Abstract
Methylglyoxal (MGO) is a highly reactive metabolite of glucose which is known to cause damage and induce apoptosis in endothelial cells. Endothelial cell damage is implicated in the progression of diabetes-associated complications and atherosclerosis. Hypericin, a naphthodianthrone isolated from Hypericum perforatum L. (St. John’s Wort), is a potent and selective inhibitor of protein kinase C and is reported to reduce neuropathic pain. In this work, we investigated the protective effect of hypericin on MGO-induced apoptosis in human umbilical vein endothelial cells (HUVECs). Hypericin showed significant anti-apoptotic activity in MGO-treated HUVECs. Pretreatment with hypericin significantly inhibited MGO-induced changes in cell morphology, cell death, and production of intracellular reactive oxygen species. Hypericin prevented MGO-induced apoptosis in HUVECs by increasing Bcl-2 expression and decreasing Bax expression. MGO was found to activate mitogen-activated protein kinases (MAPKs). Pretreatment with hypericin strongly inhibited the activation of MAPKs, including P38, JNK, and ERK1/2. Interestingly, hypericin also inhibited the formation of AGEs. These findings suggest that hypericin may be an effective regulator of MGO-induced apoptosis. In conclusion, hypericin downregulated the formation of AGEs and ameliorated MGO-induced dysfunction in human endothelial cells.
Keywords: Advanced glycation end products, Methylglyoxal, HUVECs, Hypericin, Apoptosis
INTRODUCTION
Hypericin, a powerful naturally occurring photosensitizer, is produced by Hypericum species. Hypericin, the most important active component in Hypericum perforatum, is used in the treatment of depression (Caccia, 2005). Hypericin has also recently been reported to have anti-inflammatory, antimicrobial, and selective anti-tumor photosensitizing effects (Kang et al., 2001; Ritz et al., 2007; Yow et al., 2012). In addition, hypericin suppresses phosphorylation of several important signaling molecules, such as extracellular signal-regulated kinases (ERK), nuclear factor kappa B (NF-κB), and protein kinase C (PKC), in various cell lines (Galeotti et al., 2010; Jain Swati et al., 2010; Ouyang et al., 2014).
Methylglyoxal (MGO), a highly reactive dicarbonyl compound, is an intermediate product formed during glycation of proteins by glucose and its formation involves many pathways consisting of enzymatic and non-enzymatic reactions (Yim et al., 1995; Desai et al., 2010). The main source of MGO is endogenous metabolites, but increasing evidence reveals that MGO can also be generated by exogenous sources such as foods, beverages, and cigarette smoke (Biswas et al., 2002; Degen et al., 2012). MGO can react very rapidly with proteins, resulting in the formation of a group of chemically stable substances known as advanced glycation end-products (AGEs) (Thornalley, 2005). There are reports demonstrating that increased levels of methylglyoxal and AGEs contribute to inflammation and oxidative stress that damage various organs (Rabbani and Thornalley, 2011; Sena et al., 2012; Hanssen et al., 2014). Several studies reported significantly increased levels of MGO in the blood in diabetic patients (Lapolla et al., 2003; Scheijen and Schalkwijk, 2014). Recent reports have also shown that MGO is a more potent stimulator of vascular injury than AGEs or glucose itself, which suggests that an increased level of serum MGO-derived AGEs is involved in the exacerbation of diabetic vascular complications (Bourajjaj et al., 2003; Yamawaki et al., 2008).
We performed in vitro cell screening to determine the protective effects of several compounds from a library of natural compounds against MGO-induced damage. Hypericin was the most effective anti-MGO compound screened, so we investigated its role in MGO-related glucotoxicity. The aim of the present study was to determine the protective effects of hypericin against MGO-induced cell death in HUVECs and to assess whether hypericin inhibits the formation of AGEs; our results demonstrate that hypericin can regulate apoptosis-related signaling pathways, including those involving oxidative stress and MAPKs.
