgG in the culture supernatant were determined by enzyme-linked immunosorbent assay (ELISA).
Adoptive transfer studies and keyhole limpet hemocyanin (KLH) immunization
Wild-type or CXCR5−/− Treg cells (CD4+CD25hi, 5.0×105) were adoptively transferred into Tcrb−/− mice together with CD25−GITR−CD62LhiCD44lo naïve CD4+ T cells (3.0×106). All recipient mice were s.c. immunized with KLH emulsified in CFA. Seven days later, the draining LNs were isolated and stained with PNA or anti-CD4 to visualize germinal center.
In some experiments, RV-empty vector or RV-Cxcr5-transduced Treg cells (5.0×105) were adoptively transferred into Tcrb−/− mice together with naïve CD4+ T cells isolated from B6.SJL mice (3.0×106). The recipient mice were s.c. immunized with KLH in CFA, and lymphoid cells from the draining lymph nodes were collected and analyzed for the presence of CD45.2+GFP+RFP+ donor T cells.
Western blot analysis
FACS-sorted RV-empty vector or RV-Cxcr5-transduced Treg cells were incubated in serum-free medium for overnight, then stimulated with indicated concentration of CXCL13 for 10 minutes. The cells were lysed in RIPA buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% Na deoxycholate, 0.1% SDS, and Protease/phosphatase inhibitors cocktail) and equal amounts of protein were electrophorated on Tris-Glycine NN 8–16% precast gel (NuSep Ltd., Frenchs Forest, Australia) and transferred onto polyvinylidene fluoride (PVDF) membranes. Western blot analysis was performed using the phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit monoclonal antibody and p44 MAP Kinase (Erk1) rabbit polyclonal antibody (both from Cell Signaling Technology, Danvers, MA, USA). The blots were incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG (sc-2313, Santa Cruz, Dallas, TX, USA) and detected with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Waltham, MA, USA).
Enzyme-linked immunosorbent assay (ELISA)
To measure the levels of IgG produced by B cells in vitro, total murine IgG was quantified in culture supernatants with total IgG capture antibody (Donkey anti-mouse IgG (H+L), Jackson ImmunoResearch) and HRP-conjugated total IgG detection antibody (Goat anti-mouse IgG, SouthernBiotech, Birmingham, AB, USA).
Quantitative real-time RT-PCR
Total RNA was extracted from retroviral vector transduced cells (3.0×105 cells) with TRIzol (Invitrogen) and reverse transcribed using amfiRivert reverse transcriptase (GenDEPOT, Baker, TX, USA) according to the manufacturer’s protocol. Gene expression was measured with iTaq-SYBR Green Supermix (BIORAD Laboratories, Hercules, CA, USA) and the ABI-PRISM 7900 detection system (Applied Biosystems, Foster City, CA, USA). Data were normalized to expression of the Actb gene.
Statistical analysis
Data were analyzed with GraphPad Prism 5 (GraphPad, La Jolla, CA, USA). Statistics was calculated with the two-tailed Student’s t-test. p-values below 0.05 were considered statistically significant.
