Abstract
Cordyceps bassiana is one of Cordyceps species with anti-oxidative, anti-cancer, anti-inflammatory, anti-diabetic, anti-obesity, anti-angiogenic, and anti-nociceptive activities. This mushroom has recently demonstrated to have an ability to reduce 2,4-dinitrofluorobenzene-induced atopic dermatitis symptoms in NC/Nga mice. In this study, we further examined phytochemical properties of this mushroom by column chromatography and HPLC analysis. By chromatographic separation and spectroscopic analysis, 8 compounds, such as 1,9-dimethylguanine (1), adenosine (2), uridine (3), nicotinamide (4), 3-methyluracil (5), 1,7-dimethylxanthine (6), nudifloric acid (7), and mannitol (8) were identified from 6 different fractions and 4 more subfractions. Through evaluation of their anti-inflammatory activities using reporter gene assay and mRNA analysis, compound 1 was found to block luciferase activity induced by NF-κB and AP-1, suppress the mRNA levels of cyclooxygenase (COX)-2 and tumor necrosis factor (TNF)-α. Therefore, our data strongly suggests that compound 1 acts as one of major principles in Cordyceps bassiana with anti-inflammatory and anti-atopic dermatitis activities.
Keywords: Cordyceps bassiana; Phytochemical study; 1,9-dimethylguanine; NF-κB; AP-1; Anti-inflammatory activity
INTRODUCTION
Cordyceps species are insect-born mushrooms that have been known to possess a variety of biological material. As such, the Cordyceps species has been used for a long time in Chinese and Korean traditional folk medicine as a tonic for longevity, endurance and vitality, and it has been traditionally used as a therapeutic remedy for various diseases, including eczema, skin diseases, chronic bronchitis, asthma, and tuberculosis (Ng and Wang, 2005; Zhou et al., 2009). Cordyceps bassiana is known to be a teleomorph (sexually reproducing form) of Beauveria bassiana. B. bassiana is a ubiquitous fungal pathogen of insect and fungal biocontrol agents widely used against insects (Rehner et al., 2011) and widely used as a medicine for the treatment of infantile convulsions, epilepsy, stroke, sore throat, and many sorts of wounds before being used for insect biocontrol (Madsen et al., 2007). In contrast, C. bassiana was described in China on a carpenterworm larva and connected to unequivocally B. bassiana (Rehner et al., 2011). More recently, C. bassiana was found in Korea and the evidence of the teleomorph-anamorph connection in the Cordyceps specimen was provided, in addition to in vitro production of C. bassiana (Hyun et al., 2013).
By systematic analysis of the pharmacological activities of the Cordyceps species to date, anti-oxidative, anti-cancer, anti-inflammatory, anti-diabetic, anti-obesity, anti-angiogenic, and anti-nociceptive activities have been demonstrated (Ng and Wang, 2005; Zhou et al., 2009). Recently, extracts of the artificially cultivated C. bassiana have been reported to inhibit the production of nitric oxide, interleukin (IL)-12, and interferon (IFN)-γ in lipopolysaccharide (LPS)-stimulated macrophages, splenic lymphocytes (Byeon et al., 2011a, 2011b), as well as ameliorating atopic dermatitis symptoms in 2,4-dinitrofluorobenzene-treated NC/Nga mice (Wu et al., 2011). In spite of its valuable pharmacological activities, no reports on the chemical constituents of C. bassiana have been published to date. Simultaneously, we are trying to develop an anti-atopic dermatitis remedy with this species, and have also performed research to dissect the chemical constituents of C. bassiana in order to identify the active components. Herein, isolation, structure determination, and anti-inflammatory activities of the 8 major compounds from a water extract of C. bassiana fruit body displaying anti-atopic dermatitis activity are described.
