Abstract
Auranofin has been developed as antirheumatic drugs, which is currently under clinical development for the treatment of chronic lymphocytic leukemia. Previous report showed that auranofin induced apoptosis by enhancement of annexin A5 expression in PC-3 cells. To understand the role of annexin A5 in auranofin-mediated apoptosis, we performed microarray data analysis to study annexin A5-controlled gene expression in annexin A5 knockdown PC-3 cells. Of differentially expressed genes, plasminogen activator inhibitor (PAI)-2 was increased by annexin A5 siRNA confirmed by qRT-PCR and western blot. Treatment with auranofin decreased PAI-2 and increased annexin A5 expression as well as promoting apoptosis. Furthermore, auranofin-induced apoptosis was recovered by annexin A5 siRNA but it was promoted by PAI-2 siRNA. Interestingly, knockdown of annexin A5 rescued PAI-2 expression suppressed by auranofin. Taken together, our study suggests that induction of annexin A5 by auranofin may enhance apoptosis through suppression of PAI-2 expression in PC-3 cells.
Keywords: Annexin A5, Apoptosis, Auranofin, Plasminogen activator inhibitor-2
INTRODUCTION
Auranofin is a gold compound that has been used for rheumatoid arthritis (Kean et al., 1997). As auranofin is able to prevent various crucial inflammatory pathways, it is considered as a new candidate for anticancer agents (Han et al., 2008). For example, auranofin reduces NF-κB activation via IκB kinase suppression and it decreases generation of tumor necrosis factor (TNF)-α in murine macrophage (Jeon et al., 2000). Auranofin induces apoptosis through the inhibition of IL-6-induced JAK/STAT and NF-κB pathway in myeloma cells (Stern et al., 2005; Kim et al., 2007; Nakaya et al., 2011). In addition, auranofin promotes apoptosis via FOXO3 activation or inhibition of thioredoxin reductase in human ovarian cancer cells (Marzano et al., 2007; Park et al., 2014). Phosphoinositide 3-kinase (PI3K)/Akt pathway is also inhibited by auranofin in non-small cell lung cancer cells (Li et al., 2016). Recently, Phase I/II clinical trials of auranofin to treat chronic lymphocytic leukemia has been completed (Liu et al., 2014; Clinicaltrials.gov, 2016). Moreover, our recent study suggested that auranofin leads to annexin A5 expression and translocation into mitochondria causing mitochondrial apoptosis through VDAC oligomerization in human prostate cancer cells (Park and Chun, 2014).
Annexin A5 is the annexin family protein that binds to phospholipids in a calcium-dependent manner. It was discovered from human placenta for the first time at the end of 1970s (Bohn and Kraus, 1979; Boersma et al., 2005). Annexin A5 is identified in blood vessel where it acts as a blood anticoagulation factor and it builds voltage-dependent calcium channel in phospholipid bilayers (Reutelingsperger et al., 1985; Demange et al., 1994). The property of annexin A5 that selectively binds to negative charged phospholipid phosphatidylserine (PS) has been used to distinguish the apoptotic cells from viable cells using flow cytometry analysis for many years (Koopman et al., 1994; Vermes et al., 1995). Annexin A5 induces rat Leydig cell proliferation through RhoA/Rho-associated protein kinase pathway via Ect2 protein (Jing et al., 2015). Furthermore, annexin A5 suppresses protein kinase activity by directly interacting with protein kinase C (PKC) and annexin A5-like peptide triggers apoptosis by binding to cytoplasmic domain of integrin ανβ5 (Rothhut et al., 1995; Cardó-Vila et al., 2003). However, the role of annexin A5 itself has not yet been clarified. Recently, annexin A5 has been reported as a new mediator of cisplatin-induced apoptosis by inducing voltage-dependent anion channel (VDAC) oligomerization in human kidney epithelial cells (Kwon et al., 2013; Jeong et al., 2014).
Plasminogen activator inhibitor (PAI)-2 is a member of serpin protein family that inhibits serine protease in the blood coagulation cascade in human (Kruithof et al., 1995). PAI-2 binds and inhibits urokinase-type plasminogen activator (uPA) which activates precursor plasminogen to plasmin (Irving et al., 2000). This inhibition of PAI-2 decreases extracellular matrix (ECM) degradation by regulating uPA/urokinase-type plasminogen activator receptor (uPAR) signaling (Croucher et al., 2008). Although PAI-2 is known as an inhibitor of uPA, PAI-2 is considered as an antiapoptotic protein and strong prognostic marker in various cancer cells. First evidence of PAI-2 as an antiapoptotic factor was came from linkage to Bcl-2 because of their proximity in genome and similar structure (Silverman et al., 1991; Medcalf and Stasinopoulos, 2005). Some studies show that TNF-α-induced apoptosis can be suppressed by PAI-2 expression in several cancer cells and overexpression of PAI-2 inhibits apoptosis (Kumar and Baglioni, 1991; Dickinson et al., 1995; Zhou et al., 2001). Moreover, high expression of PAI-2 in endometrial and colorectal cancer is also shown to be closely correlated with poor prognosis (Ganesh et al., 1994; Nordengren et al., 2002).
Because annexin A5 may play an important role in auranofin-mediated apoptosis and PAI-2 may have antiapoptotic roles, we wanted to determine whether annexin A5 is able to regulate PAI-2 expression. In this study, we explored how annexin A5 controls PAI-2 expression in auranofin-mediated apoptosis in PC-3 cells and we found that auranofin downregulates PAI-2 level via the induction of annexin A5 expression to augment apoptotic pathway.