RESULTS
CXCR5-deficient treg cells are inefficient in controlling germinal center reactions
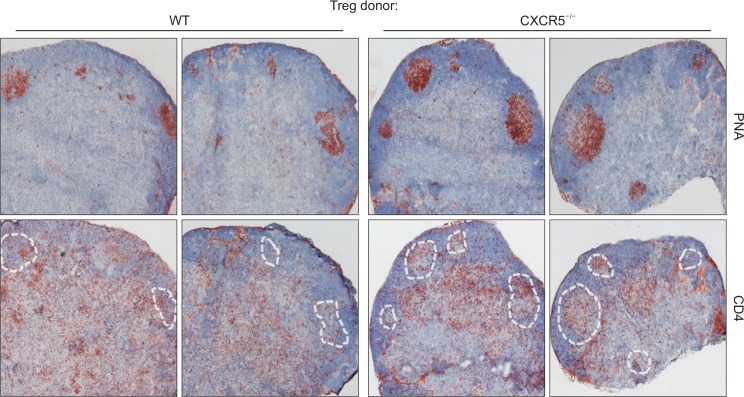
We and others previously demonstrated that follicular regulatory T cells (Tfr cells) expressing Foxp3 and CXCR5 regulates germinal center reactions (Chung et al., 2011; Linterman et al., 2011). To confirm that CXCR5 expression in Foxp3+ Treg cells is essential for the regulation of germinal center formation, we adoptively transferred naïve CD4+ T cells together with WT or CXCR5-deficient Treg cells into Tcrb-deficient mice. One day after transfer, the recipient mice were subcutaneously immunized with keyhole limpet hemocyanin (KLH) emulsified in CFA. Seven days after immunization, we found an evident PNA+ germinal center formation in the draining lymph nodes (Fig. 1). Of note, we observed an increase in the size of PNA+ germinal centers in the lymph nodes of the CXCR5-deficient Treg cell recipients compared to those of WT Treg cell recipients (Fig. 1, upper panels). Consistently, CD4+ T cells in the germinal center, presumably Tfh cells, were observed in the lymph nodes of the former group (Fig. 1, lower panels). These results indicate that CXCR5 expression in Treg cells is required for Treg cell-mediated control of germinal center reactions.
Fig. 1.
CXCR5-dependent regulation of germinal center formation by Treg cells. Naïve CD4+CD25–GITR–CD62LhiCD44lo T cells isolated from wild-type (WT) mice were mixed with CD4+CD25hi Treg cells isolated from WT or CXCR5−/− mice and adoptively transferred into Tcrb−/− mice. Twenty four hours later, the recipient mice were s.c. immunized with KLH in CFA. Seven days after immunization, germinal center formation (PNA) and CD4+ T cell localization (CD4) in the draining lymph node of the recipient mice were analyzed. Dotted areas represent germinal centers.
Generation of CXCR5-expressing treg cells
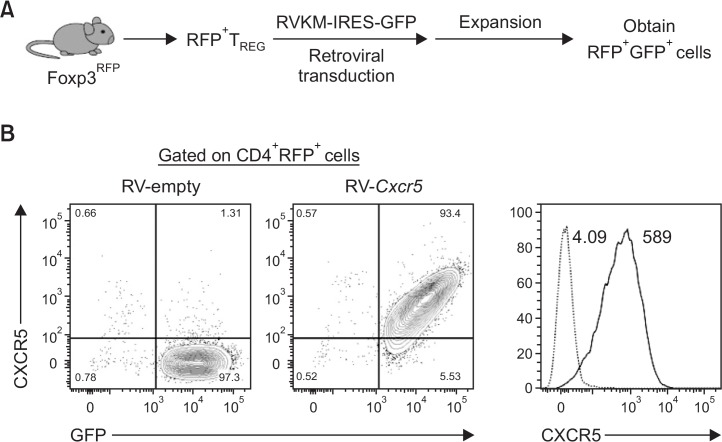
One of the key features of Tfr cells is its ability to emigrate into B cell follicles via CXCR5-mediated chemotaxis (Sage and Sharpe, 2015). Therefore, we hypothesized that enforced expression of CXCR5 onto Foxp3+ Treg cells would endow the ability of preferential migration to B cell follicles in the secondary lymphoid organs in response to the CXCL13 gradient. As a first step to test this hypothesis, we first cloned mouse Cxcr5 gene into a retroviral vector (RV) containing IRES and GFP. Foxp3+ Treg cells were isolated from Foxp3-IRES-mRFP (Foxp3RFP) reporter mice, which express a monomeric red fluorescence protein (mRFP) under the control of mouse Foxp3 promoter. We then transduced RV-empty-Gfp vector (RV-empty) or RV-Cxcr5-Gfp vector (RV-Cxcr5) into the RFP+ Treg cells in the presence of TCR stimulation by anti-CD3 and anti-CD28 Abs (Fig. 2A). Four days after the retroviral transduction, surface expression of CXCR5 on Treg cells was analyzed by flow cytometry. As depicted in Fig. 2B, most of the RV-Cxcr5-transduced Treg cells expressed CXCR5 in a GFP intensity-dependent manner while few RV-empty vector-transduced Treg cells expressed CXCR5. Therefore, while anti-CD3/anti-CD28 stimulation alone failed to do so, retroviral transduction efficiently induced the upregulation of CXCR5 on the surface of Treg cells.