MATERIALS AND METHODS
Materials
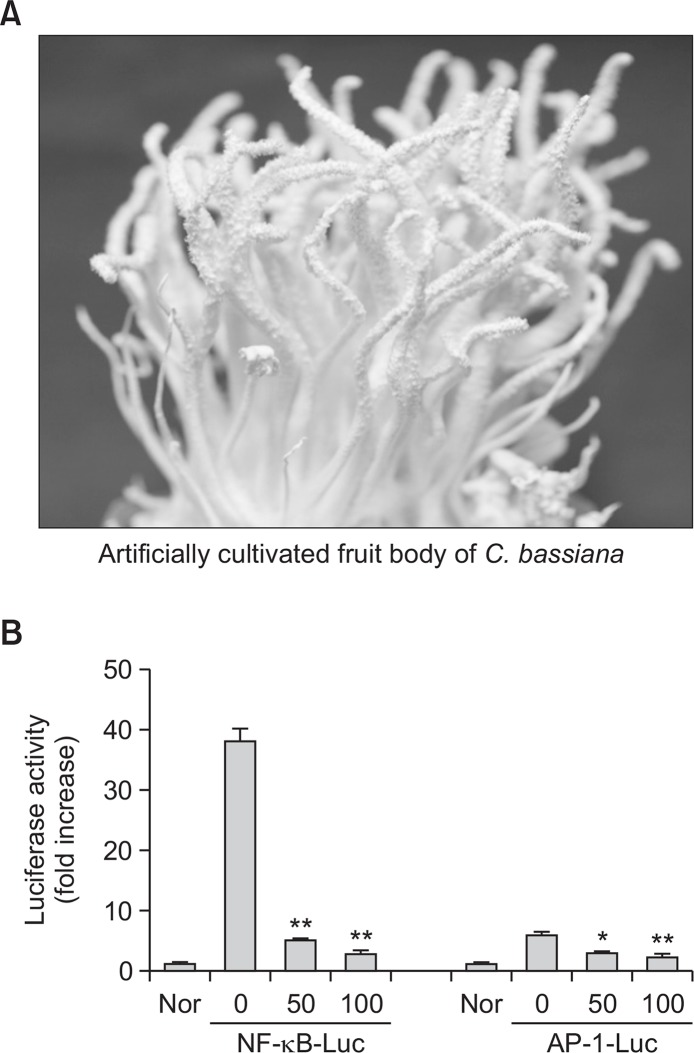
Artificially cultivated Cordyceps bassiana (C. bassiana) (Fig. 1A) were purchased from Mush-Tech (Gangwon, Korea), identified by Prof. Jae Mo Sung (Kangwon National University, Chuncheon, Korea). A voucher specimen (No. 1001) was deposited in the herbarium of Daewoong Pharmaceutical Co. Ltd. (Yongin, Korea). Lipopolysaccharide (LPS, E. coli 0111:B4) and (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Bay 11-7082 (BAY), SB203580 (SB), and U0126 (U0) were obtained from Calbiochem (La Jolla, CA, USA). Luciferase constructs containing binding promoters for NF-κB and AP-1 were gifts from Prof. Chung Hae Young (Pusan National University, Pusan, Korea) and Man Hee Rhee (Kyungpook National University, Daegu, Korea). Foetal bovine serum and RPMI 1640 were obtained from GIBCO BRL (Grand Island, NY, USA). RAW264.7 and HEK293 cells were purchased from ATCC (Rockville, MD, USA). All other chemicals were of Sigma grade. A powdered extract of C. bassiana was provided by Daewoong Pharmaceutical Co. Ltd (Yongin, Korea).
Fig. 1.
A photo of an artificially cultivated fruit body of Cordyceps bassiana and the inhibitory activity of its water extract on AP-1 and NF-κB-mediated luciferase. (A) A photo of an artificially cultivated fruit body of Cordyceps bassiana. (B) HEK293 cells co-transfected with plasmid constructs NF-κB-Luc or AP-1-Luc (each 1 μg/ml) and β-gal (as a transfection control) were treated with the water extract of Cordyceps bassiana in the presence or absence of PMA (100 nM). Luciferase activity was measured using a luminometer. *p<0.05 and **p<0.01 compared to control.
General experimental procedures
All NMR spectra were measured on an Agilent VNS-600 spectrometer or on a Bruker DPX 300 spectrometer operating at 600 or 300 MHz for 1H-NMR and 150 or 75 MHz for 13C-NMR in DMSO-d6 using TMS as an internal standard. EIMS was measured on a Hewlett Packard model 5989B GC/ MS spectrometer. HPLC (Agilent 1200 series system) composed of vacuum degasser, quaternary pump, diode array detector (DAD), manual injector, thermostatted column compartment using a Luna C18(2) 100A column (4.6×250 mm 5 μm, Phenomenex) was used for isolation and purification of compounds. Silica gels (70–230 mesh, Merck, Darmstadt, Germany), Sephadex LH-20 (GE Healthcare, Uppsala, Sweden) were used for open column chromatography. TLC was performed on silica gel 60 F254 (Merck).
