Abstract
The interleukin-1 receptor antagonist (IL-1RA) is a potential stroke treatment candidate. Intranasal delivery is a novel method thereby a therapeutic protein can be penetrated into the brain parenchyma by bypassing the blood-brain barrier. Thus, this study tested whether intranasal IL-1RA can provide neuroprotection and brain penetration in transient cerebral ischemia. In male Sprague-Dawley rats, focal cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) for 1 h. The rats simultaneously received 50 mg/kg human IL-1RA through the intranasal (IN group) or intraperitoneal route (IP group). The other rats were given 0.5 mL/kg normal saline (EC group). Neurobehavioral function, infarct size, and the concentration of the administered human IL-1RA in the brain tissue were assessed. In addition, the cellular distribution of intranasal IL-1RA in the brain and its effect on proinflammatory cytokines expression were evaluated. Intranasal IL-1RA improved neurological deficit and reduced infarct size until 7 days after MCAO (p<0.05). The concentrations of the human IL-1RA in the brain tissue 24 h after MCAO were significantly greater in the IN group than in the IP group (p<0.05). The human IL-1RA was confirmed to be co-localized with neuron and microglia. Furthermore, the IN group had lower expression of interleukin-1β and tumor necrosis factor-α at 6 h after MCAO than the EC group (p<0.05). These results suggest that intranasal IL-1RA can reach the brain parenchyma more efficiently and provide superior neuroprotection in the transient focal cerebral ischemia.
Keywords: Cerebral ischemia, Interleukin-1 receptor antagonist, Intranasal administration, Neuroinflammation
INTRODUCTION
Stroke is a major cause of death and disability in adults worldwide. Although the etiology of stroke may be ischemia or hemorrhage, ischemic stroke accounts for about 80% of all stroke cases (Chalela et al., 2004). The only approved therapy for ischemic stroke is fibrinolysis with recombinant tissue plasminogen activator (rtPA). However, rtPA can be administered in a limited number of cases because of its short therapeutic window. Therefore, additional strategies are required to manage ischemic stroke.
Inflammation is a major part of the pathophysiology of stroke (Amantea et al., 2009) and interleukin (IL)-1 is an essen tial mediator of cerebral ischemia injury (Allan et al., 2005; Simi et al., 2007). The IL-1 receptor antagonist (IL-1RA) is a competitive IL-1 antagonist and induced by the same stressors as IL-1, which can block all types of IL-1 action (Hannum et al., 1990). In animal experiments, IL-1RA administration can considerably decrease cerebral ischemia injury, even when administered peripherally to comorbid animals after the onset of ischemia (Loddick and Rothwell, 1996; Mulcahy et al., 2003; Pradillo et al., 2012). However, in a clinical trial, IL-1RA administration did not directly improve clinical outcomes such as infarct volume and survival rate in acute stroke patients (Emsley et al., 2005). One of the reasons why IL-1RA failed to demonstrate efficacy in the clinical situation may be the inadequate delivery of IL-1RA to the central nervous system. A meta-analysis of the efficacy of IL-1RA in animal models of stroke supports this assumption by demonstrating that greater neuroprotection could be achieved with central administration than with peripheral administration of IL-1RA (Banwell et al., 2009). Therefore, a more efficient route of delivery of IL-1RA to brain parenchyma is required.
The blood-brain barrier (BBB) is known to be a major barrier against the delivery of pharmacologically relevant quantities of therapeutic proteins to the brain. IL-1RA is a large (17 kDa) hydrophilic peptide. Thus, the penetration of peripherally administered IL-1RA into the brain was reported to be limited (Gutierrez et al., 1994). On the other hand, intranasal administration has been emerging as the effective delivery route of therapeutic proteins to the brain by bypassing the BBB (Malerba et al., 2011). However, a study on intranasal administration of IL-1RA to protect the brain against stroke has hardly ever been undertaken. This study was designed to compare between intranasal and systemic administrations of IL-1RA from the viewpoints of efficiency of brain penetration and neuro-protection.
MATERIALS AND METHODS
Animals
This experiment protocol was approved by the Yonsei University Animal Care and Use Committee (protocol number: 2012-0235; Seoul, Korea) and was in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. Male Sprague-Dawley rats aged 8–10 weeks, weighing 280–320 g, were obtained from Orientbio Inc. (Seongnam, Korea) and used for this study. The rats were maintained under a 12-h light-dark cycle and allowed free access to food and water before and after surgery.
Transient focal cerebral ischemia
Focal cerebral ischemia was induced by transient occlusion of the middle cerebral artery (MCA). The experimental MCA occlusion (MCAO) was performed as previously described (Belayev et al., 1996). Anesthesia was induced with intraperitoneal injection of a mixture of 30 mg/kg zoletil (Virbac Laboratory, Carros, France) and 10 mg/kg xylazine (Bayer Korea Ltd., Seoul, Korea). Zoletil is a combination of a dissociative anesthetic, tiletamine hydrochloride, and a benzodiazepine, zolazepam hydrochloride. Tiletamine is a non-competitive antagonist at the phencyclidine site of the N-methyl-D-aspartate receptor. Xylazine is an alpha-2 adrenergic agonist that exerts effects on presynaptic and postsynaptic receptors of the central and peripheral nervous systems. The rats were placed in the supine position on a heated pad, with their body temperatures maintained at 37°C ± 0.5°C according to a rectal thermometer. Under an operating microscope, the right common carotid, external carotid (EC), and internal carotid (IC) arteries were exposed through a midline incision. The EC artery was ligated, coagulated, and cut down just proximal to the lingual and maxillary artery branches. All the other branches of the EC artery were coagulated and transected. The IC artery was then isolated from the vagus nerve to prevent damage. A 4-0 monofilament nylon suture (Dermalone, United States Surgical, CT, USA) with a flame-rounded head was inserted through the IC artery via a small incision in the EC artery stump. The distance from bifurcation of the common carotid artery to the tip of the suture was approximately 18.5 mm in all the rats, which is consistent with published descriptions of the MCAO model. Cerebral blood flow was monitored by using laser Doppler flowmetry (Omega flow, FLO-C1, Neuroscience, Tokyo, Japan) with a flexible probe placed in the cortical areas supplied by the MCA (2 mm posterior and 6 mm lateral to the bregma). When the MCA was occluded by thread insertion, rats that did not show a cerebral blood flow reduction of at least 70% were excluded from the experiment (Xing et al., 2008). After 60 min of occlusion, the suture was withdrawn, the skin was sutured, and the rats were allowed to recover.