Fig. 2.
Generation of CXCR5-expressing Treg cells. (A) Schematic representation of retroviral transduction of Treg cells. RFP+ Treg cells isolated from Foxp3-IRES-mRFP (Foxp3RFP) mice were retrovirally transduced with RV-empty vector or RV-Cxcr5-expressing vector. Retrovirally transduced Treg cells were further expanded in the presence of TCR stimulation for 4 days. CD4+RFP+GFP+ cells were sorted by flow cytometry. (B) Cell surface expression of CXCR5 in gated CD4+RFP+ cells was analyzed by flow cytometry. Numbers in the histogram indicate median fluoresence intensity (MFI) of CXCR5 expression in RV-empty (Dotted)- and RV-Cxcr5 (Solid). Data are representatives of three independent experiments.
Cxcr5-transduced Treg cells maintain suppressive activity in vitro
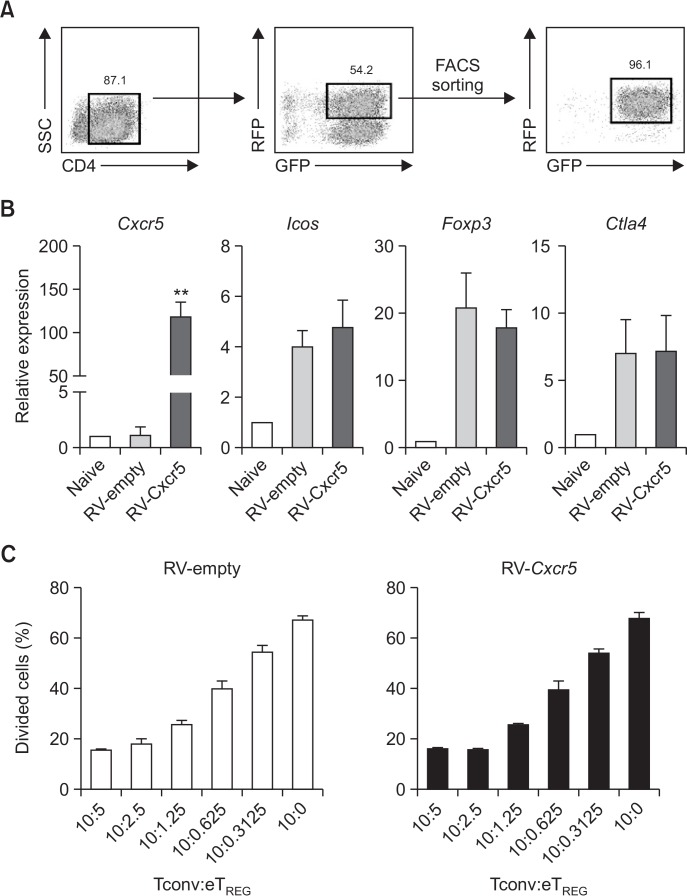
We next compared the expression of Treg cell-associated genes in the transduced cells. To this end, we purely isolated CD4+RFP+GFP+ Treg cells by flow cytometry four days after RV transduction using a sorting strategy described in Fig. 3A. As a result, we obtained >95% of RFP+GFP+ Treg cells from each transduction condition. As expected, RV-Cxcr5-transduced Treg cells exhibited a significantly up-regulated Cxcr5 transcript expression compared to RV-empty vector-transduced Treg cells. On the other hand, levels of Treg cell-associated gene transcripts, such as Foxp3, Icos and Ctla4, in Treg cells appeared to be comparable between the two cells (Fig. 3B). Moreover, RV-Cxcr5-transduced Treg cells were as potent as RV-empty vector-transduced Treg cells in suppressing the proliferation of naive CD4+ T cells in an in vitro suppression assay (Fig. 3C). These results together indicate that retroviral transduction of Cxcr5 gene efficiently induces CXCR5 expression in Treg cells without affecting their suppressive function in vitro.