Extraction and isolation
Powdered water extract of fruit bodies of C. bassiana (4.0 kg) was treated with MeOH (3 times, each with 10 liters) by using reflux apparatus for 3 h at 60°C that yielded a crude fraction (605 g) upon removal of the solvent in vacuo. The methanol fraction (165 g) was separated by silica gel column chromatography and eluted with CHCl3-MeOH mixtures of increasing polarity (100:1→20:1) to afford 10 fractions (Fr. 1~10). Fraction 4 was purified by Sephadex LH-20 column chromatography using MeOH-Water (9:1) to produce compound 5. Fraction 6 was separated on a Sephadex LH-20 column chromatography with MeOH-Water to generate 8 subfractions (Fr.6-1~Fr.6-2). Fraction 6-5 was separated by silica gel column chromatography and eluted with CHCl3-acetone mixtures to afford 18 subfractions (Fr.6-5-1~Fr.6-5-18). Subfraction 6-5-6 was re-crystallized from MeOH to afford compound 7. Fractions 8 and 9 were recrystallized from MeOH to afford compound 6 and compound 1. The remaining fraction 9 was separated using a Sephadex LH-20 column chromatography with MeOH-Water to generate 8 subfractions (Fr.9-1~Fr.9-8). Fraction 9–8 was recrystallized from MeOH to afford compound 2. Fraction 9-6 was further purified by semi-preparative reverse-phase HPLC on a Phenomenex Luna C18 column (250×10.0 mm, 5 μM, Phenomenex) and eluted with Water-MeOH (flow rate, 2.0 ml/ min; 5–20% MeOH 30 min; UV, 254 nm) to produce compound 3 (tR=12.8 min) and compound 4 (tR=18.3 min). Fraction 10 was recrystallized from MeOH to afford compound 8.
HPLC profiling of the extract
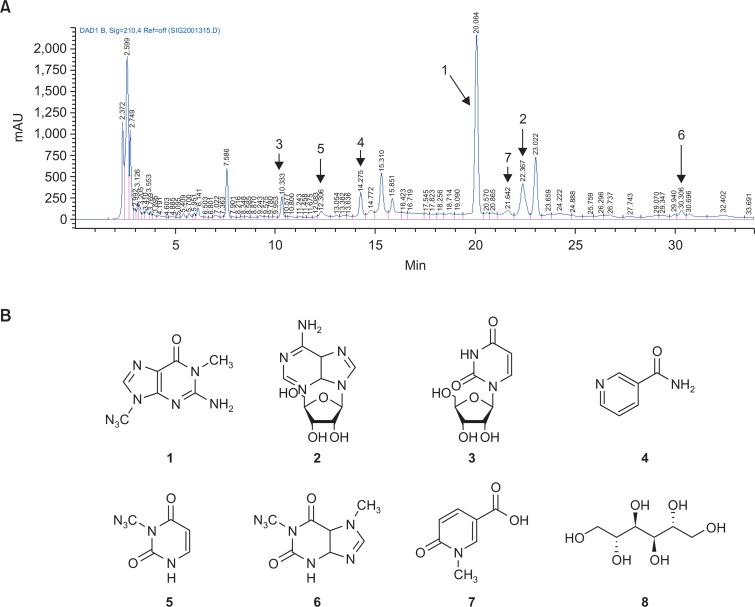
Metabolic profiling was performed on an Agilent 1200 serious HPLC coupled to an Agilent DAD detector. A Luna C18 column (250 mm×4.6 mm i.d., 5 μm; Phenomenex, Torrance, CA, USA) was used and the mobile phase consisted of water (A) and acetonitrile (B) at a flow rate of 0.9 ml/min, as reported previously (Vo et al., 2015). The gradient elution started at 20% B and increased linearly to 100% B in 30 min followed by an isocractic 100% for a further 15 min. The column temperature was set to 20°C. The detection wavelength was 210 nm. The retention times for each compound were 20.66 (1), 22.37 (2), 10.33 (3), 14.27 (4), 12.30 (5), 30.30 (6), and 21.64 (7) min.