Treatments
The rats were randomly divided into 3 groups. All of the rats underwent MCAO/reperfusion as described earlier. The IN group intranasally received 50 mg/kg human IL-1RA (Kineret, Amgen Manufacturing, Ltd., Thousand Oaks, CA, USA). Intra-nasal administration of 0.5 mL/kg Kineret (undiluted solution, approximately 100 μL) was performed in 8- to 10-μL drops by using a pipette, treating each nare every 5 min for 60 min from the start of MCAO. In addition, 0.5 mL/kg 0.9% saline was administered intraperitoneally at the start of MCAO. The IP group received 50 mg/kg IL-1RA intraperitoneally at the start of MCAO and the same volume of 0.9% saline intranasally in the same manner as did the IN group. The EC group received 0.5 mL/kg 0.9% saline through both the intranasal and intraperitoneal routes.
The administration dose of human IL-1RA was determined based on previous studies. The systemic administration of 100 mg/kg human IL-1RA showed neuroprotective effects in rat models of cerebral ischemia when given at the beginning of ischemia (Garcia et al., 1995a; Relton et al., 1996; Greenhalgh et al., 2010). We used half of this dose (50 mg/kg) in the present study to investigate whether the neuroprotective properties of IL-1RA could also be guaranteed at a lower dose. In order to compare the amount of IL-1RA reaching the brain parenchyma according to route of administration, the same dose was used for intranasal administration.
Neurobehavioral assessment
Neurological deficits were assessed before and 1, 2, 4, and 7 days after MCAO. Two examiners who were blinded to the treatment conditions consecutively and independently performed the neurological examinations of the animals. Adherence to a predetermined time excluded behavioral changes based on the circadian rhythm. The neurological examination consisted of 6 tests developed and described by Garcia et al. (1995b). The score assigned to each rat at the completion of the evaluation equaled the sum of all the scores in the following 6 tests: (1) spontaneous activity; (2) symmetry in movement of the 4 limbs; (3) forepaw outstretching; (4) climbing; (5) body proprioception; and (6) response to vibrissae touch. The final maximum neurological score was 18.
The rota-rod test was used to assess the recovery of impaired motor function after MCAO. This accelerating rota-rod test (ENV-577, Med Associates Inc., Georgia, VT, USA) was conducted as described by Hunter et al. (2000). The exercise time, which is the time the animals remained on an accelerating rota-rod cylinder, was measured. Speed was increased from 4 to 40 rpm within 5 min. The trial ended if the animal fell off the rungs or gripped the device and spun around for 2 consecutive revolutions without attempting to walk on the rungs. The exercise time on the rota-rod was measured for each rat 5 times per day before and 1, 2, 4, and 7 days after MCAO.
Infarct volume
On the seventh day after MCAO, the animals were anesthetized and decapitated. The brains were quickly isolated and sectioned into 2-mm-thick serial coronal slices. The brain slices were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma Aldrich, St Louis, MO, USA) in the dark at 37°C for 30 min and fixed with 4% paraformaldehyde (PFA, Sigma Aldrich) overnight. The posterior surface of each slice was photographed and analyzed by using a computer-assisted image analysis system (Optimas ver 6.1, Optimas, Bothell, WA, USA). The lesion volume was calculated by multiplying the area by the thickness of the slices. We adopted a previously described method to eliminate the contribution of edema to the ischemic lesion and to correct for the individual difference in brain volumes by using the percentage of infarct volume in the ipsilateral hemisphere volume (Belayev et al., 1996). Edema volume was calculated by subtracting the volume of the contralateral hemisphere from that of the ipsilateral hemisphere. The percentage of edema volume was calculated as (edema volume/contralateral hemisphere volume).
Determination of the administered human IL-1RA level (enzyme-linked immunosorbent assay)
Serum and brain tissues, including the striatum and cortex, were obtained during appointment and were stored in aliquots at −80°C until use for different assays. Serum levels of human IL-1RA were measured by using high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits (Quantikine ELISA, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. To measure the human IL-1RA level in brain tissue, total proteins of brain tissues, including the striatum and cortex, were extracted by using a tissue protein extraction reagent (T-PER Tissue Protein Extraction Reagent, Thermo Scientific, Waltham, MA, USA). In brief, the weight of the striatal and cortical tissues was measured, and the reagent was added (20-μL reagent per 1-mg tissue). Inhibitor cocktail (100X Halt protease and phosphate inhibitor cocktail, Thermo 1861281, Thermo Scientific) were also added to the reagent for protection of intact active cellular proteins from degradation, and the striatal and cortical tissues were then homogenized. After centrifugation, the supernatant was collected. Protein concentration was determined by using the BCA Protein Assay Kit (Thermo Scientific) according to the manufacturer’s methods. To calculate the protein yield in a maximum of 20,000 pg/mL, we measured the sample area by using the manufacturer’s instruction standard. The human IL-1RA levels in brain tissue lysate were assayed in high sensitivity ELISA kits (Quantikine ELISA, R&D Systems) according to the manufacturer’s instructions. Samples were added at 100 μL per plate and incubated for 2 h at room temperature. After washing with a wash buffer, 200 μL of human IL-1RA conjugates was added to each sample and incubated for 2 h. After reaction, the wavelength was read at 450 nm by using a microplate reader.
Assessment of proinflammatory cytokine expression (reverse transcriptase polymerase chain reaction)
Total RNA was prepared from the cortex of the ipsilateral hemisphere by using an RNeasy Mini Kit (Qiagen, Austin, Texas, USA) according to the manufacturer’s directions. Briefly, recommended amounts of tissue (25 mg) were placed in the appropriate lysis buffer supplied with the kit (add 10 μL of β-mercaptoethanol to 1 mL of lysis buffer). At this point, the manufacturer’s protocol was followed. The RNA was eluted with 30–50 μL of RNase-free H2O. The samples were immediately aliquoted and stored at −80°C. RNA was quantified by measuring A260 absorbance in NanoDrop ND-1000 (Thermo Scientific). Purity was assessed by calculating the A260/A280 ratio. cDNA was synthesized with 1 μg of total RNA from each sample by using a Maxime Reverse Transcriptase (RT) PreMix kit (OligodT Primer, iNtRON Biotechnology, Inc., Seongnam, Korea). The diluted cDNA was amplified with SYBR Green Polymerase Chain Reaction (PCR) Master Mix (Applied Bio-systems, Foster City, CA, USA) in a final volume of 50 μL. The PCR was performed by using an ABI prism 7500 sequence detector (Applied Biosystems). The PCR program consisted of initial denaturation at 95°C for 10 s and 40 cycles at 95°C for 5 s, 60°C for 34 s, 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. The primers for b-actin (forward, 5′-GGCATCCTGACCCTGA-AGTA-3′ and reverse, 5′-GGGGTGTTGAAGGTCTCAAA-3′), IL-1β (forward, 5′-CACCTCTCAAGCAGAGCACAG-3′ and reverse, 5′-GGGTTCCATGGTGAAGTCAAC-3′), TNF-α (forward, 5′-CAGGAGAAAGTCAGCCTCCT-3′ and reverse, 5′-TCATA-CCAGGGCTTGAGCTCA-3′), and IL-6 (forward, 5′-CGAAAGT-CAACTCCATCTGCC-3′ and reverse, 5′-GGCAACTGGCTG-GAAGTCTCT-3′) were purchased from Bioneer Inc (Seongnam, Korea). The cycling threshold (Ct) values of IL-1β, IL-6, and TNF-α were normalized to Ct values of b-actin. All the samples were run in triplicate, and the animals that underwent sham surgery without MCAO served as controls.