Fig. 3.
Retroviral transduction of Cxcr5 on Treg cells does not affect Treg cell signature genes expression or their suppressive ability. (A) Cell sorting strategy and sorting purity of retrovirally transduced Treg cells. After five days of retroviral transduction, CD4+RFP+GFP+ cells were sorted by flow cytometry. (B) Quantitative RT-PCR analysis of indicated genes were conducted in RV-empty vector- or RV-Cxcr5-transduced Treg cells. Naïve CD4+ T cells were included as a control. (C) Cell proliferation dye-labeled responder conventional T cells (Tconv; CD4+CD25– T cells) were stimulated with anti-CD3 Ab in the presence of irradiated T cell-depleted splenocytes for 3 days. Titrated number of RV-empty vector- or RV-Cxcr5-transduced Treg cells were added to the culture. Cell proliferation was measured by flow cytometry. Data are representatives of three independent experiments (**p<0.01).
CXCR5 on the Cxcr5-transduced Treg cells is stable in vivo
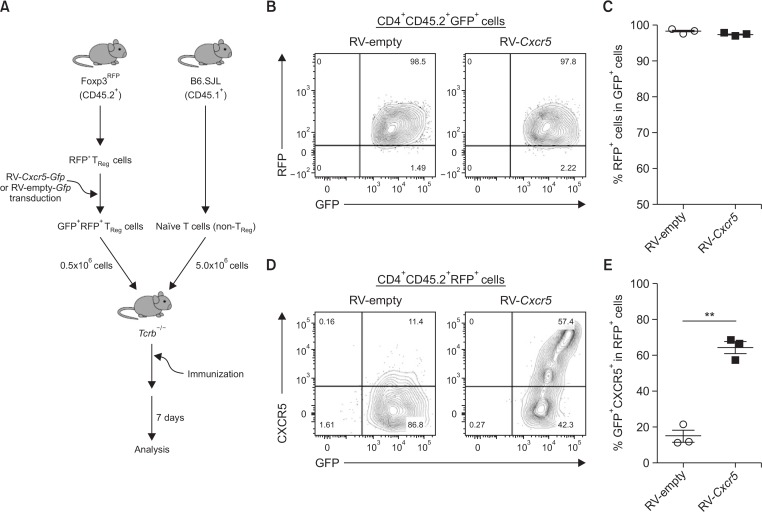
Treg cells are known to be converted into conventional T cells in response to inflammatory cytokines by downregulating Foxp3 (Zhou et al., 2009; Komatsu et al., 2014). Instability of Foxp3 expression is one of the concerns for the clinical application of Treg cells (Trzonkowski et al., 2015). Therefore, we next sought to determine the stability of Foxp3 as well as CXCR5 in the transduced Treg cells in vivo. To this end, RFP+GFP+ Treg cells among the retrovirally transduced cells were isolated as shown in Fig. 3A, before being adoptively transferred into TCRβ-deficient mice. Naïve CD4+ T cells isolated from CD45.1+ congenic mice were co-transferred into the same recipients. The recipient mice were then subcutaneously immunized with KLH (Fig. 4A). Seven days later, we analyzed CD4+CD45.2+ T cells in the draining lymph nodes. As depicted in Fig. 4B, 4C, over 95% of the RV-Cxcr5-transduced as well as RV-empty-transduced donor T cells retained RFP expression, indicating that the transduced T cells maintained their Foxp3 expression in vivo even after active immunization. More importantly, about 60% of RV-Cxcr5-transduced CD4+RFP+GFP+ Treg cells expressed CXCR5, while only about 15% of RV-empty vector-transduced cells did so (Fig. 4D, 4E), indicating that the Cxcr5-transduced Treg cells remarkably maintained CXCR5 on their surface in the recipient mice in vivo. Collectively, according to this experimental setting, these results demonstrate that the expression of Foxp3 and CXCR5 was largely stable in vivo.
Fig. 4.