Luciferase reporter gene activity assay
HEK293 cells (1×106 cells/ml) were transfected in 12-well plates with NF-κB-Luc or AP-1-Luc (each 1 μg/ml), as well as β-galactosidase (0.25 μg/ml), using the PEI method, as reported previously (Chen et al., 2014). After 24 h, the transfected cells were treated with DWJ503 in the presence or absence of PMA (100 nM) or TNF-α (10 ng/ml) for NF-κB-Luc and AP-1-Luc assays, and the cells were harvested and lysed to determine luciferase activity 1 day later. Luciferase assays were performed using the Luciferase Assay System (Promega), as reported previously (Yang et al., 2015). Luciferase activity was normalized to β-galactosidase activity.
mRNA analysis by semiquantitative reverse transcription-polymerase chain reaction
To determine cytokine mRNA expression levels, total RNA was isolated from LPS-treated RAW264.7 cells with TRIzol Reagent (GIBCO BRL), according to the manufacturer’s instructions (Moon et al., 2014). Total RNA was stored at −70°C until use. Semi-quantitative RT reactions were performed as reported previously (Lee et al., 2014). The results were expressed as the ratio of the optimal density to the density of GAPDH. The primers (Bioneer, Daejeon, Korea) used are listed in Table 1.
Table 1.
Primer sequences used in the semiquantitative RT-PCR analyses
| Gene | Primer sequences | |
|---|---|---|
| Semiquantitative PCR | ||
| TNF-α | F | 5′-TTGACCTCAGCGCTGAGTTG-3′ |
| R | 5′-CCTGTAGCCCACGTCGTAGC-3′ | |
| COX-2 | F | 5′-CACTACATCCTGACCCACTT-3′ |
| R | 5′-ATGCTCCTGCTTGAGTATGT-3′ | |
| GAPDH | F | 5′-CACTCACGGCAAATTCAACGGCAC-3′ |
| R | 5′-GACTCCACGACATACTCAGCAC-3′ | |
Statistical analysis
All data are presented as the mean ± SEM of 3 different experiments performed with 4 samples (Fig. 1B, 3A, 3B, 3D) for in vitro experiments. For statistical comparisons, these results were analyzed using ANOVA/Scheffe’s post hoc test and a Kruskal–Wallis/Mann–Whitney test. A p-value of <0.05 was considered statistically significant. All statistical tests were performed using SPSS (SPSS Inc., Chicago, IL, USA). Similar experimental data were also observed by an additional independent set of in vitro experiments performed with the same numbers of samples.
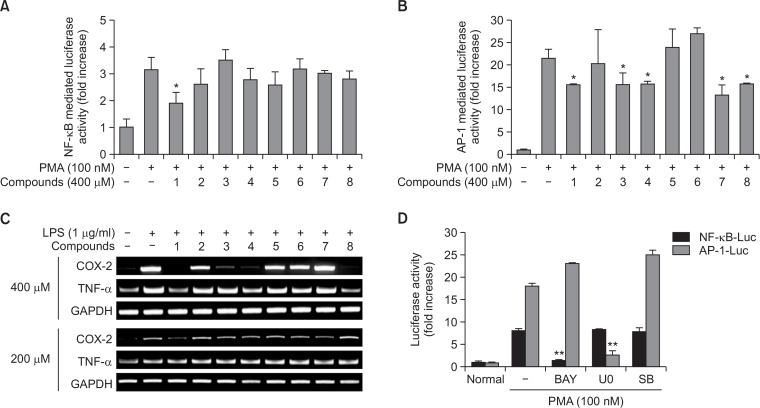
Fig. 3.
The inhibitory effects of compounds 1–8 on the activation of NF-κB and AP-1, and the mRNA expression of pro-inflammatory genes. (A, B, D) HEK293 cells co-transfected with plasmid constructs NF-κB-Luc or AP-1-Luc (each 1 μg/ml) and β-gal (as a transfection control) were treated with compounds 1–8 or standard compounds [Bay 11-7082 (BAY), U0126 (U0), and SB203580 (SB)] in the presence or absence of PMA (100 nM). Luciferase activity was measured using a luminometer. (C) The mRNA levels of COX-2 and TNF-α were determined by semiquantitative PCR. *p<0.05 and **p<0.01 compared to control.