Immunohistochemistry
Brain tissue samples were obtained at 6 h after MCAO and fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS). After fixation, the isolated brain tissues were soaked in 30% sucrose at 4°C overnight. For cryosection, the samples were embedded in optimum cutting temperature compound (Tissue-Tek, Sakura, Japan) and frozen at −80°C. The brain tissues were sectioned coronally at a 20-μm thickness by using a cryotome (Leica Instruments GmbH, Nussloch, Germany) and placed on Muto silane-coated microscope slides (Muto Pure Chemicals, Tokyo, Japan). For immunohistochemistry of NeuN, Iba-1, IL-1β, and IL-1ra, the slides were defrosted and immersed in 0.3% Triton X-100 for 1 h for antigen retrieval and washed with PBS 3 times for 5 min. Then, the samples were incubated with EtOH at −20°C for 10 min and dried for 30 sec. Bovine serum albumin (BSA) solution (5%) was used for the blocking process. Tissue sections were incubated overnight at 4°C with primary antibodies, diluted in 2% BSA with PBS such as monoclonal mouse anti-NeuN (1:50; Millipore, Bedford, MA, USA), monoclonal mouse anti-Iba-1 (1:50; Abcam, Cambridge, UK), polyclonal rabbit anti-IL-1β (1:50, Abcam), and polyclonal goat anti-IL-1ra (R&D Systems). The next day, fluorescein isothiocyanate-conjugated anti-mouse IgG (1:5,000; Millipore), rhodamine-conjugated anti-goat IgG (1:5,000; Millipore), rhodamine-conjugated anti-rabbit IgG antibodies (1: 5,000; Millipore) were incubated at room temperature for 1 h as secondary antibodies. The slides were washed with PBS. 4′6-Diamidino-2-phenylindole was used for counterstaining, and slides were covered with VECTASHIELD mounting medium (Vecta Laboratories, CA, USA). The tissue sections were observed under an LSM700 confocal laser scanning microscope (Carl Zeiss, Thornwood, NY, USA).
Statistical analysis
All data were presented as mean (standard error of the mean [SEM]). The 2 groups were compared by performing an independent 2-tailed t test. One-way analysis of variance with Bonferroni post hoc tests was used for comparisons between the 3 groups. Serial data from neurobehavioral tests were compared by using repeated-measures analysis of variance with Bonferroni correction. Statistical analyses were performed with IBM SPSS Statistics 20 (IBM Software Group, Chicago, IL, USA). A p-value of <0.05 was considered statistically significant.
RESULTS
Neurobehavioral function and infarct size
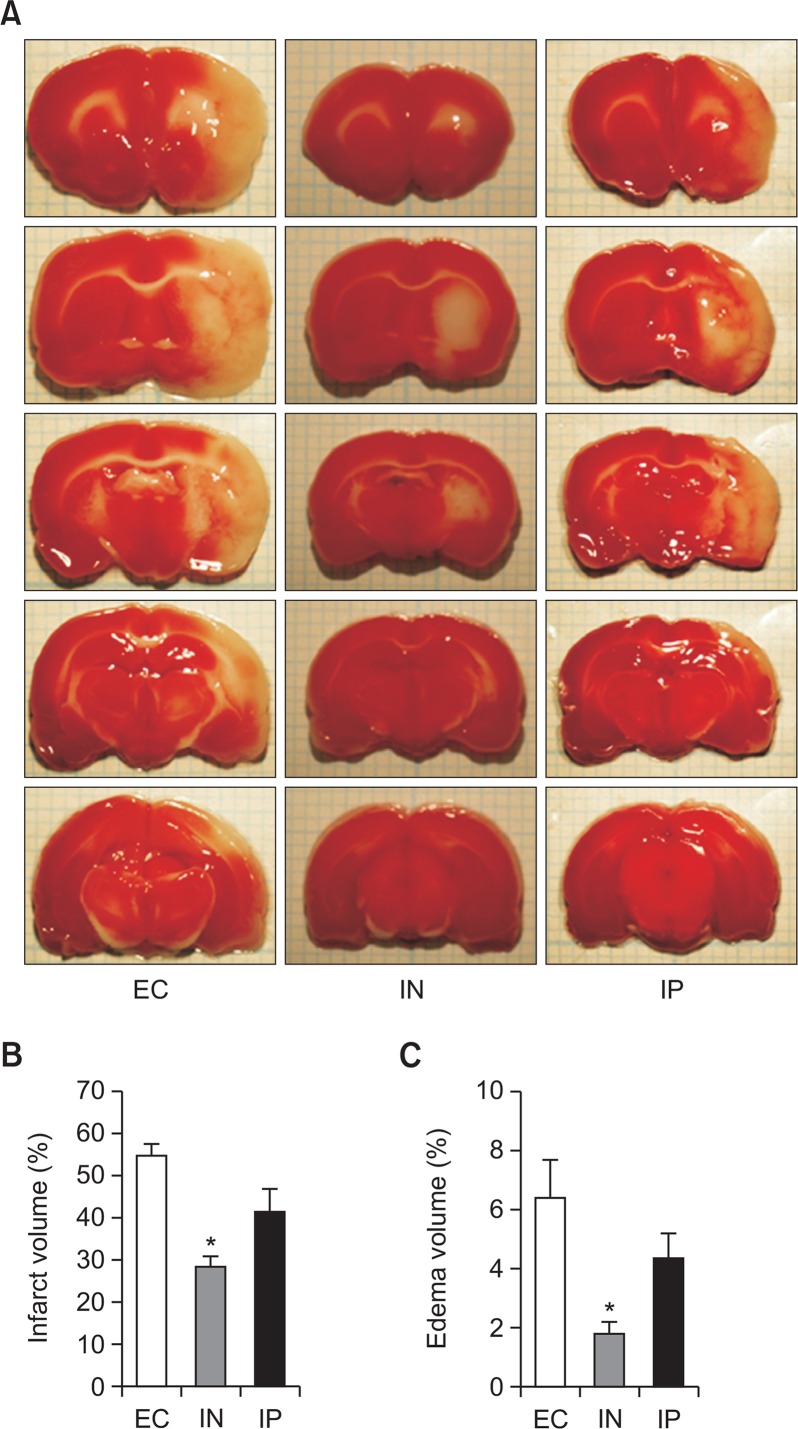
In order to evaluate the effects of intranasal IL-1RA on the neurological outcome of transient focal cerebral ischemia, neurobehavioral function, infarct, and edema volume were assessed. Two rats in the EC group and one in the IP group died before the completion of the assessments. The comparisons for infarct and edema size were performed by using one-way analysis of variance with the Bonferroni post hoc test (Fig. 1). On the seventh day after MCAO, the IN group had significantly lesser infarct volume than the EC group (p=0.001). However, the difference in infarct volume between the IN and IP groups did not reach statistical significance (p=0.087), and neither did the difference between the EC and IP groups (p=0.099; Fig. 1B). In addition, a similar pattern was observed in the difference in edema volume between the 3 groups (p=0.002 for the IN group vs. the EC group; p=0.154 for the IN group vs. the IP group; and p=0.382 for the IP group vs. EC group) as shown in Fig. 1C.