MATERIALS AND METHODS
Reagents
Auranofin was purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) and RPMI 1640 medium were from HyClone (Logan, UT, USA). Neon transfection system and BCA protein assay kit were from Thermo Scientific (Rockford, IL, USA). Enhanced chemiluminescence (ECL) kit was from Bionote (Gyeonggi, Korea). EZ-Cytox enhanced cell viability assay kit was from Daeil Lab Service Co., Ltd (Seoul, Korea). PAI-2 antibody was purchased from Abcam (Cambridge, UK). Poly (ADP-ribose) polymerase antibody was from Cell Signaling Technology (Beverly, MA, USA). Annexin A5, β-actin, Bcl-2 and caspase 3 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG was from Bethyl (Montgomery, TX, USA). HRP-conjugated goat anti-mouse IgG was from AbClon (Seoul, Korea). MuseTM annexin V dead cell kit was from Millipore (Bedford, MA, USA). Moloney murine leukemia virus (M-MLV) reverse transcriptase and RNase inhibitor (RNasin) were purchased from Promega (Madison, WI, USA). TaKaRa Ex-Taq DNA polymerase was obtained from TaKaRa Bio (Shiga, Japan). SYBR green I PCR kit, PAI-2 siRNA were from Qiagen (Valencia, CA, USA). Annexin A5 siRNA was from Dharmacon (Lafayette, CO, USA). All others chemicals were of the highest purity or molecular biology grade available from commercial sources.
Cell culture
The human prostate cancer cell line, PC-3 cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 unit/ml penicillin, and 100 μg/ml streptomycin in a humidified incubator (5% CO2/95% air) at 37°C. DMSO was used as the vehicle and did not exceed 0.1% (v/v). Cells were treated with auranofin and incubated in a humidified CO2 incubator at 37°C for the designated time. After incubation, cells were harvested and washed with phosphate-buffered saline (PBS). Cells were centrifuged at 13000 rpm for 2 min and the pellets were preserved in −70°C.
Cell viability assay
Cells (7×103 cells/well) were added onto a 96-well flat-bottom microplate, and were incubated for the designated time at 37°C. After treatment, the cells were incubated with 10% of cell viability assay reagent for 1.5 h. After incubation at 37°C, the absorbance was measured at 450 nm by using a Sunrise™ microplate reader (Tecan, Männedorf, Switzerland).
Apoptosis assay
Cells were harvested with 0.05% trypsin-EDTA and then centrifuge at 13000 rpm for 2 min and washed once with 1 ml of PBS. The resulting pellets were resuspended with 100 μl of PBS containing 1% FBS and 100 μl of MuseTM annexin V dead cell kit. The resulting mixture was kept for 20 min at room temperature. The samples were analyzed immediately using MuseTM cell analyzer (Millipore).
Transient transfection
Cells were transfected with plasmid or siRNA using Neon transfection system as recommended by the manufacturer. Briefly, cells were collected approximately 1×106 cells washed in PBS and then resuspended in resuspension buffer from Neon transfection system kit. Transfection was carried out with 5 μg of vector or 37.5 nM of siRNA in RPMI medium containing 10% FBS without antibiotics for 24 h at 37°C. After transfection, cells were maintained in RPMI medium containing 10% FBS for 24 h. Plasmid containing annexin A5 cDNA was modified from pcDNA3.1zeo. Annexin A5 siRNA was SMARTpool. The target sequence of PAI-2 siRNA was as follows: 5′-CAGATCCAGAAGGGTAGTTAT-3′.
RT-PCR and qRT-PCR
Total RNA was extracted using Ribospin™ Total RNA kit (GeneAll, Seoul, Korea). Whole RNA (500 ng) was reverse transcribed at 37°C for 1 h for 20 μl volume containing 5x RT buffer, 10 mM dNTPs, 40 units of RNase inhibitor, 200 units of M-MLV reverse transcriptase, and 100 pmole of oligo-dT primer. Subsequently, 0.8 μl of the reaction mixture from each sample was amplified with 10 pmole of each oligonucleotide primer, 0.2 mM dNTPs, 1.5 mM MgCl2, and 1.25 units of Taq DNA polymerase in a final volume of 25 μl. PCR was performed as follows: one cycle of 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec, annealing according to each primer’s melting temperature(Tm) for 30 sec, and extension at 72°C for 15 sec. Human PAI-2 cDNA was amplified using a sense primer (5′-AACCCCAGGCAGTAGACTTC-3′) and an antisense primer (5′-ACAGCATTCACCAGGACCAT-3′). Human 18S rRNA cDNA was amplified using a sense primer (5′-GTAACCCGTTGAACCCCATT-3′) and an antisense primer (5′-CCATCCAATCCAATCGGTAGTAGCG-3′). Human annexin A5 cDNA was amplified using a sense primer (5′-CTTGGGCACAGATGAGGAGAGCA-3′) and an antisense primer (5′-AAGCCGAGAGGGTTTCATCAGAGC-3′). The number of amplification cycles was optimized in preliminary experiments to ensure that the PCR reaction did not reach plateau. PCR products were analyzed using 2% (w/v) agarose gel electrophoresis and a ChemiDoc XRS (Bio-Rad, Hercules, CA, USA). To determine the concentration of the expressed mRNA, quantitative real-time PCR (qRT-PCR) was performed using a Rotor-Gene Q (Qiagen) with a SYBR green I PCR kit as recommended by the manufacturer. Each reaction contained 5 μl of the 2X SYBR green Premix Ex Taq, 10 pmoles of each oligonucleotide primer and 10 ng cDNA in a final volume of 10 μl. qRT-PCR was performed as follows: one cycle of 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 5 sec, annealing and extension at 55°C (PAI-2) or 58°C (annexin A5) for 10 sec. Melting curve analysis was performed to verify amplification specificity. For data analysis, the comparative Ct (threshold cycle) method was used to calculate the relative changes in gene expression.