CXCR5 expression in Cxcr5-transduced Treg cells is stable in vivo. (A) RV-empty vector- or RV-Cxcr5-transduced Treg cells were co-transferred with naïve CD4+ T cells isolated from B6.SJL congenic mice into Tcrb−/− mice followed by subcutaneous immunization of the recipient mice with KLH. Seven days later, the transferred Treg cells in the draining lymph nodes were analyzed by flow cytometry after gating on CD4+CD45.2+GFP+ cells. (B, C) RFP expression in gated CD4+CD45.2+RFP+ were analyzed. (D, E) Flow cytometric analysis of CXCR5 expression in gated CD4+CD45.2+RFP+ cells. Data are representatives of two independent experiments (**p<0.01).
Cxcr5-transduced Treg cells efficiently migrate toward the CXCL13 gradient and suppress antibody production from B cell
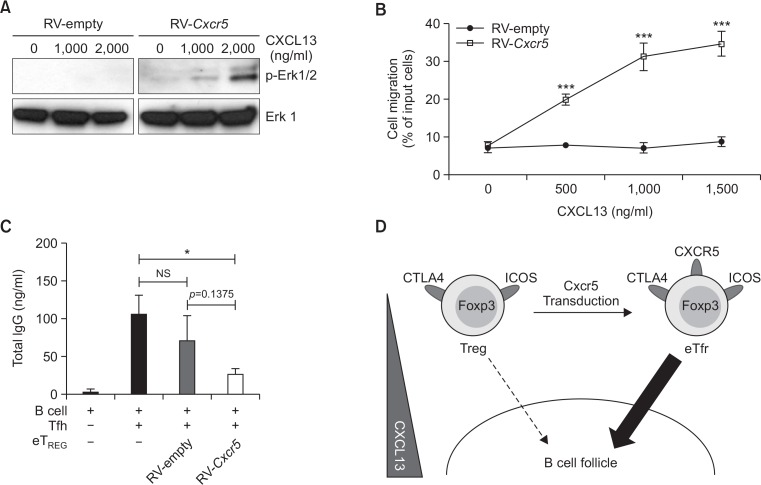
We next determined if the Cxcr5-transduced Treg cells act like Tfr cells in terms of their migratory property as well as their B cell suppressive activity. CXCL13 is a chemokine enriched in B cell follicles whose receptor is CXCR5 (Gunn et al., 1998; Legler et al., 1998). CXCL13 has been shown to activate Erk1/2 mitogen-activated protein kinase (MAPK) pathway upon stimulation via CXCR5 (Muller and Lipp, 2001). Therefore, we addressed whether CXCL13 activates the Erk1/2 signaling pathway in the RV-Cxcr5-transduced Treg cells. CD4+RFP+GFP+ cells isolated from retroviral vector-transduced Treg cells were stimulated with different concentrations of CXCL13 for 10 minutes. Then, we analyzed the phosphorylation of Erk1/2 by immunoblot. As depicted in Fig. 5A, CXCL13 stimulation induced an evident phosphorylation of Erk1/2 in the Cxcr5-transduced Treg cells in a dose-dependent manner, indicating that the CXCR5 on the transduced Treg cells was functional. By contrast, little phosphorylation of Erk 1/2 was detectable in the RV-empty vector-transduced Treg cells (Fig. 5A). We next determined whether the transduced Treg cells migrate toward the CXCL13 gradient by employing an in vitro tran-swell chemotaxis assay. While RV-empty vector-transduced Treg cells failed to migrate into the CXCL13-containing bottom well regardless of CXCL13 concentration in the transwell system, the RV-Cxcr5-transduced Treg cells efficiently migrated into the CXCL13-containing well in a dose-dependent manner (Fig. 5B). These results clearly demonstrate that CXCR5 on the RV-Cxcr5-transduced Treg cells was functionally active.
Fig. 5.