RESULTS
Isolation and identification of chemical constituents
Since the total water extract of C. bassiana (Cb-WE) displayed strong inhibitory activities of transcriptional activation mediated by NF-κB and AP-1 (Fig. 1B), we further explored the compounds that can act as an anti-inflammatory principle in C. bassiana using column chromatography and HPLC. Indeed, as Supplementary Fig. 1, Fig. 2A show, we could separate 7 compounds from 6 different fractions and 4 more subfractions. Compound 8 was not detected under UV spectrum light due to the absence of a chromophore. Through column chromatographic separation, after all, 8 compounds were isolated as 1,9-dimethylguanine (1), adenosine (2), uridine (3), nicotinamide, 3-methyluracil (5), 1,7-dimethylxanthine (6), nudifloric acid (7), and mannitol (8) (Fig. 2B).
Fig. 2.
Identification and isolation of ingredients from the methanol layer of Cordyceps bassiana water extract. (A) HPLC chromatogram of C. bassiana water extract (254 nm). (B) Chemical structures of purified compounds 1–8.
Effect of chemical constituents on inflammatory responses
Continuously, we evaluated the anti-inflammatory activities of 8 compounds using the same reporter gene assay and RT-PCR analysis of inflammatory genes. As Fig. 3 shows that compound 1 blocked luciferase activity induced by NF-κB activation, whilst compounds 1, 3, 4, 7, and 8 significantly suppressed AP-1-mediated luciferase activity, suggesting that these compounds may have an anti-inflammatory function. To confirm these activities, we next examined whether these compounds are able to modulate the expression of TNF-α and COX-2. At 200 μM, compound 1 clearly suppressed the mRNA levels of COX-2, whereas compounds 1, 3, 4, and 8 at 400 μM strongly suppressed COX-2 expression and compounds 1, 4, and 8 diminished the mRNA expression of TNF-α.
DISCUSSION
After establishing a technology to artificially cultivate the fruit bodies of C. bassiana, our group has focused on exploring the pharmacological activity of this mushroom and have attempted to expand the medical and neutraceutical applications of C. bassiana (Wu et al., 2011). Because Cb-WE strongly inhibited transcriptional activation triggered by both NF-κB and AP-1 (Fig. 1B), representative redox-sensitive transcription factors inducing inflammatory gene expression (Lee et al., 2011; Gil et al., 2015), anti-inflammatory principle in C. bassiana was further isolated using column chromatography and HPLC. By this work, in fact, 8 compounds could be identified, including 1,9-dimethylguanine (1) (Sigel et al., 2000), adenosine (2) (Ciuffreda et al., 2007), uridine (3) (Bagno et al., 2008), nicotinamide (Jung et al., 2012), 3-methyluracil (5) (Wong et al., 2002), 1,7-dimethylxanthine (6) (Jiang et al., 1998), nudifloric acid (7) (Yuyama and Suzuki, 1991), and mannitol (8) (Bock and Pedersen, 1983), respectively, based on their spectroscopic data (Fig. 2B). As mentioned earlier, chemical investigation of C. bassiana has never been previously carried out. Thus, compounds 1–8, which were isolated in this study, are all report for the first time from C. bassiana, despite the fact that their nucleic acids and glycosides were reported to be isolated from the Cordyceps species and adenosine (2), uridine (3), nicotinamide (4), 1,7-dimethylxanthine (6), and mannitol (8) have been already reported from various Cordyceps species. In this study, 1,9-dimethylguanine (1), 3-methyluracil (5), and nudifloric acid (7) were isolated for the first time from the Cordyceps species. According to our results, the occurrence of 1,9-dimethylguanine (1), 3-methyluracil (5), and nudifloric acid (7) in the Cordyceps bassiana may possibly serve as useful chemotaxonomic markers for species of this genus.
We next investigated the anti-inflammatory activities of the isolated constituents at the transcriptional levels. As Fig. 3 indicates, compound 1 was found to suppress NF-κB-mediated luciferase activity, whereas others [compounds 1, 3, 4, 7, and 8] targeted to block AP-1-mediated luciferase activity, implying that these compounds may have an anti-inflammatory function. This possibility was also confirmed by testing the levels of TNF-α and COX-2 genes, representative inflammatory genes mediated by the activation of NF-κB and AP-1 (Yu et al., 2012). At 200 μM, compound 1 was found to inhibit the mRNA levels of COX-2, but at 400 μM, compounds 1, 3, 4, and 8 inhibited COX-2 expression and compounds 1, 4, and 8 downregulated TNF-α expression. Based on our data, these results strongly suggest that compound 1 seems to have potential activities acting as a major principle with anti-inflammatory activity. It has been previously reported that C. bassiana has the ability to ameliorate anti-atopic dermatitis symptoms (Wu et al., 2011) and to suppress NF-κB/AP-1-dependent cytokine production (Byeon et al., 2011a, 2011b). Therefore, whether the compound 1 can be further developed as anti-atopic dermatitis drug with anti-inflammatory activity will be further elucidated.