MATERIALS AND METHODS
Materials
MGO and 2′,7′-dichlorofluorescein diacetate (DCF-DA) were obtained from Sigma (St. Louis, MO, USA). Hypericin was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Endothelial growth medium (EGM-2) was purchased from Lonza (Walkersville, MD, USA). p38, phospho-p38, ERK1/2, phospho-ERK1/2, JNK, and phospho-JNK were purchased from Cell Signaling Technology (Danvers, MA, USA). Bcl-2, Bax and p53 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
The human umbilical vein endothelial cell line (HUVECs, Lot # 60319874) was purchased from the American Type Culture Collection (ATCC, VA, USA). HUVECs were cultured under standard cell culture conditions (37°C in a humidified incubator containing 5% CO2) in EGM-2 supplemented with 4% FBS. The passage number of all the cells used was between 5 and 8.
Cell viability analysis and morphological examination
Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. HUVECs were seeded in 96-well plates at 1.0×104 cells/well and incubated for 24 h at 37°C. The cells were then pretreated with hypericin for 1 h, followed by treatment with MGO for 24 h. Morphological changes in the HUVECs were observed with an IncuCyte ZOOM imaging system (Essen Bioscience, MI, USA) 24 h after incubation with MGO. MTT solution was added at a final concentration of 0.1 mg/ml. This was followed by a 2 h incubation in the CO2 incubator at 37°C. The medium was gently removed and the formazan crystals were dissolved in 100 μl/well dimethyl sulfoxide. The absorbance at 570 nm was measured using a microplate reader (Molecular Devices, CA, USA).
Detection of intracellular ROS
DCF-DA probe was used to measure the intracellular ROS scavenging activity of hypericin. Briefly, 2.0×105 cells were seeded in a 12-well plate and incubated overnight at 37°C. After 24 h, cells were pre-incubated with hypericin for 30 min, followed by incubation with MGO for 1 h. The medium was removed and the cells were washed with PBS. Then, medium containing 10 μM DCF-DA was added for 20 min at 37°C. After washing with PBS, cells were photographed using a JuLI live-cell imaging system (NanoEnTek, Seoul, Korea). The fluorescence intensity was assessed using the computer software program Image J software (NIH, Bethesda, MD, USA) not by human eyes, to avoid potential subjective errors.
Western blotting
Changes in the levels of proteins related to MAPKs and apoptosis in HUVECs were evaluated by Western blotting. HUVECs were lysed in PRO-PREPTM (iNtRON Biotechnology, Seongnam, Korea) containing phosphatase inhibitor. Total protein concentration was determined using the Bradford assay. Samples containing equal amounts of proteins were separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Blots were blocked in 5% skim milk for 1 h at room temperature and exposed to the primary antibodies overnight at 4°C. They were then incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Densities of the resulting bands were detected with ECL reagents using a ChemiDoc XRS+imaging system (Bio-Rad, CA, USA).
Inhibition of AGEs formation
The AGEs formation assay was used to investigate inhibition of protein glycation; the protocol was slightly modified from that published by Kiho et al. (2005). BSA (5 mg/mL) was incubated with MGO (2 mM) in PBS buffer, pH 7.4, in the presence or absence of hypericin (1 and 10 μM). Sodium azide (0.02%) was added to the reaction mixture and the reaction was allowed to proceed for 7 days. The formation of AGEs was determined using fluorescence at an excitation/emission wavelength of 355/460 nm with a VICTOR™ X3 multilabel plate reader (Perkin Elmer, Waltham, MA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Values are given as mean ± S.D. Statistical analysis of results was performed using one-way ANOVA followed by Bonferroni’s test. A p-value<0.05 was considered statistically significant.