Cxcr5-transduced Treg cells can migrate in a CXCL13 dose-dependent manner and suppress antibody production by B cells in vitro. (A) Serum starved RV-empty vector- or RV-Cxcr5-transduced Treg cells were stimulated with titrated doses of CXCL13 for 10 mins. Phosphorylation of Erk1/2 was analyzed by Western blot. (B) Transwell migration of RV-empty vector- or RV-Cxcr5-transduced Treg cells to CXCL13-enriched lower chamber was analyzed. Titrated doses of CXCL13 were added to the lower chamber. (C) Cells recovered from lower chamber in (B) were co-cultured with B220+GL7–IgD+ naïve B cells and CD4 +PD-1+CXCR5+ Tfh cells in the presence of 2 μg/ml soluble anti-CD3ε and anti-IgM Abs for 6 days. Total IgG levels were measured by ELISA. (D) Illustration of the effect of Cxcr5-transduction on Treg cells for Treg cell signature gene expression and chemotaxis toward CXCL13. Data are representatives of two independent experiments (*p<0.05, ***p<0.001).
We finally determined whether the Cxcr5-transduced Treg cells control Tfh cell-mediated antibody production from B cell. To mimic a germinal center environment, we employed a transwell culture system. In brief, B220+GL7–IgD+ naïve B cells were co-cultured with FACS-sorted CD4+PD-1+CXCR5+ Tfh cells in the presence of soluble anti-CD3, anti-IgM, and CXCL13 to mimic germinal center reactions in the bottom well. FACS-sorted GFP+RFP+ cells from the RV-empty- or RV-Cxcr5-transduced Treg cell population were placed in the upper well. After 1 hour of incubation, the upper wells were removed and the bottom wells were further cultured for 6 days. As expected, Tfh cells efficiently stimulated B cells to produce IgG in the absence of Treg cells. However, adding RV-Cxcr5-transduced Treg cells into upper wells significantly inhibited IgG production from B cells while RV-empty-transduced Treg cells failed to do so (Fig. 5C). Taken together, in this experimental setting, these results suggest that stable expression of CXCR5 on the Treg cells by Cxcr5 transduction drives chemotactic activity toward the CXCL13 gradient to suppress Tfh cell-mediated IgG production from B cells in vitro (Fig. 5D).
DISCUSSION
In the present study, we aimed to determine if ectopic expression of CXCR5 on Treg cells can induce Tfr cell properties. We demonstrate that (i) CXCR5-deficient Treg cells were inefficient in suppressing germinal center reactions, (ii) retroviral transduction of Cxcr5 did not affect the expression of Treg cell-related genes nor its suppressive activity, (iii) expression of CXCR5 and Foxp3 in the Cxcr5-transduced Treg cells was largely stable when transferred in vivo, (iv) CXCR5 on the Cxcr5-transduced Treg cells was functionally active, and (v) Cxcr5-transduced Treg cells sufficiently suppressed the production of IgG from B cells stimulated with Tfh cells in our transwell studies. Thus, the Cxcr5-transduced Treg cells resemble some features of Tfr cells including the expression of CXCR5 and the responsiveness toward the CXCL13 gradient as well as B cell suppressive activity. Therefore, our findings suggest that these genetically engineered Tfr-like cells might be a promising alternative to genuine Tfr cells which can be developed as novel cellular immunotherapeutics for the treatment of antibody-mediated immune disorders in humans.
Due to the low frequency of Treg cells in the peripheral blood of human patients, polyclonal Treg cells were expanded from precursor cells ex vivo by TCR stimulation or IL-2 cytokine stimulation or both for clinical application of Treg cells. In the current study, Cxcr5-transduced Treg cells were further expanded by TCR stimulation for four more days after retroviral transduction. It is possible that exogenous IL-2 can induce vigorous proliferation of Cxcr5-transduced Treg cells after transduction. Interestingly, however, it has been demonstrated that Stat5 activation by exogenous IL-2 inhibited Tfh cell development by promoting Blimp-1, a critical negative regulator of Bcl6 (Johnston et al., 2012; Nurieva et al., 2012). Therefore, it needs to be tested if IL-2 can be used for the expansion of Cxcr5-transduced Treg cells in vitro without affecting the stability and function of Tfr cells.