Western blot analysis
Cells were solubilized with lysis buffer (pH 7.4) containing 50 mM Tris.Cl, 150 mM NaCl and 1% Nonidet P-40. Protein concentration was measured using BCA protein assay reagents. Extracted proteins (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels, and were electrophoretically transferred onto polyvinylidene difluoride (PVDF) membrane. Membranes were blocked in Tris-buffered saline containing 5% (w/v) nonfat dried milk for 3 h at 4°C and then were incubated for overnight with primary antibody at a 1:1000 dilution in Tris-buffered saline. After incubating with HRP-conjugated secondary antibody for 2 h at 4°C, proteins were visualized by an ECL and the band intensity was analyzed by ChemiDoc XRS densitometer and quantified by Quantity One software (Bio-Rad, Richmond, CA, USA).
Microarray data analysis
Affymetrix (Affymetrix, Santa Clara, USA) GeneChip® Human Gene 2.0 ST oligonucleotide arrays were used for global gene expression analysis. The sample preparation was performed according to the instructions and recommendations provided by the manufacturer. Total RNA was isolated using Ribospin™ Total RNA kit. RNA quality was assessed by Agilent 2100 bioanalyzer using the RNA 6000 Nano Chip (Agilent Technologies, Amstelveen, The Netherlands), and quantity was determined by ND-1000 Spectrophotometer (NanoDrop Technologies, Inc., DE, USA). Briefly, 300 ng of total RNA from each sample was converted to double-strand cDNA using a random hexamer incorporating a T7 promoter, amplified RNA (cRNA) was generated from the double-stranded cDNA template through an IVT (in-vitro transcription) reaction and purified with the Affymetrix sample cleanup module. cDNA was regenerated through a random-primed reverse transcription using a dNTP mix containing dUTP. The cDNA was then fragmented by UDG and APE 1 restriction endonucleases and end-labeled by terminal transferase reaction incorporating a biotinylated dideoxynucleotide. Fragmented end-labeled cDNA was hybridized to the GeneChip® Human Gene 2.0 ST arrays for 17 h at 45°C and 60 rpm. After hybridization, the chips were stained and washed in a Genechip Fluidics Station 450 (Affymetrix) and scanned by using a Genechip Array scanner 3000 7G (Affymetrix). Expression data were normalized and log2 transformed using the robust multichip average (RMA) method implemented in the Bioconductor package RMA (Bolstad et al., 2003; Irizarry et al., 2003). To reduce noise for the significance analysis, probe sets that did not show detection call rate at least 50% of the samples in the comparison were filtered out. Highly expressed genes that showed a 2-fold change in expression were selected. The results were classified using hierarchical clustering algorithms implemented in TMEV software 4.0 (Eisen et al., 1998).
Statistical analysis
Statistical analysis was implemented by using one-way analysis of variance, followed by Dunnett’s pairwise multiple comparison t-test with GraphPad Prism software (GraphPad Software Inc., San Diego, USA) when appropriate. The difference was considered statistically significant at *p<0.05.
RESULTS
Depletion of PAI-2 expression promotes apoptosis
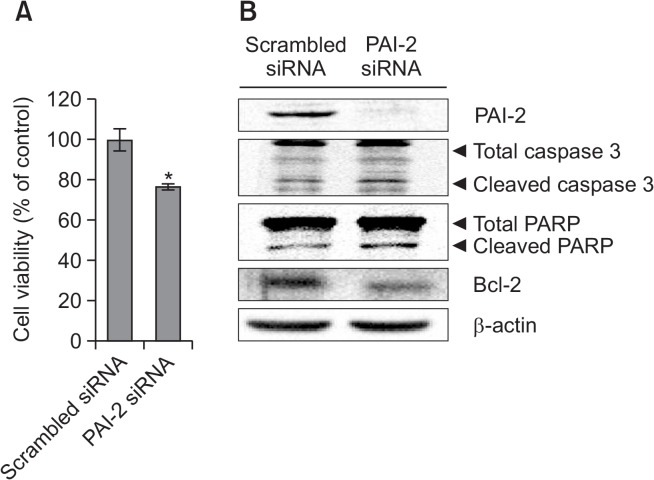
To confirm the function of PAI-2 as an antiapoptotic factor in PC-3 cells, PAI-2 mRNA level was knocked down in PC-3 cells using siRNA (37.5 nM) for 48 h. Cells treated with PAI-2 siRNA showed decreased cell viability about 20% relative to control group (Fig. 1A). Moreover, we examined the protein levels of caspase 3, PARP, or Bcl-2 in PAI-2 knockdown cells. Cleaved caspase 3 and PARP levels were increased and Bcl-2 level was decreased after transfection of PAI-2 siRNA (Fig. 1B). These results indicated that PAI-2 is able to inhibit apoptosis in PC-3 cells.
Fig. 1.
Knockdown of PAI-2 increases apoptosis in PC-3 cells. (A) Cell viability assay. PC-3 cells were transfected with PAI-2 siRNA for 48 h. After incubation, the absorbance was measured at 450 nm. The percentage of cells surviving in each group relative to the control was calculated. The data are showed as a mean ± SD (n=3). *Significantly different from control (p<0.05). (B) Western blot analysis. PC-3 cells were transfected with PAI-2 siRNA for 48 h. Cells were harvested and total cellular proteins were extracted. Extracted proteins were separated by SDS-PAGE (10%) and western blot analysis was performed with specific antibodies. β-actin level was determined as loading control.