Fig. 1.
Neuroprotective effects of intranasal IL-1RA against transient cerebral ischemia. Brain damage is assessed by using TTC staining 7 days after the ischemic insult (cerebral ischemia for 1 h). The EC, IN, and IP groups received vehicle, intranasal IL-RA, and intraperitoneal IL-1RA, respectively. Representative TTC staining images of brain sections for each group (A). Quantitative comparisons of infarct volume (B) and edema volume (C) (n=5 in the EC group, n=7 in the IN group, and n=6 in the IP group). Values are shown as mean (SEM). *p<0.005, versus the EC group by oneway analysis of variance with Bonferroni post hoc test.
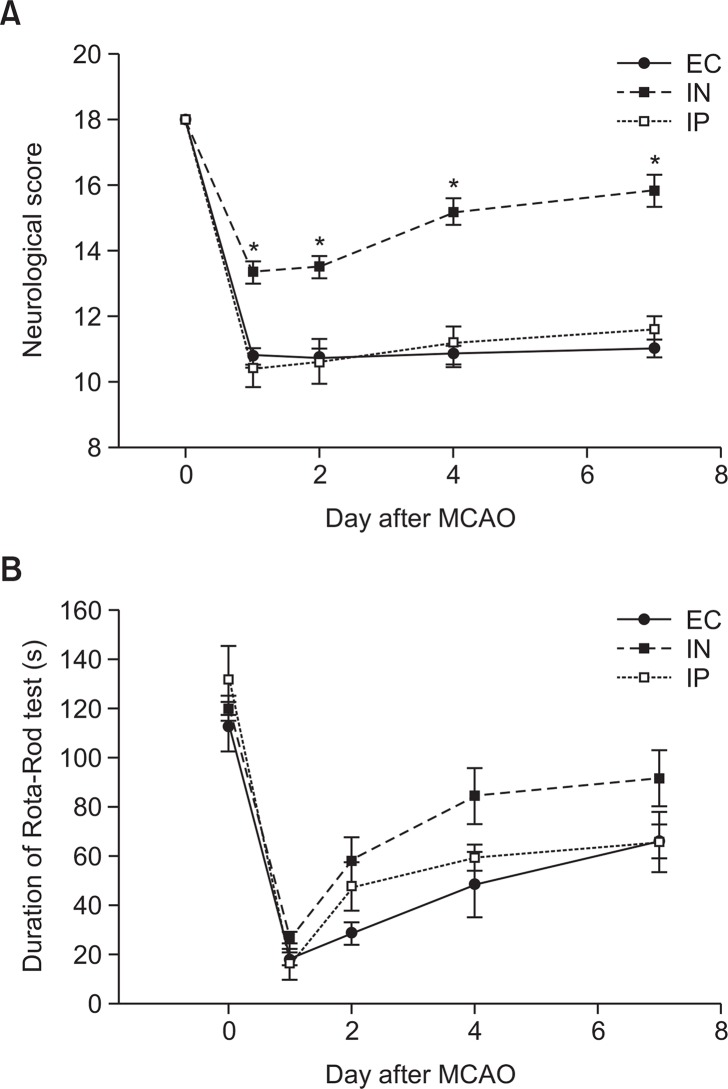
The neurological score before MCAO was 18 in all the experimental rats because no neurological deficit was present. By the seventh day after MCAO, the IN group showed significantly greater neurological scores than the EC and IP groups (p<0.001 by repeated-measures analysis of variance). No significant difference in neurological score was observed between the IP and EC groups during the period (Fig. 2A). In the rota-rod test, no statistically significant difference in the change in the exercise duration after MCAO was observed between the 3 groups (p=0.064 by repeated-measures analysis of variance). However, the mean exercise durations in the IN group on days 4 and 7 after MCAO were greater than those in other groups, although the differences were not statistically significant (Fig. 2B).
Fig. 2.
Neurobehavioral function evaluation after transient cerebral ischemia and IL-1RA administration. Neurobehavioral function is assessed by using an 18-point neurological scoring system (A) and a rota-rod test (B) (n=5 in EC group, n=7 in IN group, and n=6 in the IP group). The IN group maintained significantly greater neurological scores than the EC and IP groups by 7 days after MCAO (p<0.001 by repeated-measures analysis of variance). In the rota-rod test, the difference in the change in exercise duration after MCAO between the 3 groups did not have any statistical significance. Values are shown as mean (SEM). *p<0.01, versus the EC and IP groups at each time point by one-way analysis of variance with Bonferroni post hoc test.