Conclusively, we have isolated 8 compounds, 1,9-dimethylguanine (1), adenosine (2), uridine (3), nicotinamide (4), 3-methyluracil (5), 1,7-dimethylxanthine (6), nudifloric acid (7), and mannitol (8) from the water extract of Cordyceps bassiana. By evaluation of their anti-inflammatory activities, compound 1 has been revealed to be a major player in the action of Cordyceps bassiana. Therefore, further investigation of the immunopharmacological activities of this compound will be continued in our future projects.
Acknowledgments
This work was supported by the BK21 plus program through the National Research Foundation (NRF) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. NRF-2016R1A6A1A03007648), Korea.
REFERENCES
- Bagno A, Rastrelli F, Saielli G. Predicting the NMR spectra of nucleotides by DFT calculations: cyclic uridine monophosphate. Magn Reson Chem. 2008;46:518–524. doi: 10.1002/mrc.2204. [DOI] [PubMed] [Google Scholar]
- Byeon SE, Lee J, Yoo BC, Sung GH, Kim TW, Park HJ, Cho JY. p38-targeted inhibition of interleukin-12 expression by ethanol extract from Cordyceps bassiana in lipopolysaccharide-activated macrophages. Immunopharmacol. Immunotoxicol. 2011a;33:90–96. doi: 10.3109/08923973.2010.482137. [DOI] [PubMed] [Google Scholar]
- Byeon SE, Lee SY, Kim AR, Lee J, Sung GH, Jang HJ, Kim TW, Park HJ, Lee SJ, Hong S, Cho JY. Inhibition of cytokine expression by a butanol extract from Cordyceps bassiana. Pharmazie. 2011b;66:58–62. [PubMed] [Google Scholar]
- Bock K, Pedersen C. Carbon-13 nuclear magnetic resonance of monosaccharides. Adv Carbohydr Chem Biochem. 1983;41:27–66. doi: 10.1016/S0065-2318(08)60055-4. [DOI] [Google Scholar]
- Chen L, Xiao H, Wang ZH, Huang Y, Liu ZP, Ren H, Song H. miR-29a suppresses growth and invasion of gastric cancer cells in vitro by targeting VEGF-A. BMB Rep. 2014;47:39–44. doi: 10.5483/BMBRep.2014.47.1.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda P, Casati S, Manzocchi A. Complete (1)H and (13)C NMR spectral assignment of alpha- and beta-adenosine, 2′-deoxyadenosine and their acetate derivatives. Magn Reson Chem. 2007;45:781–784. doi: 10.1002/mrc.2036. [DOI] [PubMed] [Google Scholar]
- Gil M, Pak HK, Park SJ, Lee AN, Park YS, Lee H, Lee H, Kim KE, Lee KJ, Yoon DH, Chung YS, Park CS. Engagement of CD99 reduces AP-1 activity by inducing BATF in the human multiple myeloma cell line RPMI8226. Immune Netw. 2015;15:260–267. doi: 10.4110/in.2015.15.5.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun SH, Lee SY, Park SJ, Kim DY, Chun YJ, Sung GH, Kim SH, Choi HK. Alteration of media composition and light conditions change morphology, metabolic profile, and beauvericin biosynthesis in cordyceps bassiana mycelium. J Microbiol Biotechnol. 2013;23:47–55. doi: 10.4014/jmb.1208.08058. [DOI] [PubMed] [Google Scholar]
- Jiang M, Kameda K, Han LK, Kimura Y, Okuda H. Isolation of lipolytic substances caffeine and 1,7-dimethylxanthine from the stem and rhizome of Sinomenium actum. Planta Med. 1998;64:375–377. doi: 10.1055/s-2006-957456. [DOI] [PubMed] [Google Scholar]
- Jung JY, Kim IY, Kim YN, Kim JS, Shin JH, Jang ZH, Lee HS, Hwang GS, Seong JK. 1H NMR-based metabolite profiling of diet-induced obesity in a mouse mode. BMB Rep. 2012;45:419–424. doi: 10.5483/BMBRep.2012.45.7.248. [DOI] [PubMed] [Google Scholar]