Annexin A5 suppresses PAI-2 expression
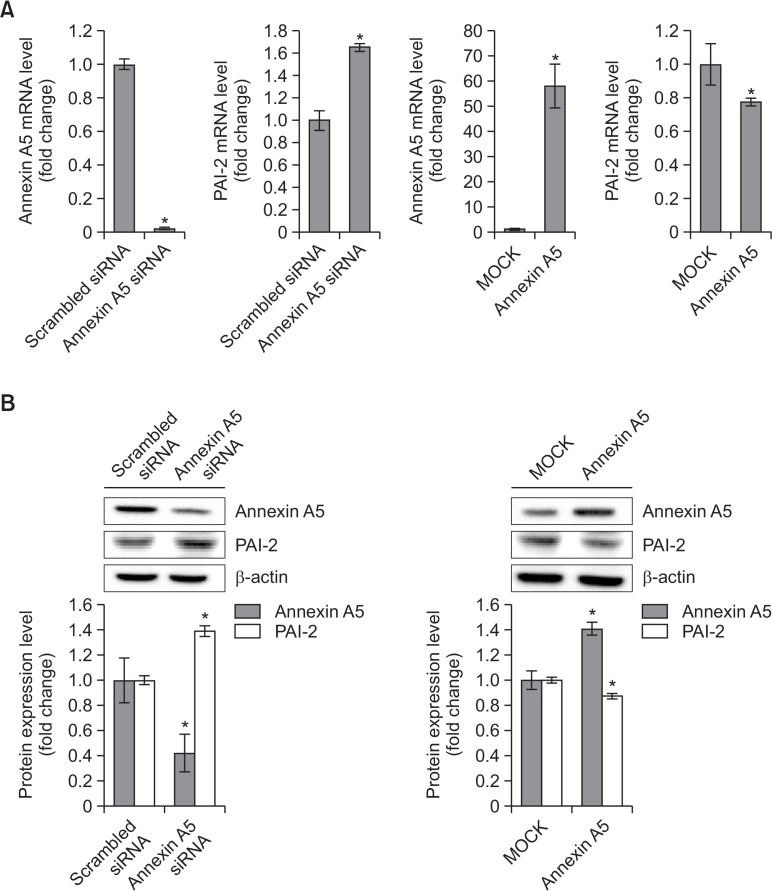
To identify whether annexin A5 regulates specific gene expression in PC-3 cells, cells were transfected with annexin A5 siRNA (37.5 nM) for 48 h. Annexin A5-depleted cells were analyzed with Affymetrix GeneChip® Human Gene 2.0 ST Array. Of approximately 30,000 differentially expressed genes in microarray data, 33 genes were increased when annexin A5 was knocked down in PC-3 cells (Table 1). Because PAI-2 gene expression was significantly increased (2.6-fold) in knockdown cells and PAI-2 is considered as an antiapoptotic factor in cancer cells, we chose this gene to see whether annexin A5 modulates PAI-2 gene expression to control apoptosis in PC-3 cells. To confirm that annexin A5 expression affects PAI-2 level, annexin A5 siRNA (37.5 nM) or annexin A5 overexpression plasmid (5 μg) was used. As results, PAI-2 mRNA level was increased 1.8-fold in knockdown cells of annexin A5 and decreased about 20% when cells were overexpressed with annexin A5 (Fig. 2A). As shown in Fig. 2B, protein level of PAI-2 was also upregulated about 1.4-fold in annexin A5 siRNA-treated cells and downregulated about 10% in annexin A5 overexpressing cells. These results indicate that annexin A5 may inhibit PAI-2 mRNA and protein expression in PC-3 cells.
Table 1.
Microarray data analysis in annexin A5 knockdown PC-3 cells
| Gene symbol (representative) | Log2 ratio | Absolute fold change | Gene description |
|---|---|---|---|
| HSD3B2 | 1.62 | 3.07 | Hydroxy-Δ5-steroid dehydrogenase, 3β- and steroid Δ-isomerase 2 |
| PAI-2 | 1.41 | 2.66 | Plasminogen activator inhibitor-2 |
| KRTAP3-1 | 1.34 | 2.53 | Keratin associated protein 3-1 |
| VTRNA1-1 | 1.33 | 2.52 | Vault RNA 1-1 |
| LOC646813 | 1.32 | 2.49 | DEAH (Asp-Glu-Ala-His) box helicase 9 pseudogene |
| CCL3L3 | 1.27 | 2.41 | Chemokine (C-C motif) ligand 3-like 3 |
| OR52I1 | 1.26 | 2.39 | Olfactory receptor, family 52, subfamily I, member 1 |
| TBC1D4-AS1 | 1.24 | 2.36 | TBC1D4 antisense RNA 1 |
| TAS2R20 | 1.22 | 2.33 | Taste receptor, type 2, member 20 |
| TRNAI6 | 1.21 | 2.32 | Transfer RNA isoleucine 6 (anticodon UAU) |
| SNRPN | 1.21 | 2.31 | Small nuclear ribonucleoprotein polypeptide N |
| AMY2A | 1.21 | 2.31 | Amylase, α 2A (pancreatic) |