RESULTS
Effects of hypericin on MGO-induced apoptosis and ROS production
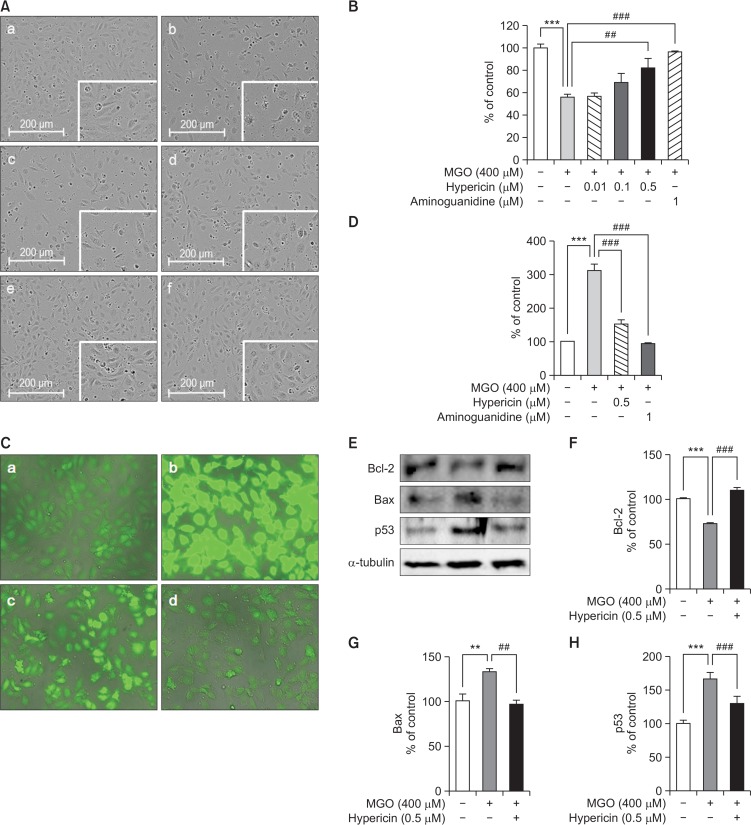
MGO-induced cell morphological changes in HUVECs after treatment with hypericin were observed. As shown in Fig. 1A, the percentage of apototic cells was increased (43%) in HUVEC incubated for 24 h in the EGM-2 media containing 400 μM MGO. In addition to decrease in cell number, typical morphological features such as shrinkage, fragmentation and rounding of the cell were observed at this time. But, treatment with hypericin rescued the morphological changes such as a loss of confluence and decreased the number of floating cell fragments induced by MGO. To determine the effect of hypericin on MGO-induced cytotoxicity in HUVECs, we performed an MTT assay. HUVEC cell viability was significantly reduced after MGO treatment, but hypericin treatment restored cell viability (Fig. 1B). Moreover, pretreatment with 0.5 μM hypericin increased cell viability.
Fig. 1.
The effect of hypericin on MGO-induced cytotoxicity and oxidative stress in HUVECs. (A) The representative photographs of MGO-treated HUVECs without (−) or with (+) hypericin. (a) control; (b) 400 μM MGO; (c) MGO+0.1 μM hypericin; (d) MGO+0.5 μM hypericin; (e) MGO+1 μM hypericin; (f) MGO+aminoguanidine. (B) The cell viability of HUVECs treated with MGO and hypericin. Cell viability was analyzed by the MTT assay. The percentage of cell viability is presented as mean ± SD of three independent experiments. (C) The protective effect of hypericin on MGO-induced ROS generation. HUVECs were pretreated with hypericin for 1 h and then treated with 400 μM MGO for 2 h. ROS generation was detected by staining with the fluorescent dye DCF-DA. (a) control; (b) 400 μM MGO; (c) MGO+hypericin; (d) MGO+aminoguanidine. (D) Fluorescent intensity was measured using Image J software. (E) The effect of hypericin on Bcl-2, Bax and p53 protein expression in MGO-treated HUVECs as assessed by Western blot. Cells were incubated without (−) or with (+) hypericin for 1 h and then treated with 400 μM MGO for 24 h. Tubulin was used as an internal control. (F) The relative band intensity of Bcl-2. (G) The relative band intensity of Bax. (H) The relative band intensity of p53. Bar values are presented as the mean ± SD of three independent experiments (**p<0.01 vs. control, ***p<0.001 vs. control, ##p<0.01 and ###p<0.001 vs. 400 μM MGO treatment only).
It is known that MGO can increase intracellular ROS levels and may induce cell death. Therefore, we measured ROS formation in HUVECs following treatment with MGO. We also estimated the inhibitory effect of hypericin on ROS formation in HUVECs following treatment with MGO. We measured the antioxidative effect of hypericin using DCF-DA. As shown in Fig. 1C, we found that pretreatment with hypericin significantly reduced ROS generation in MGO treated HUVECs.