Administered human IL-1RA concentration in brain parenchyma and serum
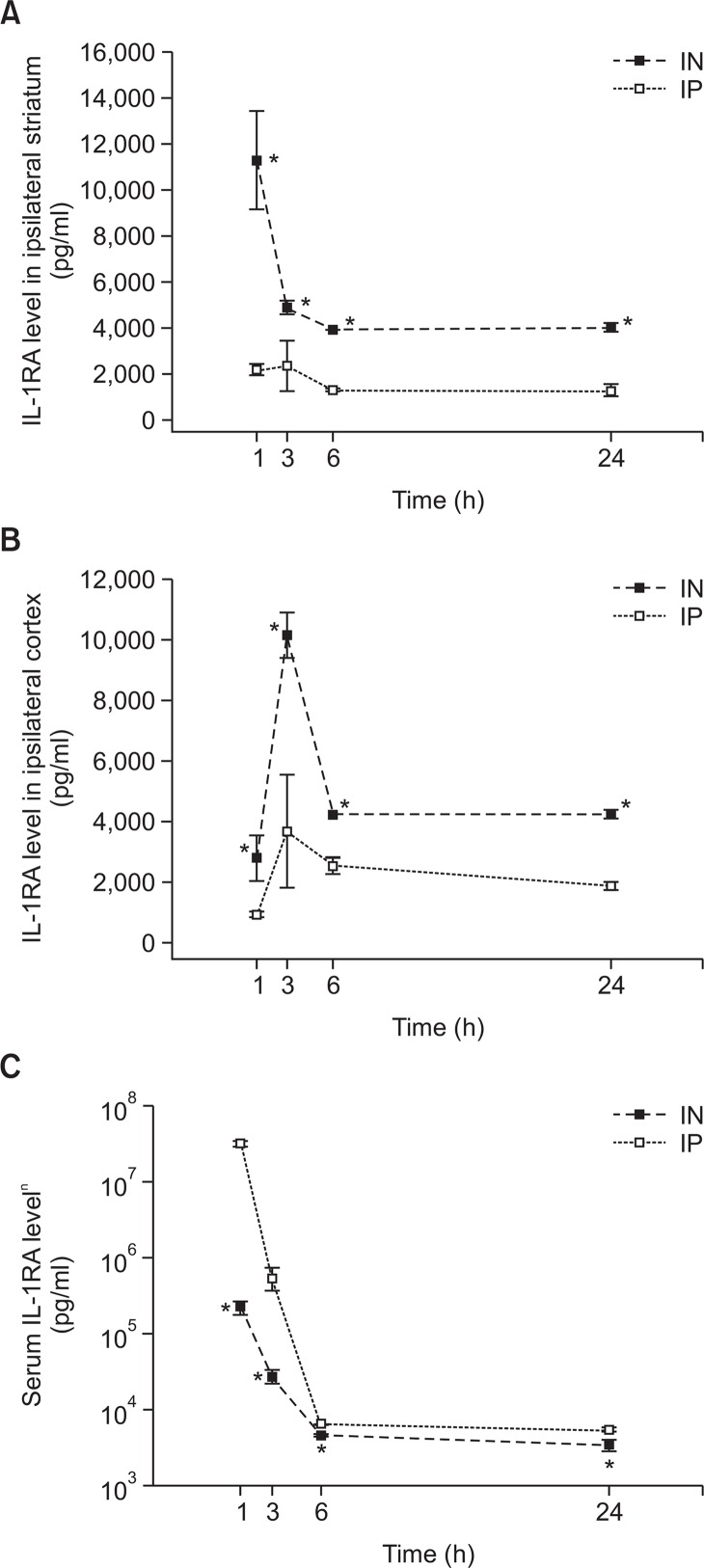
Human IL-1RA concentrations in brain parenchyma, including the cortex and striatum, and serum were measured at 1, 3, 6, and 24 h after MCAO. Fig. 3 shows the human IL-1RA concentrations in serum and brain tissue. After intranasal administration, human IL-1RA already reached significant concentrations in both of the striatum and cortex at the first sampling time (1 h after MCAO; Fig. 3A, 3B). In the striatum, the IL-1RA concentration at 1 h after MCAO was highest (11,265 ± 2,105 pg/mL) among the measurement time points. On the other hand, the IL-1RA concentration in the cortex was the highest at 3 h after MCAO (10,130 ± 750 pg/mL). After intraperitoneal administration, the IL-1RA concentration was highest at 3 h after MCAO in both the striatum (2,369 ± 1,097 pg/mL) and cortex (3,682 ± 1,863 pg/mL). In the all measurement time points, the IL-1RA concentrations were significantly greater in the IN group than in the IP group (by independent 2-tailed t test).
Fig. 3.
Level of human IL-1RA in the ipsilateral hemisphere (striatum [A] and cortex [B]) and the serum (C) from the rats with transient cerebral ischemia after intranasal (■) or intraperitoneal (□) administration of human IL-1RA (50 mg/kg). Human IL-1RA was not detected in the EC group over all the measurement time points. Values are shown as mean concentration (SEM). In each group, 5 rats were assessed at each measurement time point. *p<0.05, versus the IP group at each time point by independent 2-tailed t test.
Fig. 3C depicts the serum levels of human IL-1RA. The IL-1RA levels at 1 h after MCAO were highest in both groups (IN group: 222,274 ± 47,209 pg/mL [222 ng/mL]; IP group: 31,661,574 ± 2,403,415 pg/mL [31,662 ng/mL]). At all the measurement time points, the serum concentrations of IL-1RA were significantly higher in the IP group than in the IN group (by independent 2-tailed t test). Especially the IP group had more than 142 times greater IL-1RA serum concentration than the IN group at 1 h after MCAO.
According to the manufacturer, the anti-human IL-1RA anti-body used in the ELISA in this study shows no cross-reactivity with rat IL-1RA. We also confirmed that IL-1RA was not detected in the EC group over all the measurement time points.
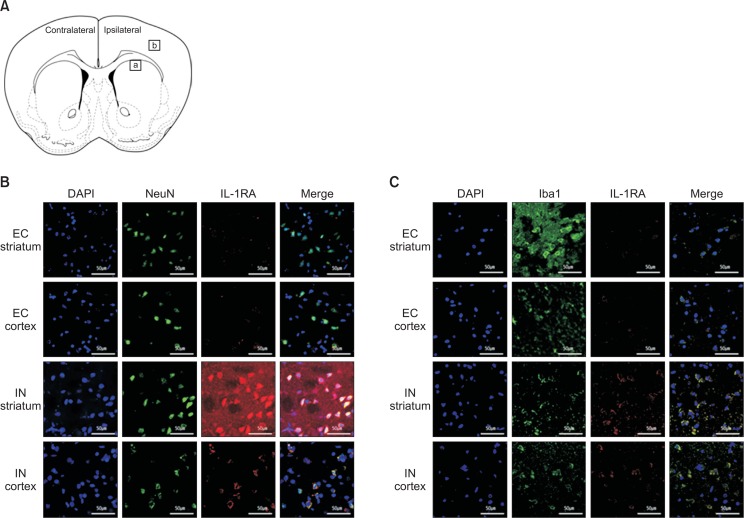
Distribution of the administered human IL-1RA and IL-1β
Cellular distribution of intranasal IL-1RA was assessed by using immunohistochemistry 6 h after MCAO. Fig. 4 shows the immunoreactivity of IL-1RA, which coincides with the NeuN (Fig. 4B) and Iba1 staining (Fig. 4C) in the ipsilateral striatum and cortex. The EC group rarely had IL-1RA staining in both the striatum and cortex. On the other hand, the IN group demonstrated strong immunoreactivity of IL-RA, which was largely colocalized with NeuN (Fig. 4A) and Iba-1 (Fig. 4B). According to the manufacturer of the anti-human IL-1RA antibody used in the immunohistochemistry, cross-reactivity with rat IL-1RA has not been reported yet (only less than 15% cross-reactivity with mouse was described). However, minimal immunoreactivity was observed in the EC group.