| CLYBL | 1.19 | 2.29 | Citrate lyase β like |
| CRYZL1 | 1.18 | 2.27 | Crystallin, ζ (quinone reductase)-like 1 |
| FAM74A2 | 1.17 | 2.25 | Family with sequence similarity 74, member A2 |
| OR5T2 | 1.16 | 2.23 | Olfactory receptor, family 5, subfamily T, member 2 |
| YME1L1 | 1.15 | 2.22 | YME1-like 1 ATPase |
| SNORD114-4 | 1.14 | 2.20 | Small nucleolar RNA, C/D box 114-4 |
| SNORD20 | 1.13 | 2.19 | Small nucleolar RNA, C/D box 20 |
| RNA5SP38 | 1.12 | 2.18 | RNA, 5S ribosomal pseudogene 38 |
| MYH2 | 1.07 | 2.11 | Myosin, heavy chain 2, skeletal muscle, adult |
| PRAMEF3 | 1.07 | 2.10 | PRAME family member 3 |
| KRTAP13-4 | 1.07 | 2.09 | Keratin associated protein 13-4 |
| TRIM43B | 1.06 | 2.09 | Tripartite motif containing 43B |
| IL13RA2 | 1.05 | 2.07 | Interleukin 13 receptor α2 |
| MS4A4E | 1.03 | 2.04 | Membrane-spanning 4-domains, subfamily A, member 4E |
| IFI44 | 1.02 | 2.03 | Interferon-induced protein 44 |
| RNU7-60P | 1.01 | 2.02 | RNA, U7 small nuclear 60 pseudogene |
| PCDH11X | 1.01 | 2.01 | Protocadherin 11 X-linked |
| OR52M1 | 1.00 | 2.00 | Olfactory receptor, family 52, subfamily M, member 1 |
| F3 | 1.00 | 2.00 | Coagulation factor III (thromboplastin, tissue factor) |
| HIST1H3J | 1.00 | 2.00 | Histone cluster 1, H3j |
| RNA5SP409 | 1.00 | 2.00 | RNA, 5S ribosomal pseudogene 409 |
Cells were transfected with annexin A5 siRNA for 48 h. Gene expressions were analyzed with Affymetrix GeneChip® Human Gene 2.0 ST Array. Cut off ≥ 2-fold (p<0.05).
Fig. 2.
PAI-2 may be downregulated by annexin A5 in PC-3 cells. (A) qRT-PCR. PC-3 cells were transfected with annexin A5 siRNA or annexin A5 overexpression plasmid for 48 h. After harvesting, total RNA was isolated and qRT-PCR was performed to determine annexin A5 and PAI-2 mRNA expression levels. 18S rRNA was a RNA control. The data are showed as a mean ± SD (n=3). *Significantly different from control (p<0.05). (B) Western blot analysis. PC-3 cells were transfected with annexin A5 siRNA or annexin A5 overexpression plasmid for 48 h. Cells were harvested and total cellular proteins were extracted. Extracted proteins were separated by SDS-PAGE (10%) and western blot analysis was performed with specific antibodies. β-actin level was determined as loading control. Quantity one software were used to measure the intensities of protein bands. Relative protein expression level was determined by the ratio of target protein to β-actin. The data are showed as a mean ± SD (n=3). *Significantly different from control (p<0.05).