Next, we investigated whether MGO can affect expression of apoptosis related proteins such as. Bcl-2, Bax and p53 in HUVECs. As shown in Fig. 1D, treatment with MGO decreased Bcl-2 protein expression, but enhanced protein expressions of Bax and p53 in HUVECs. Also, treatment with hypericin has downregulated the expression of Bax and p53 and upregulated the expression of Bcl-2. These data suggest that hypericin may prevent MGO-induced apoptosis in HUVECs.
Effects of hypericin on MAPK activation
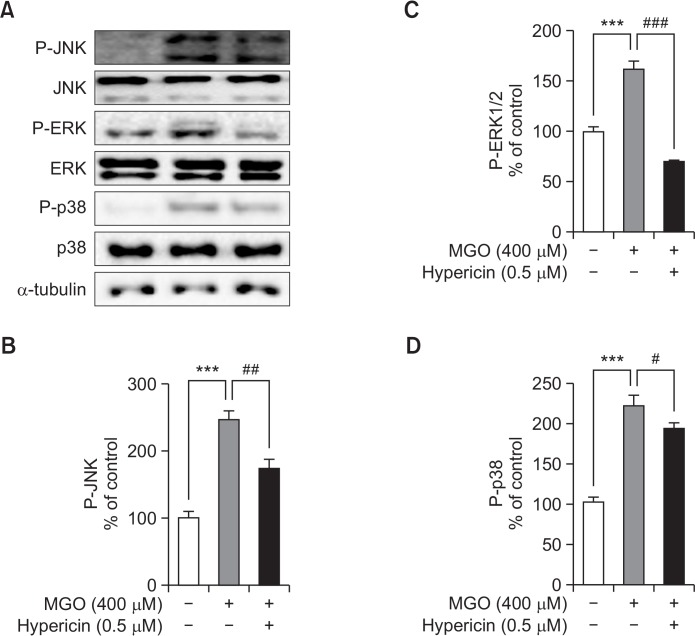
Activation of MAPK plays an important role in MGO-induced apoptosis in various types of cells. In this study, we investigated three proteins in MAPK subfamilies, p38, ERK1/2, and JNK, in MGO-treated HUVECs, and evaluated the effect of hypericin on activation of MAPK cascades induced by MGO. As shown in Fig. 2, there was a significant increase in p38, ERK1/2, and JNK activation in MGO-treated cells, whereas pretreatment with hypericin prior to MGO stimulation reduced the activation of all three MAPKs.
Fig. 2.
The effects of hypericin on MAPK signaling in HUVECs. Levels of the total and phosphorylated forms of MAPKs were detected by Western blot. Cells were incubated without (−) or with (+) hypericin for 1 h and then treated with 400 μM MGO for 1 h. (A) Representative Western blots of MAPKs. (B) Relative band intensity of p-JNK. (C) Relative band intensity of p-ERK. (D) Relative band intensity of p-p38. Bar values are presented as the mean ± SD of three independent experiments (***p<0.001 vs. control, #p<0.05, ##p<0.01 and ###p<0.001 vs. 400 μM MGO treatment only).
Inhibitory effects of hypericin on the formation of AGEs
The AGEs formation assay was used to measure fluorescence at an excitation wavelength of 355 nm and an emission wavelength of 460 nm; aminoguanidine was used as a positive control. As shown in Table 1, we found that formation of AGEs was significantly increased in cells treated with BSA-MGO. Hypericin (10 μM) significantly attenuated the formation of AGEs. However, 1 μM hypericin did not produce a significant difference in AGEs formation.
Table 1.
Inhibitory effect of hypericin on AGE formation
| Sample | AGEs Formation (%) |
|---|---|
| Control | 12.24 ± 0.14 |
| MGO (400 μM) | 100.00 ± 0.89*** |
| Hypericin (1 μM) | 102.67 ± 0.11 |
| Hypericin (10 μM) | 80.06 ± 0.64### |
| Aminoguanidine (1 mM) | 28.82 ± 0.21### |
The percent of AGEs formation is presented as the mean ± SD of three independent experiments.
p<0.001 vs. control and
p< 0.001 vs. 400 μM MGO treatment only.