Several preclinical studies proved that the inhibitory function of Ag-specific Treg cells is superior to that of polyclonal Treg cells in suppressing autoimmune diseases or graft rejection (MacDonald et al., 2016). Ag-specific Treg cells were generally prepared by ex vivo expansion of precursor cells by antigenic stimulation. In the present study, however, it remains unclear whether the CXCR5-transduced Treg cells can suppress germinal center reactions in an antigen-specific manner in vivo, since we started with polyclonal Treg cells. Chimeric antigen receptor (CAR) technique has been emerged as a promising tool to generate T cells recognizing a specific antigen ex vivo (Elinav et al., 2009; Blat et al., 2014). In particular, CAR has been extensively investigated for the last two decades in developiong Ag-specific T cell adoptive immunotherapy against cancer (Geldres et al., 2016; Guo et al., 2016). CAR is a retrovirally transduced artificial T cell antigen receptor that consists of single-chain variable fragment (ScFv), hinge region, transmembrane domain, costimulatory domain and TCR ζ-chain (Geldres et al., 2016). It has been suggested that if the CAR can recognize tissue-specific antigens in the inflammed tissue, Treg cells can be activated in vivo without knowing the target autoantigens (Blat et al., 2014). In this regard, we propose that the use of CAR technique will endow antigen-specificity into the engineered Treg cells. For instance, surface molecules expressed on resting or activated B cells, such as CD19 and GL7, would be attractive targets for the development of CAR-mediated Ag-specific Tfr adoptive immunotherapy.
There still reamins an issue with stability and persistence of the engineered Tfr cells for their clinical application. The stability of Treg cells in vivo has been a matter of debate for the last ten years. Several mouse studies have demonstrated that a small number of CD4+Foxp3– “exFoxp3” T cells originated from Foxp3+ Treg cells contribute to the development of autoimmune diseases in diabetes or arthritis animal models (Zhou et al., 2009; Bailey-Bucktrout et al., 2013; Komatsu et al., 2014). On the contrary, others suggested that only a minor subset of Treg cells have potential to be pathogenic after losing Foxp3, while the majority of remaining Treg cells are stable in vivo (Rubtsov et al., 2010; Miyao et al., 2012). We found that Cxcr5-transduced Treg cells stably express Foxp3 as well as CXCR5 without converting to Tfh cells or other pathogenic non-Treg cells when transferred in vivo. But it needs to be addressed whether Cxcr5-transduced Treg cells stably express Foxp3 and persist in the long term under severe inflammatory settings like lupus-prone BXD2 mice (Kim et al., 2015).
Compared to conventional Treg cells, Tfr cells highly express several costimulatory molecules including ICOS, PD-1, CTLA-4 and GITR (Chung et al., 2011; Linterman et al., 2011; Wollenberg et al., 2011; Sage et al., 2013, 2014; Wing et al., 2014). In addition, increased Ki-67 expression reveals that Tfr cells have enhanced proliferative potential (Wollenberg et al., 2011; Sage et al., 2013). These results suggest that Tfr cells represent not just the Treg cells that are selectively located in GC regions but they are activated Treg cells with enhanced suppressive activity. Recently, however, it has been shown that Tfr cells directly suppress B cells through CTLA4 and the suppressive activity of Tfr cells against B cells was comparable to that of conventional Treg cells (Sage et al., 2014; Wing et al., 2014). Since the suppressive mechanism by which Tfr cells inhibit B cells was similar to that by conventional Treg cells in those studies, we propose that Cxcr5-transduction itself might be sufficient to confer B cell suppressive Tfr-like activity on Treg cells as long as the Treg cells are properly activated during and after the transduction. Hence, careful consideration on Treg cell homing properties should be given when developing cellular immunotherapy based on Treg cells.
It still remains to be elucidated whether Tfr cells have unique characteristics other than CXCR5 expression that are essential for the regulation of the germinal center reaction. In this regard, Bcl6, a Tfh cell defining transcription factor, would be one of the attractive candidates for Tfr engineering to assure full differentiation of Tfr cells. Unfortunately, however, since Bcl6 transduction itself was not sufficient to induce CXCR5 expression in naïve T cells, it has been demonstrated that Bcl6 transduction alone could not initiate Tfh cell programing in naïve T cells (Crotty, 2011). A recent study demonstrated that achaete-scute homologue-2 (ASCL2) facilitates early Tfh differentiation by inducing upregulation of CXCR5 (Liu et al., 2014). It will be interesting to test whether Ascl2 transduction can ensure the generation of stable Tfr-like cells from conventional Treg cells without affecting Foxp3 expression and suppressive function.