Auranofin inhibits PAI-2 expression level via annexin A5 induction
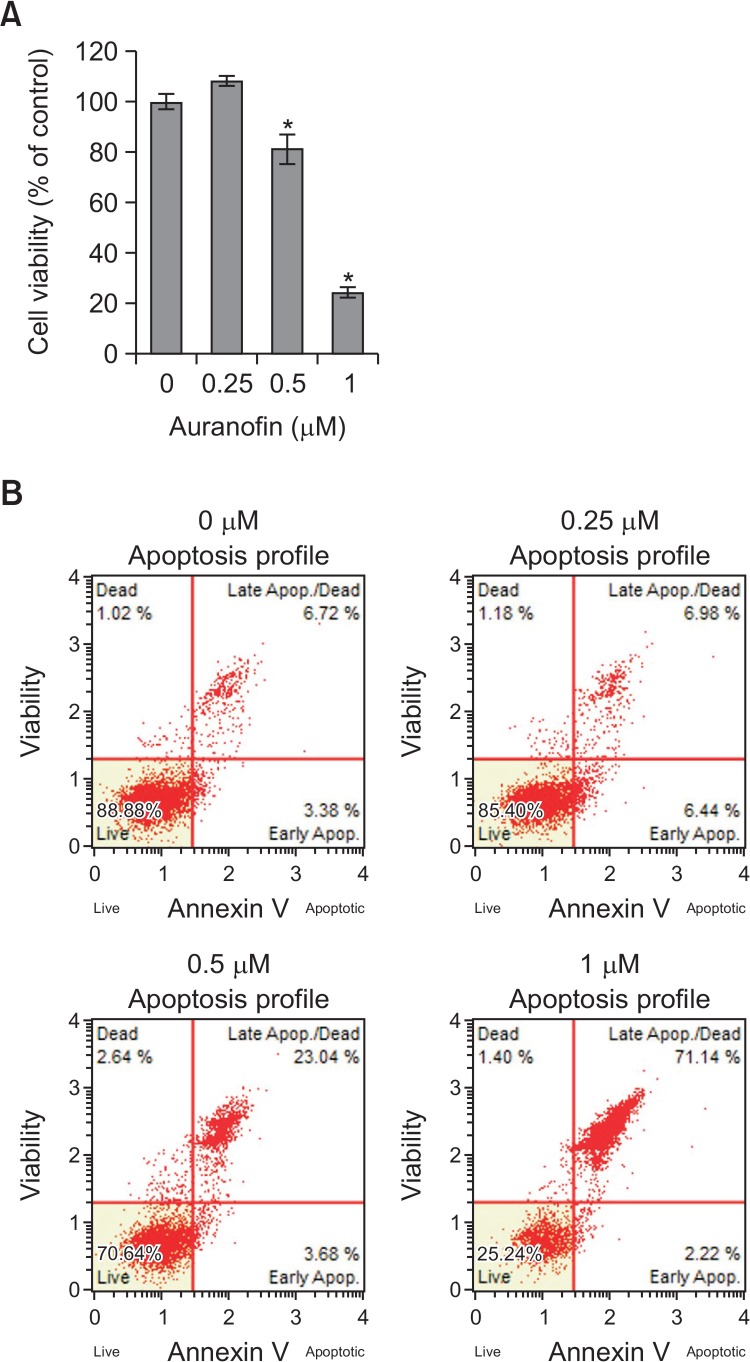
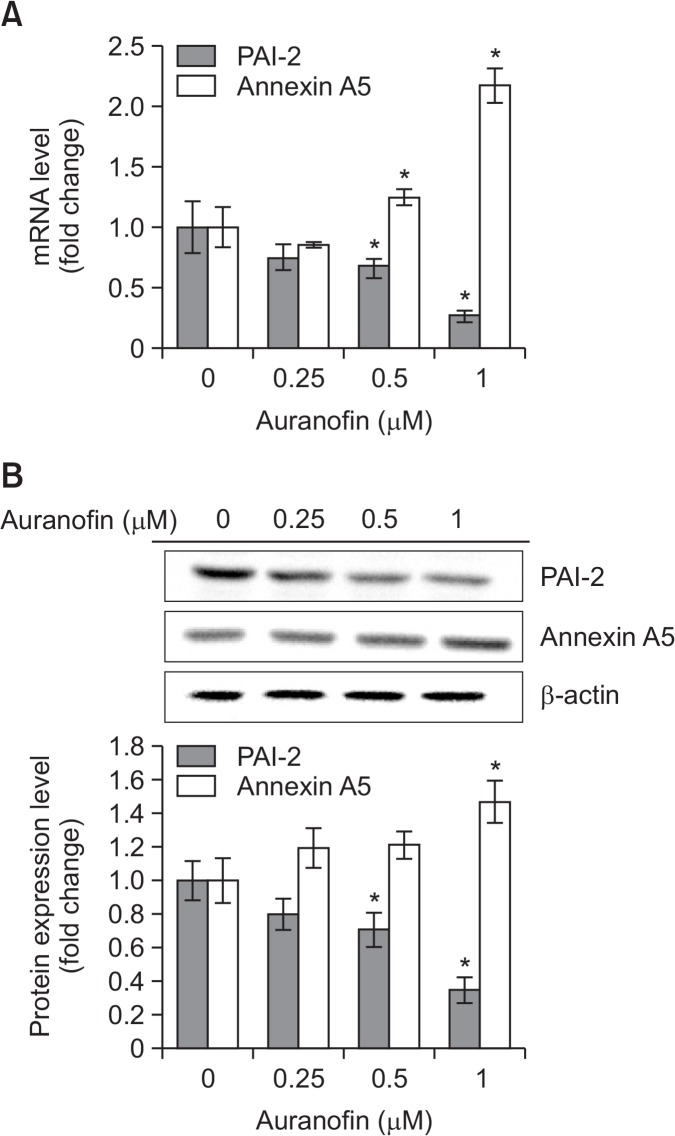
To examine that auranofin inhibits cell proliferation and drives apoptosis in PC-3 cells, various concentrations (0, 0.25, 0.5, or 1 μM) of auranofin were treated and cell viability and apoptosis assay was performed (Fig. 3). When cells were treated with auranofin, cell viability was decreased about 20% at 0.5 μM and 75% at 1 μM (Fig. 3A). To confirm whether auranofin is able to promote apoptosis in PC-3 cells, flow cytometry analysis was performed. The percentage of total apoptotic cell was about 30% at 0.5 μM and 75% at 1 μM (Fig. 3B). These data showed that auranofin induces apoptosis in PC-3 cells in a concentration-dependent manner. To identify whether annexin A5 and PAI-2 are expressed in an opposite way in auranofin-induced apoptosis, the mRNA and protein levels of annexin A5 and PAI-2 were measured by qRT-PCR and western blot analysis, respectively. Treatment with auranofin of PC-3 cells for 24 h led to increase of annexin A5 mRNA and protein expression and decrease of PAI-2 mRNA and protein expression in concentration-dependent manners (Fig. 4). These findings suggest that annexin A5 and PAI-2 may play an antagonistic role each other in auranofin-induced apoptosis in PC-3 cells.
Fig. 3.
Auranofin induces apoptosis in PC-3 cells. (A) Cell viability assay. PC-3 cells were treated with auranofin at various concentrations (0, 0.25, 0.5, or 1 μM) for 24 h. After incubation, the absorbance was measured at 450 nm. The percentage of cells surviving in each group relative to the control was calculated. The data are showed as a mean ± SD (n=3). *Significantly different from control (p<0.05). (B) Apoptosis assay using flow cytometry. PC-3 cells were treated with auranofin at various concentrations (0, 0.25, 0.5, or 1 μM) for 24 h. The cells were stained with MuseTM annexin V dead cell kit. After incubation for 20 min at room temperature, MuseTM cell analyzer assessed the percentage of apoptotic cells.
Fig. 4.