DISCUSSION
MGO is one of the most significant reactive carbonyl species formed by the triose phosphate glycolytic intermediates of glucose metabolism (Lo et al., 2006). Elevated MGO levels have been reported in vascular endothelial cells cultured in high-glucose media (Mukohda et al., 2012). MGO is related to diabetic vascular complications, and it triggers cellular injury and apoptosis in endothelial cells (Figarola et al., 2014). Recently, several natural compounds found in plants have been reported to be protective against MGO-induced cytotoxicity (Hu et al., 2013; Zhu et al., 2014). We screened a library of naturally occurring compounds for those that potentially ameliorated MGO-induced toxicity in HUVECs. In this study, we propose that hypericin, the most active ingredient in Hypericum perforatum, protects against MGO-induced apoptosis and oxidative damage. To evaluate the antiapoptotic effect of hypericin, we pretreated HUVECs with different concentrations (0.1–1 μM) of hypericin for 1 h followed by 400 μM MGO treatment for 24 h. We found that 0.5 μM hypericin protected against MGO-induced cell toxicity (Fig. 1B). Treatment with high concentrations of hypericin (more than 1 μM) had no effect on MGO-induced cell death (data not shown).
It is well known that intracellular ROS is generated in vascular endothelial cells in response to MGO treatment (Figarola et al., 2014). Antioxidant activity of Hypericum perforatum L. and its components have been reported (Gioti et al., 2009). Furthermore, Cakir et al. reported that hypericin has an anti-oxidative effect (Cakir et al., 2003). Therefore, we investigated whether hypericin ameliorates MGO-induced ROS generation. First, we found that ROS levels were significantly increased by treatment with 400 μM MGO. Also, MGO-induced ROS generation was significantly reduced by pretreatment with hypericin (Fig. 1C). It has been reported that the antioxidant activity of phenolic compounds is mainly governed by number of total hydroxyl groups (Weng and Wang, 2000). Since hypericin contains many hydroxyl groups, we expected hypericin to have antioxidant activity (Cakir et al., 2003). Namely, hypericin may be one of the antioxidants from Hypericum perforatum L.
Alteration of the ratio of the expression of one or more members of the Bcl-2 protein family is significant in determining whether apoptosis occurs, because Bcl-2 family member proteins play critical roles in regulating the process of apoptosis (Murphy et al., 2000). p53 protein can regulate the function of mitochondria and oxidative stress (Lin and Beal, 2006). Also, p53 protein can directly activate Bax protein by translocating to mitochondria (Chipuk et al., 2004). In this study, exposure of HUVECs to 400 μM MGO decreased Bcl-2 and increased Bax and p53 expression in MGO-treated HUVECs. However, pretreatment with hypericin attenuated the increase in Bax and p53 proteins expression, and increased Bcl-2 protein expression (Fig. 1D). It is interesting that they found that potential effect of hypericin in HUVECs was strongly associated with positive regulation for p53 activation, suggesting a correlation with oxidative stress and apoptosis.
In endothelial cells, MGO may induce cytotoxicity via activation of several key molecules including ERK, JNK, and p38, which are involved in the MAPK signaling pathway (Akhand et al., 2001). MAPKs play a major role in the process of apoptosis and phosphorylate and regulate the activity of the Bcl-2 family in response to a variety of stresses (Liu et al., 2012). We confirmed that treatment with MGO activated phosphorylation of MAPKs and observed that hypericin downregulated activation of MAPKs in 400 μM MGO-treated HUVECs. These results show that hypericin modulates the MAPK signaling pathway, and may thus protect MGO-treated HUVECs from apoptosis.
To investigate the protective effect of hypericin against MGO-induced apoptosis, we performed annexin V-FITC/PI assay using flow cytometry. However, PI baseline was shifted to the necrosis zone, since hypericin is a red-colored naphthodianthrone derivative. So we could not get the reliable annexin V-FITC/PI assay data. However, the proteins expressions of Bcl-2, Bax, p53 and MAPKs by treatment of MGO were changed. Furthermore, many previously studies reported that MGO-mediated diabetic vascular complications might be due to apoptosis in endothelial cells (Phalitakul et al., 2013; Figarola et al., 2014; Lv et al., 2014). This indicates that MGO-induced cytotoxicity is prevented by incubation with hypericin in HUVECs, which seems to act by regulating cell apoptotic proteins.