Fig. 4.
Distribution of human IL-1RA in the striatum and cortex at 6 h after intranasal administration. A coronal section of the rat brain (A). The square areas represent the location where the following immunostaining images were taken (a, striatum and b, cortex). After intranasal administration, human IL-RA was detected with strong immunoreactivity and largely co-localized with NeuN (B) and Iba-1 (C) in the striatum and cortex of the rats in the IN group. On the other hand, the immunoreactivity in the EC group is minimal.
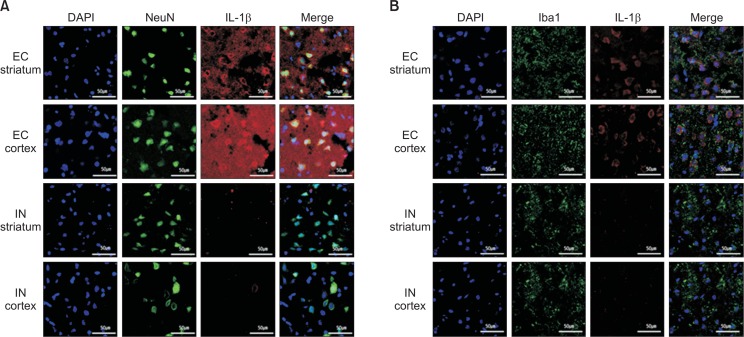
Fig. 5 exhibits the IL-1β expression 6 h after MCAO in the cortex and striatum. The EC group had greater IL-1β immuno-reactivity, which was colocalized with NeuN (Fig. 5A) and Iba1 (Fig. 5B) when compared with the IN group, which imposed that intranasal IL-1RA suppressed the IL-1β expression.
Fig. 5.
The effect of intranasal IL-1RA administration on IL-1β expression. In the EC group, IL-1β is detected with strong immunoreactivity and largely co-localized with NeuN (A) and Iba-1(B) in the striatum and cortex. However, the IL-1β expression level is remarkably reduced at 6 h after intranasal administration of IL-1RA in the IN group.
Inflammatory cytokine expression after intranasal administration of IL-1RA
The effects of intranasal IL-1RA on the mRNA levels of IL-1β and TNF-α after transient focal cerebral ischemia were evaluated by using semiquantitative RT-PCR. The values were expressed as a ratio in comparison with the sham control. As shown in Fig. 6, the IN group had significantly lower mRNA levels of IL-1β and TNF-α than the EC group at 6 h after MCAO (by independent 2-tailed t test).
Fig. 6.
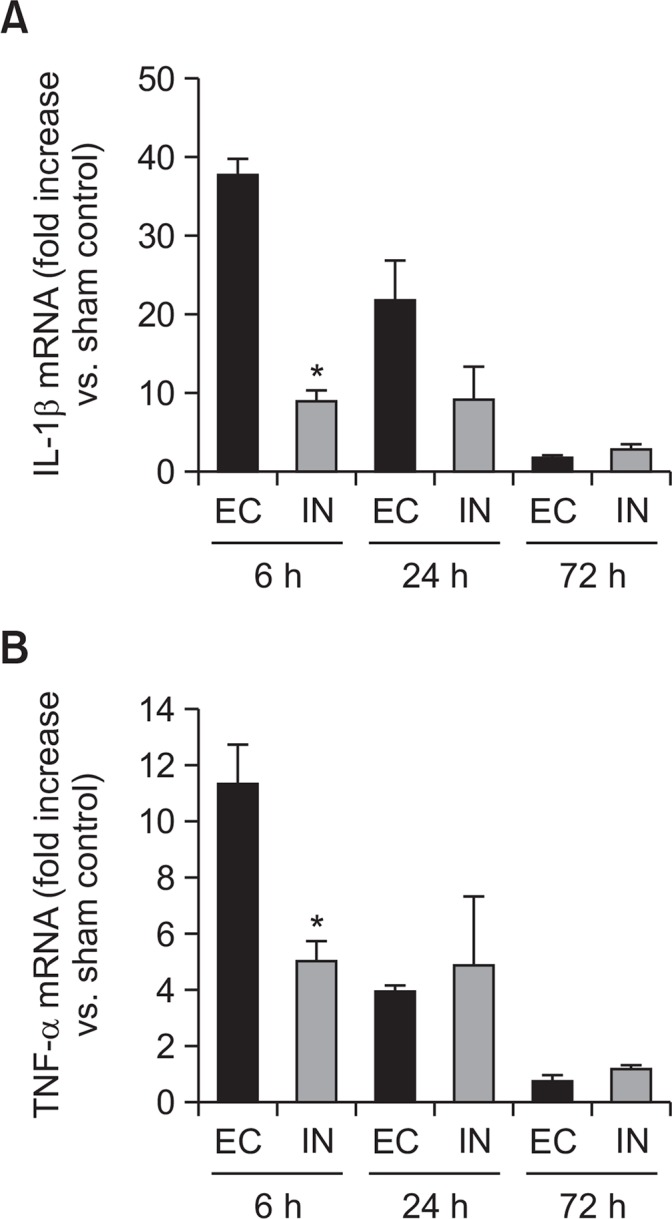
Time course of inflammatory cytokine expressions in the ischemic hemisphere after transient cerebral ischemia. Quantitative mRNA expressions of IL-1β and TNF-α according to intranasal IL-1RA administration. (A) IL-1β and (B) TNF-α expressions. Intranasal administration of IL-1RA more significantly reduced the expression of IL-1β and TNF-α mRNA at 6 h after administration than the EC group. In each group, 4 rats were assessed at each measurement time point. Values are expressed as ratios versus the sham controls without MCAO (means [SEM]). *p<0.05, versus the EC group by independent 2-tailed t test.