Auranofin suppresses PAI-2 expression through annexin A5 induction in PC-3 cells. (A) qRT-PCR. PC-3 cells were treated with auranofin at various concentrations (0, 0.25, 0.5, or 1 μM) for 24 h. After harvesting, total RNA was isolated and qRT-PCR was performed to determine annexin A5 and PAI-2 mRNA expression levels. 18S rRNA was used as a RNA control. The data are showed as a mean ± SD (n=3). *Significantly different from control (p<0.05). (B) Western blot analysis. PC-3 cells were treated with auranofin at various concentrations (0, 0.25, 0.5, or 1 μM) for 24 h. Cells were harvested and total cellular proteins were extracted. Extracted proteins were separated by SDS-PAGE (10%) and western blot analysis was performed with specific antibodies. β-actin level was determined as a loading control. Quantity one software were used to measure the intensities of protein bands. Relative protein expression level was determined by the ratio of target protein to β-actin. The data are showed as a mean ± SD (n=3). *Significantly different from control (p<0.05).
Relationship between annexin A5 and PAI-2 in auranofin-induced apoptosis
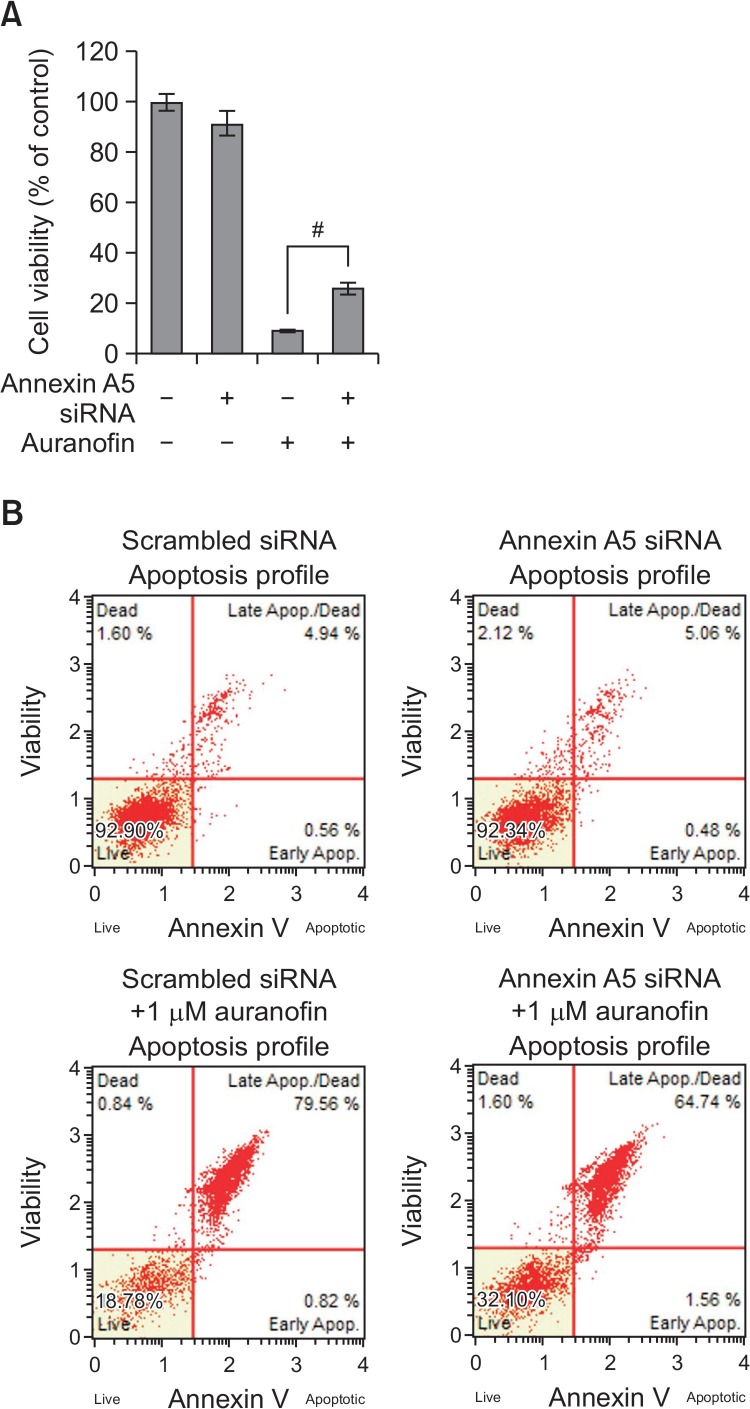
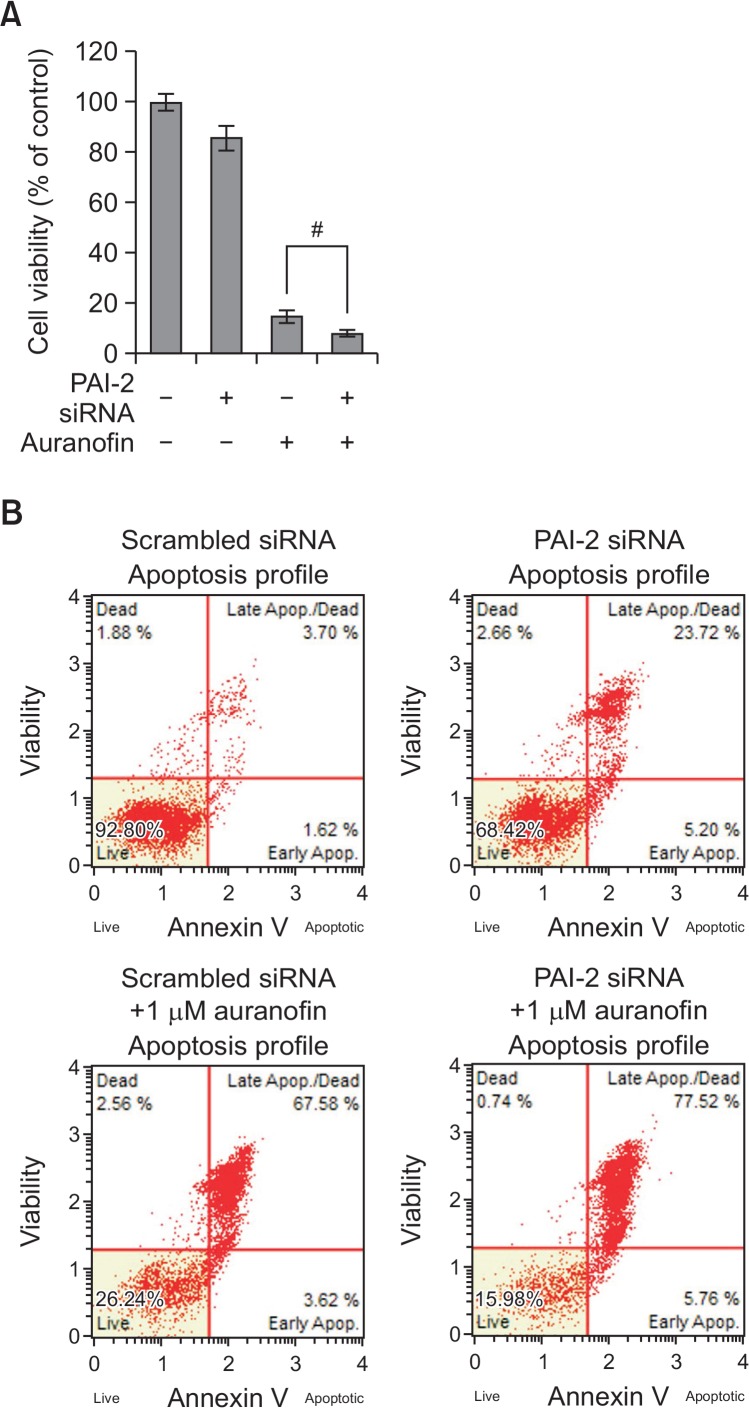
To examine the role of annexin A5 in auranofin-treated PC-3 cells, annexin A5 siRNA (37.5 nM) was transfected with or without auranofin (1 μM) into cells. As shown in Fig. 5A, treatment with auranofin in the presence of annexin A5 siRNA recovered cell viability about 20% compared to scrambled siRNA-treated cells. Apoptosis assay using flow cytometry also revealed that auranofin-induced apoptosis was prevented by annexin A5 siRNA about 15% (Fig. 5B). These results demonstrate that annexin A5 may play an important role in auranofin-induced apoptosis. To further determine the function of PAI-2, cells were treated with auranofin (1 μM) for 24 h in the presence of PAI-2 siRNA (37.5 nM). As shown in Fig. 6A, treatment with PAI-2 siRNA resulted in decreasing cell viability about 10% compared to the control cells. Flow cytometry analysis also showed that increased apoptosis by auranofin was enhanced by knockdown of PAI-2 about 10% (Fig. 6B). These data show that PAI-2 may act as an antiapoptotic factor in auranofin-induced apoptosis. In an effort to investigate relationship between annexin A5 