MGO, a dicarbonyl compound, is more reactive than are reducing sugars such as glucose. This highly reactive compound is a major precursor in the formation of AGEs (Desai and Wu, 2007). One of the major AGEs adducts, Nε-(carboxymethyl) lysine, is formed from MGO (Thornalley, 1996). In endothelial cells, increased levels of AGEs cause oxidative stress, mitochondrial dysfunction, cellular dysfunction, and, ultimately, cell death (Li et al., 2011). That hypericin inhibits the formation of AGEs suggests that it can ameliorate AGEs-related endothelial cell dysfunction. Table 1 shows the percent inhibition of AGEs formation brought about by treatment with hypericin. After treatment with 10 μM hypericin and MGO with BSA, the formation of AGEs was decreased by 20% on 7 day. However, the potency of hypericin was less than that of aminoguanidine, which was used as a positive control. Hypericin is a red-colored anthraquinone-derived naphthodianthrone. AGEs formation is inhibited by phenolic acids carrying multiple hydroxyl groups (Xie and Chen, 2013). Phenolic compounds, such as caffeic acid and chlorogenic acid, exhibit a significant inhibitory effect on AGEs formation (Gugliucci et al., 2009). Likewise, anthraquinones with ortho or meta dihydroxyl groups seem to inhibit the formation of AGEs and aldose reductase (Yoo et al., 2010). Furthermore Luc Sero et al. reported that Hypericum perforatum L. decreased the AGEs formation (Sero et al., 2013). Therefore, we suggest that hypericin may be an active component from Hypericum perforatum L. for decrease of AGE formation. Given all of these observations, hypericin, a red-colored naphthodianthrone, may directly scavenge AGEs.
In conclusion, our results support the notion that hypericin protects against MGO-induced apoptosis in cultured HUVECs via scavenging of ROS and reducing the activation of cell apoptosis via the MAPK pathway. Therefore, our results suggest that hypericin may be a useful tool to prevent or reverse MGO-induced vascular damage.
Acknowledgments
This research was supported by iPET (Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries), Ministry of Agriculture, Food and Rural Affairs (NO. 115045-3).
This research was supported by High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (114006041HD020).
REFERENCES
- Akhand AA, Hossain K, Mitsui H, Kato M, Miyata T, Inagi R, Du J, Takeda K, Kawamoto Y, Suzuki H, Kurokawa K, Nakashima I. Glyoxal and methylglyoxal trigger distinct signals for map family kinases and caspase activation in human endothelial cells. Free Radic Biol Med. 2001;31:20–30. doi: 10.1016/S0891-5849(01)00550-0. [DOI] [PubMed] [Google Scholar]
- Biswas S, Gairola CG, Das SK. Passive cigarette smoke and the renal glyoxalase system. Mol Cell Biochem. 2002;229:153–156. doi: 10.1023/A:1017992403656. [DOI] [PubMed] [Google Scholar]
- Bourajjaj M, Stehouwer CD, van Hinsbergh VW, Schalkwijk CG. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem Soc Trans. 2003;31:1400–1402. doi: 10.1042/bst0311400. [DOI] [PubMed] [Google Scholar]
- Caccia S. Antidepressant-like components of Hypericum perforatum extracts: an overview of their pharmacokinetics and metabolism. Curr Drug Metab. 2005;6:531–543. doi: 10.2174/138920005774832641. [DOI] [PubMed] [Google Scholar]
- Cakir A, Mavi A, Yildirim A, Duru ME, Harmandar M, Kazaz C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L. by activity-guided fractionation. J Ethnopharmacol. 2003;87:73–83. doi: 10.1016/S0378-8741(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Degen J, Hellwig M, Henle T. 1,2-dicarbonyl compounds in commonly consumed foods. J Agric Food Chem. 2012;60:7071–7079. doi: 10.1021/jf301306g. [DOI] [PubMed] [Google Scholar]
- Desai K, Wu L. Methylglyoxal and advanced glycation endproducts: new therapeutic horizons? Recent Pat Cardiovasc Drug Discov. 2007;2:89–99. doi: 10.2174/157489007780832498. [DOI] [PubMed] [Google Scholar]
- Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J, Untereiner A, Wu L. Oxidative stress and aging: is methylglyoxal the hidden enemy? Can J Physiol Pharmacol. 2010;88:273–284. doi: 10.1139/Y10-001. [DOI] [PubMed] [Google Scholar]
- Figarola JL, Singhal J, Rahbar S, Awasthi S, Singhal SS. LR-90 prevents methylglyoxal-induced oxidative stress and apoptosis in human endothelial cells. Apoptosis. 2014;19:776–788. doi: 10.1007/s10495-014-0974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N, Vivoli E, Bilia AR, Vincieri FF, Ghelardini C. St. John’s Wort reduces neuropathic pain through a hypericin-mediated inhibition of the protein kinase Cgamma and epsilon activity. Biochem Pharmacol. 2010;79:1327–1336. doi: 10.1016/j.bcp.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Gioti EM, Fiamegos YC, Skalkos DC, Stalikas CD. Antioxidant activity and bioactive components of the aerial parts of Hypericum perforatum L. from Epirus, Greece. Food Chem. 2009;117:398–404. doi: 10.1016/j.foodchem.2009.04.016. [DOI] [Google Scholar]
- Gugliucci A, Bastos DH, Schulze J, Souza MF. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia. 2009;80:339–344. doi: 10.1016/j.fitote.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Hanssen NM, Wouters K, Huijberts MS, Gijbels MJ, Sluimer JC, Scheijen JL, Heeneman S, Biessen EA, Daemen MJ, Brownlee M, de Kleijn DP, Stehouwer CD, Pasterkamp G, Schalkwijk CG. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype. Eur Heart J. 2014;35:1137–1146. doi: 10.1093/eurheartj/eht402. [DOI] [PubMed] [Google Scholar]
- Hu T-Y, Liu C-L, Chen J-Y, Hu M-L. Curcumin ameliorates methylglyoxal-induced alterations of cellular morphology and hyperpermeability in human umbilical vein endothelial cells. J. Funct. Foods. 2013;5:745–754. doi: 10.1016/j.jff.2013.01.020. [DOI] [Google Scholar]
- Jain SS, Murch SJ, Bird RP, Saxena PK. Optimized St. John’s Wort (Hypericum perforatum L.) Germplasm Lines Exert Cytotoxicity in HT-29 Colon Cancer Cells via Downregulation of NF-κB. J Complementary Integr Med. 2010;7 doi: 10.2202/1553-3840.1283. [DOI] [Google Scholar]
- Kang BY, Chung SW, Kim TS. Inhibition of interleukin-12 production in lipopolysaccharide-activated mouse macrophages by hpyericin, an active component of Hypericum perforatum. Planta Med. 2001;67:364–366. doi: 10.1055/s-2001-14333. [DOI] [PubMed] [Google Scholar]
- Kiho T, Kato M, Usui S, Hirano K. Effect of buformin and metformin on formation of advanced glycation end products by methylglyoxal. Clin. Chim. Acta. 2005;358:139–145. doi: 10.1016/j.cccn.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Lapolla A, Flamini R, Dalla Vedova A, Senesi A, Reitano R, Fedele D, Basso E, Seraglia R, Traldi P. Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin Chem Lab Med. 2003;41:1166–1173. doi: 10.1515/CCLM.2003.180. [DOI] [PubMed] [Google Scholar]
- Li BY, Li XL, Cai Q, Gao HQ, Cheng M, Zhang JH, Wang JF, Yu F, Zhou RH. Induction of lactadherin mediates the apoptosis of endothelial cells in response to advanced glycation end products and protective effects of grape seed procyanidin B2 and resveratrol. Apoptosis. 2011;16:732–745. doi: 10.1007/s10495-011-0602-4. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang Y, Ye YC, Shi QF, Chai K, Tashiro S, Onodera S, Ikejima T. Activation of ERK-p53 and ERK-mediated phosphorylation of Bcl-2 are involved in autophagic cell death induced by the c-Met inhibitor SU11274 in human lung cancer A549 cells. J Pharmacol Sci. 2012;118:423–432. doi: 10.1254/jphs.11181FP. [DOI] [PubMed] [Google Scholar]
- Lo CY, Li S, Tan D, Pan MH, Sang S, Ho CT. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol Nutr Food Res. 2006;50:1118–1128. doi: 10.1002/mnfr.200600094. [DOI] [PubMed] [Google Scholar]