DISCUSSION
The present study demonstrated that intranasal delivery of IL-1RA significantly reduced the size of infarct lesion and alleviated neurological impairment by 7 days after transient focal cerebral ischemia. However, systemic administration of IL-1RA neither significantly decreased the infarct size nor improved neurological impairment. The administered human IL-1RA concentrations of brain tissue were significantly higher in the intranasal than in the systemic administration at all measurement points for 24 h after cerebral ischemia. On the other hand, in the serum, the administered IL-1RA was detected significantly less in the intranasal than in the systemic administration at every measurement time point. Finally, our results showed that the intranasally administered IL-1RA was deposited in neurons and activated microglia, and suppressed inflammatory cytokines after cerebral ischemia. Therefore, the superior neuroprotective efficacy of intranasal administration to systemic administration may have resulted from the greater delivery of IL-1RA into brain tissues, which can suppress neuroinflammation after transient focal cerebral ischemia.
Previous experimental studies already demonstrated the efficacy of IL-1RA in cerebral ischemia model 2 decades ago (Martin et al., 1994; Garcia et al., 1995a; Relton et al., 1996). Furthermore, in a recent investigation, IL-1RA administration was reported to reduce neuroinflammation and ischemic brain injury in aged and obese rats (Pradillo et al., 2012), elevating IL-1RA closer to the standards for preclinical neuroprotective and restorative drug development (Fisher et al., 2009). In addition, systemic administration of IL-1RA at a clinically safe dose to patients with subarachnoid hemorrhage (SAH) maintained the IL-1RA concentration in the cerebrospinal fluid (CSF) at which IL-1RA was reported to provide neuroprotection against transient cerebral ischemia in rats (Clark et al., 2008). In spite of these experimental backgrounds, the phase II clinical trial of IL-1RA in acute stroke patients failed to prove efficacy (Emsley et al., 2005). According to pharmacokinetic data of IL-1RA in rats, the CSF concentration of IL-1RA was only 2–5.1% of the plasma concentration after transient cerebral ischemia (Clark et al., 2008; Greenhalgh et al., 2010). The human data of the patients with SAH demonstrated that the CSF concentration of IL-1RA was also only 4% of that in plasma at best (Gueorguieva et al., 2008). Furthermore, the penetration of IL-1RA into brain tissue only appeared in the lesion with BBB breakdown when the rats received systemic administration of IL-1RA, and the CSF concentration of IL-1RA may not reflect the level of brain parenchyma (Greenhalgh et al., 2010). Therefore, one of the possible causes of failure in the clinical trial of IL-1RA may be the insufficient delivery of IL-1RA to the brain tissue (Banwell et al., 2009). Thus, the efficiency of the delivery should be improved and the delivery to the brain parenchyma, not in the CSF, should be assessed. This investigation assessed and compared the delivery of IL-1RA to the brain according to the administration pathway by measuring the IL-1RA concentrations in the brain tissue.
Until recently, the intranasal administration of various therapeutic proteins has been investigated not only in stroke models (Fletcher et al., 2009; Sun et al., 2016) but also in other neurological disease models (Malerba et al., 2011). Drugs that are administered through the nasal route may be absorbed into the systemic circulation across the nasal membrane or transported directly to the brain tissue along the olfactory and trigeminal nerves (Dhuria et al., 2010). Although it is possible that after intranasal administration, the drug is absorbed into the systemic circulation and subsequently transported into the brain across the BBB, large and hydrophilic therapeutics such as IL-1RA cannot easily enter the blood circulation, as revealed by our results. When the same IL-1RA dose was given through the intraperitoneal or intranasal route, the peak serum concentration of IL-1RA in the IN group was only about 0.7% of that in the IP group, although the IL-1RA level in the brain tissue was significantly higher in the IN group than in the IP group. Direct delivery to the brain after intranasal administration is supposed to involve 2 mechanisms (Dhuria et al., 2010). One is an extracellular pathway in which the drug is rapidly transported to the olfactory bulb or brain stem along the olfactory or trigeminal nerve through intercellular clefts. The other is by endocytosis of the drug into olfactory sensory neurons and intracellular transport along axons to the olfactory bulb, which is known to be a much slower process. Our results show that IL-1RA was already detected in the cortex and striatum 1 h after intranasal administration and that the IL-1RA level reached the peak at about 1 h after intranasal administration in the striatum and 3 h after intranasal administration in the cortex. A previous report indicated that a protein administered intranasally might take more than 8 h to reach brain tissue through endocytosis and axonal transport (Furrer et al., 2009). Therefore, our results imposed that after intranasal administration, IL-1RA might be transported to the brain tissue largely by the rapid pathway through intercellular clefts. In addition, the time to the peak IL-1RA level after intranasal administration was faster in the striatum than in the cortex, which is probably because IL-1RA is distributed from the olfactory bulb and brain stem to the whole brain and that the striatum is more adjacent to the olfactory bulb and brain stem than the cortex. On the other hand, the peak IL-1RA level in the brain tissue of the IP group was only 21–36% of that of the IN group, which may be because the penetration of the IL-1RA into brain tissue when administered systemically occurred only in the area with damaged BBB. Finally, the neuroprotective efficacy against transient focal cerebral ischemia in the IN group was superior to that in the IP group, which may be because of the difference in the delivery of IL-1RA to brain tissue. In a few previous studies, IL-1RA was reported to provide significant neuroprotection even when systemically administered at a lower dose than that administered in the present study (Pradillo et al., 2012; Xia et al., 2014). However, IL-1RA was administered 1.5–12 h after cerebral ischemia induction in these studies, compared to immediately after ischemia induction in the present study. Because IL-1RA has a short half-life in plasma (Kim et al., 1995), the timing of administration may have affected the outcome. Furthermore, they assessed infarct size and neurological function only at 24 h after cerebral ischemia. Because the inflammatory process after cerebral ischemia evolves for many days (Iadecola and Anrather, 2011), the assessment at 24 h after injury may be insufficient; thus, neurological outcomes were assessed for 7 days in this study.