- Lee N, Jung YS, Lee HY, Kang N, Park YJ, Hwang JS, Bahk YY, Koo J, Bae YS. Mouse neutrophils express functional umami taste receptor T1R1/T1R3. BMB Rep. 2014;47:649–654. doi: 10.5483/BMBRep.2014.47.11.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YG, Lee J, Byeon SE, Yoo DS, Kim MH, Lee SY, Cho JY. Functional role of Akt in macrophage-mediated innate immunity. Front. Biosci. (Landmark Ed.) 2011;16:517–530. doi: 10.2741/3702. [DOI] [PubMed] [Google Scholar]
- Liang C, Ding Y, Song SB, Kim JA, Cuong NM, Ma JY, Kim YH. Oleanane-triterpenoids from Panax stipuleanatus inhibit NF-κB. J Ginseng Res. 2013;37:74–79. doi: 10.5142/jgr.2013.37.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen AM, Hansen VM, Meyling NV, Eilenberg J. Human exposure to airborne fungi from genera used as biocontrol agents in plant production. Ann Agric Environ Med. 2007;14:5–24. [PubMed] [Google Scholar]
- Moon SJ, Jeong JH, Jhun JY, Yang EJ, Min JK, Choi JY, Cho ML. Ursodeoxycholic Acid ameliorates pain severity and cartilage degeneration in monosodium iodoacetate-induced osteoarthritis in rats. Immune Netw. 2014;14:45–53. doi: 10.4110/in.2014.14.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005;57:1509–1519. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Minnis AM, Sung GH, Luangsa-ard JJ, Devotto L, Humber RA. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia. 2011;103:1055–1073. doi: 10.3852/10-302. [DOI] [PubMed] [Google Scholar]
- Sigel RK, Freisinger E, Lippert B. Effects of N7-methylation, N7-platination, and C8-hydroxylation of guanine on H-bond formation with cytosine: platinum coordination strengthens the Watson-Crick pair. J Biol Inorg Chem. 2000;5:287–299. doi: 10.1007/PL00010657. [DOI] [PubMed] [Google Scholar]
- Vo HT, Cho JY, Choi YE, Choi YS, Jeong YH. Kinetic study for the optimization of ginsenoside Rg3 production by heat treatment of ginsenoside Rb1. J Ginseng Res. 2015;39:304–313. doi: 10.1016/j.jgr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Bachki A, Banerjee K, Leyland-Jones B. Identification of N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-5-carboxamide as components in urine extracts of individuals consuming coffee. J Pharm Biomed Anal. 2002;30:773–780. doi: 10.1016/S0731-7085(02)00384-9. [DOI] [PubMed] [Google Scholar]
- Wu G, Li L, Sung GH, Kim TW, Byeon SE, Cho JY, Park CW, Park HJ. Inhibition of 2,4-dinitrofluorobenzene-induced atopic dermatitis by topical application of the butanol extract of Cordyceps bassiana in NC/Nga mice. J Ethnopharmacol. 2011;134:504–509. doi: 10.1016/j.jep.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lee J, Rhee MH, Yu T, Baek KS, Sung NY, Kim Y, Yoon K, Kim JH, Kwak YS, Hong S, Kim JH, Cho JY. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015;39:61–68. doi: 10.1016/j.jgr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Yi YS, Yang Y, Oh J, Jeong D, Cho JY. The Pivotal Role of TBK1 in Inflammatory Responses Mediated by Macrophages. Mediators Inflamm. 2012;2012:979105. doi: 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama S, Suzuki T. The excretion of N′-methyl-2-pyridone-5-carboxylic acid and related compounds in human subjects after oral administration of nicotinic acid, trigonelline, and N′-methyl-2-pyridone-5-carboxylic acid. Adv Exp Med Biol. 1991;294:475–479. doi: 10.1007/978-1-4684-5952-4_48. [DOI] [PubMed] [Google Scholar]
- Zhou X, Gong Z, Su Y, Lin J, Tang K. Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol. 2009;61:279–291. doi: 10.1211/jpp.61.03.0002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.