In summary, we found that forced expression of CXCR5 endowed Tfr-like features in Treg cells. These engineered Tfr-like cells efficiently migrated along with CXCL13 gradient and effectively suppressed B cell antibody production. Our study provides new insights into the development of adoptive Treg cell immunotherapy for the regulation of autoimmune disorders.
Acknowledgments
We thank Mr. Inbo Shim for editing manuscript. This work was supported by research grants SNU invitation for distinguished scholar (to YC), 2014R1A2A1A11054364 (to YC) from the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST), 1R01HL118361 (to YC and RAW) from National Institutes of Health.
REFERENCES
- Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, Danilenko DM, Caplazi P, Wong M, Fulcher DA, Cook MC, King C, Tangye SG, de Sauvage FJ, Ghilardi N. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther. 2014;22:1018–1028. doi: 10.1038/mt.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J-H, Chung Y. Regulatory T cells in B cell follicles. Immune Netw. 2014;14:227–236. doi: 10.4110/in.2014.14.5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, Massaad MJ, Garcia-Lloret M, Hanna-Wakim R, Dbaibo G, Alangari AA, Alsultan A, Al-Zahrani D, Geha RS, Chatila TA. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135:217–227. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, Neelapu SS, Dong C. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Li J, Yang P, Luo B, Wu Q, Zajac AJ, Wildner O, Hsu HC, Mountz JD. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol. 2014;66:2601–2612. doi: 10.1002/art.38735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, Andris F. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113:2426–2433. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Adam N, Waks T, Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology. 2009;136:1721–1731. doi: 10.1053/j.gastro.2009.01.049. [DOI] [PubMed] [Google Scholar]
- Geldres C, Savoldo B, Dotti G. Chimeric antigen receptor-redirected T cells return to the bench. Semin Immunol. 2016;28:3–9. doi: 10.1016/j.smim.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang Y, Han W. Chimeric Antigen Receptor-Modified T Cells for Solid Tumors: Challenges and Prospects. J Immunol Res. 2016;016:3850839. doi: 10.1155/2016/3850839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YU, Lim H, Jung HE, Wetsel RA, Chung Y. Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells. PLoS ONE. 2015;10:e0120294. doi: 10.1371/journal.pone.0120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, Nakatsukasa H, Neelapu SS, Chen W, Clevers H, Tian Q, Qi H, Wei L, Dong C. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507:513–518. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB. Induction of CD4+ regulatory and polarized effector/helper T cells by dendritic cells. Immune Netw. 2016;16:13–25. doi: 10.4110/in.2016.16.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, Broady R, Levings MK. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of Foxp3+ T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Muller G, Lipp M. Signal transduction by the chemokine receptor CXCR5: structural requirements for G protein activation analyzed by chimeric CXCR1/CXCR5 molecules. Biol Chem. 2001;382:1387–1397. doi: 10.1515/BC.2001.171. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, Huang H, Wen R, Wang J, Li HS, Watowich SS, Qi H, Dong C, Wang D. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015;36:410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest. 2015;125:2194–2202. doi: 10.1172/JCI78084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, ten Brinke A, Bushell A, Cools N, Geissler EK, Gregori S, Marieke van Ham S, Hilkens C, Hutchinson JA, Lombardi G, Madrigal JA, Marek-Trzonkowska N, Martinez-Caceres EM, Roncarolo MG, Sanchez-Ramon S, Saudemont A, Sawitzki B. Hurdles in therapy with regulatory T cells. Sci Transl Med. 2015;7:304ps18. doi: 10.1126/scitranslmed.aaa7721. [DOI] [PubMed] [Google Scholar]
- Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- Wahren-Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM, Kogimtzis A, Kenefeck R, Sansom DM, Walker LS. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci USA. 2015;112:524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]