As a response to ischemic brain injury, cytokines are secreted from immune cells to trigger an inflammatory response. The cytokines that are produced in the early phase after cerebral ischemia are reported to be IL-1β and TNF-α (Lambertsen et al., 2012). Our results demonstrated that IL-1RA administration may reduce the expression of IL-1β and TNF-α in the early phase, which might affect overall inflammatory cascade after cerebral ischemic injury. In addition, IL-1β is known to be implicated in neuroinflammation and worsen cerebral ischemic injury by evoking excitotoxicity in neurons and increasing the production of reactive oxygen and proteases in glial cells (Galea and Brough, 2013). Therefore, in order to reduce the possibility of an inflammatory response, the IL-1 activity in neurons and microglia should be blocked. In our results, the administered IL-1RA was co-localized with neurons and activated microglia; thus, IL-1RA administration might block the effect of IL-1β on neurons and microglias.
There is a concern that successful application of intranasal administration in rodents may not be reproduced in humans (Grassin Delyle et al., 2012), which can be the limitation of this study. The anatomy of the nasal cavity of rodents quite differs from that of humans; the proportion of the olfactory epithelium in the nasal cavity is much lower in humans than in rodents (3% vs. 50%) (Malerba et al., 2011). The olfactory epithelium is innervated by the olfactory nerve and thus is important in direct transport to the brain through the extracellular pathway. However, direct evidence exists regarding the transport from the nose to the brain in human studies (Illum, 2004). Furthermore, various modifications can be made to improve transport to the brain, such as changes in drug formulation and permeation enhancers (Illum, 2004; Malerba et al., 2011). Therefore, intranasal administration of IL-1RA can be a robust candidate method for ischemic stroke management.
In conclusion, intranasal administration of IL-1RA was a more efficient route to reach brain parenchyma and showed superior efficacy in neuroprotection against transient cerebral ischemia in the rats when compared with systemic administration. Therefore, intranasal delivery of IL-1RA should be investigated further as a potential therapeutic option for ischemic stroke.
Acknowledgments
This study was financially supported by the “Dongwha Holdings” Faculty Research Assistance Program of Yonsei University College of Medicine for 2013 (No. 6-2013-0067), the Basic Science Research Program through the National Research Foundation of Korea (NRF) that was funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1002001) to J.H.L. and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2014R1A2A2A01007289) to K.B.-N.
REFERENCES
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis. 2009;18:269–276. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.STR.27.9.1616. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Merino JG, Warach S. Update on stroke. Curr Opin Neurol. 2004;17:447–451. doi: 10.1097/01.wco.0000137536.06986.f9. [DOI] [PubMed] [Google Scholar]
- Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, Tyrrell PJ, Hopkins SJ, Rothwell NJ. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab. 2008;28:387–394. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatr. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher L, Kohli S, Sprague SM, Scranton RA, Lipton SA, Parra A, Jimenez DF, Digicaylioglu M. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111:164–170. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- Furrer E, Hulmann V, Urech DM. Intranasal delivery of ESBA105, a TNF-alpha-inhibitory scFv antibody fragment to the brain. J Neuroimmunol. 2009;215:65–72. doi: 10.1016/j.jneuroim.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Galea J, Brough D. The role of inflammation and interleukin-1 in acute cerebrovascular disease. J Inflamm Res. 2013;6:121–128. doi: 10.2147/JIR.S35629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Relton JK. Interleukin-1 receptor antagonist decreases the number of necrotic neurons in rats with middle cerebral artery occlusion. Am J Pathol. 1995a;147:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995b;26:627–634. doi: 10.1161/01.STR.26.4.627. [DOI] [PubMed] [Google Scholar]
- Grassin Delyle S, Buenestado A, Naline E, Faisy C, BlouquitLaye S, Couderc L, Le Guen M, Fischler M, Devillier P. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol Ther. 2012;134:366–379. doi: 10.1016/j.pharmthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Greenhalgh AD, Galea J, Dénes A, Tyrrell PJ, Rothwell NJ. Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br J Pharmacol. 2010;160:153–159. doi: 10.1111/j.1476-5381.2010.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueorguieva I, Clark SR, McMahon CJ, Scarth S, Rothwell NJ, Tyrrell PJ, Hopkins SJ, Rowland M. Pharmacokinetic modelling of interleukin-1 receptor antagonist in plasma and cerebrospinal fluid of patients following subarachnoid haemorrhage. Br J Clin Pharmacol. 2008;65:317–325. doi: 10.1111/j.1365-2125.2007.03026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J Neuroimmunol. 1994;55:153–160. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, Parsons AA. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–816. doi: 10.1016/S0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56:3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- Kim DC, Reitz B, Carmichael DF, Bloedow DC. Kidney as a major clearance organ for recombinant human inter-leukin-1 receptor antagonist. J Pharm Sci. 1995;84:575–580. doi: 10.1002/jps.2600840511. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Malerba F, Paoletti F, Capsoni S, Cattaneo A. Intranasal delivery of therapeutic proteins for neurological diseases. Expert Opin Drug Deliv. 2011;8:1277–1296. doi: 10.1517/17425247.2011.588204. [DOI] [PubMed] [Google Scholar]
- Martin D, Chinookoswong N, Miller G. The interleukin-1 receptor antagonist (rhIL-1ra) protects against cerebral infarction in a rat model of hypoxia-ischemia. Exp Neurol. 1994;130:362–367. doi: 10.1006/exnr.1994.1215. [DOI] [PubMed] [Google Scholar]
- Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol. 2003;140:471–476. doi: 10.1038/sj.bjp.0705462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradillo JM, Denes A, Greenhalgh AD, Boutin H, Drake C, McColl BW, Barton E, Proctor SD, Russell JC, Rothwell NJ, Allan SM. Delayed administration of interleukin-1 receptor antagonist reduces ischemic brain damage and inflammation in comorbid rats. J Cereb Blood Flow Metab. 2012;32:1810–1819. doi: 10.1038/jcbfm.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton JK, Martin D, Thompson RC, Russell DA. Peripheral administration of Interleukin-1 Receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol. 1996;138:206–213. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans. 2007;35:1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- Sun BL, He MQ, Han XY, Sun JY, Yang MF, Yuan H, Fan CD, Zhang S, Mao LL, Li DW, Zhang ZY, Zheng CB, Yang XY, Li YV, Stetler RA, Chen J, Zhang F. Intranasal delivery of granulocyte colony-stimulating factor enhances its neuroprotective effects against ischemic brain injury in rats. Mol Neurobiol. 2016;53:320–330. doi: 10.1007/s12035-014-8984-2. [DOI] [PubMed] [Google Scholar]
- Xia Y, Song S, Min Y, Zhong Y, Sheng Y, Li R, Liu Q. The effects of anakinra on focal cerebral ischemic injury in rats. CNS Neurosci Ther. 2014;20:879–881. doi: 10.1111/cns.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, Zhang S. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39:2362–2369. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]