- Lv Q, Gu C, Chen C. Venlafaxine protects methylglyoxal-induced apoptosis in the cultured human brain microvascular endothelial cells. Neurosci Lett. 2014;569:99–103. doi: 10.1016/j.neulet.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Mukohda M, Okada M, Hara Y, Yamawaki H. Exploring mechanisms of diabetes-related macrovascular complications: role of methylglyoxal, a metabolite of glucose on regulation of vascular contractility. J Pharmacol Sci. 2012;118:303–310. doi: 10.1254/jphs.11R12CP. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000;7:102–111. doi: 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Zhai Z, Li H, Liu X, Qu X, Li X, Fan Q, Tang T, Qin A, Dai K. Hypericin suppresses osteoclast formation and wear particle-induced osteolysis via modulating ERK signalling pathway. Biochem Pharmacol. 2014;90:276–287. doi: 10.1016/j.bcp.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Phalitakul S, Okada M, Hara Y, Yamawaki H. Vaspin prevents methylglyoxal-induced apoptosis in human vascular endothelial cells by inhibiting reactive oxygen species generation. Acta Physiol. (Oxf.) 2013;209:212–219. doi: 10.1111/apha.12139. [DOI] [PubMed] [Google Scholar]
- Rabbani N, Thornalley PJ. Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol. 2011;22:309–317. doi: 10.1016/j.semcdb.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Ritz R, Wein HT, Dietz K, Schenk M, Roser F, Tatagiba M, Strauss WS. Photodynamic therapy of malignant glioma with hypericin: comprehensive in vitro study in human glioblastoma cell lines. Int J Oncol. 2007;30:659–667. [PubMed] [Google Scholar]
- Scheijen JL, Schalkwijk CG. Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: evaluation of blood specimen. Clin Chem Lab Med. 2014;52:85–91. doi: 10.1515/cclm-2012-0878. [DOI] [PubMed] [Google Scholar]
- Sena CM, Matafome P, Crisostomo J, Rodrigues L, Fernandes R, Pereira P, Seica RM. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res. 2012;65:497–506. doi: 10.1016/j.phrs.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Sero L, Sanguinet L, Blanchard P, Dang BT, Morel S, Richomme P, Seraphin D, Derbre S. Tuning a 96-well microtiter plate fluorescence-based assay to identify AGE inhibitors in crude plant extracts. Molecules. 2013;18:14320–14339. doi: 10.3390/molecules181114320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification-A role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci. 2005;1043:111–117. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- Weng XC, Wang W. Antioxidant activity of compounds isolated from Salvia plebeia. Food Chem. 2000;71:489–493. doi: 10.1016/S0308-8146(00)00191-6. [DOI] [Google Scholar]
- Xie Y, Chen X. Structures required of polyphenols for inhibiting advanced glycation end products formation. Curr Drug Metab. 2013;14:414–431. doi: 10.2174/1389200211314040005. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Saito K, Okada M, Hara Y. Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. Am J Physiol, Cell Physiol. 2008;295:C1510–C1517. doi: 10.1152/ajpcell.00252.2008. [DOI] [PubMed] [Google Scholar]
- Yim HS, Kang SO, Hah YC, Chock PB, Yim MB. Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. J Biol Chem. 1995;270:28228–28233. doi: 10.1074/jbc.270.47.28228. [DOI] [PubMed] [Google Scholar]
- Yoo NH, Jang DS, Lee YM, Jeong IH, Cho JH, Kim JH, Kim JS. Anthraquinones from the roots of Knoxia valerianoides inhibit the formation of advanced glycation end products and rat lens aldose reductase in vitro. Arch Pharm Res. 2010;33:209–214. doi: 10.1007/s12272-010-0204-7. [DOI] [PubMed] [Google Scholar]
- Yow CM, Tang HM, Chu ES, Huang Z. Hypericin-mediated photodynamic antimicrobial effect on clinically isolated pathogens. Photochem Photobiol. 2012;88:626–632. doi: 10.1111/j.1751-1097.2012.01085.x. [DOI] [PubMed] [Google Scholar]
- Zhu D, Wang L, Zhou Q, Yan S, Li Z, Sheng J, Zhang W. (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Mol Nutr Food Res. 2014;58:2249–2260. doi: 10.1002/mnfr.201400533. [DOI] [PubMed] [Google Scholar]