and PAI-2 in auranofin-mediated apoptosis, cells transfected with annexin A5 siRNA were treated with auranofin (1 μM) for 24 h to induce apoptosis. After treatment with annexin A5 siRNA and 1 μM of auranofin, PAI-2 mRNA and protein levels were determined. As shown in Fig. 7A, PAI-2 mRNA level was increased about 1.8-fold compared to the control level. Auranofin suppressed PAI-2 mRNA expression about 40% but annexin A5 siRNA significantly recovered auranofin-mediated PAI-2 mRNA suppression about 2-fold. Western blot data also showed that annexin A5 siRNA prevents auranofin-mediated PAI-2 downregulation (Fig. 7B). Taken together, these results suggest that induction of annex-in A5 by auranofin may suppress PAI-2 expression.
Fig. 5.
Knockdown of annexin A5 prevents auranofin-mediated apoptosis in PC-3 cells. (A) Cell viability assay. PC-3 cells were transfected with annexin A5 siRNA for 24 h and then treated with auranofin (1 μM) for 24 h. After incubation, the absorbance was measured at 450 nm. The percentage of cells surviving in each group relative to the control was calculated. The data are showed as a mean ± SD (n=3). #Significantly different from auranofin-treated cells (p<0.05). (B) Apoptosis assay using flow cytometry. PC-3 cells were transfected with annexin A5 siRNA for 24 h and then treated with auranofin (1 μM) for 24 h. The cells were stained with MuseTM annexin V dead cell kit. After incubation for 20 min at room temperature, MuseTM cell analyzer assessed the percentage of apoptotic cells.
Fig. 6.
Knockdown of PAI-2 expression enhances auranofin-mediated apoptosis in PC-3 cells. (A) Cell viability assay. PC-3 cells were transfected with PAI-2 siRNA for 24 h and then treated with auranofin (1 μM) for 24 h. After incubation, the absorbance was measured at 450 nm. The percentage of cells surviving in each group relative to the control was calculated. The data are showed as a mean ± SD (n=3). #Significantly different from auranofin-treated cells (p<0.05). (B) Apoptosis assay using flow cytometry. PC-3 cells were transfected with PAI-2 siRNA for 24 h and then treated with auranofin (1 μM) for 24 h. The cells were stained with MuseTM annexin V dead cell kit. After incubation for 20 min at room temperature, MuseTM cell analyzer assessed the percentage of apoptotic cells.
Fig. 7.
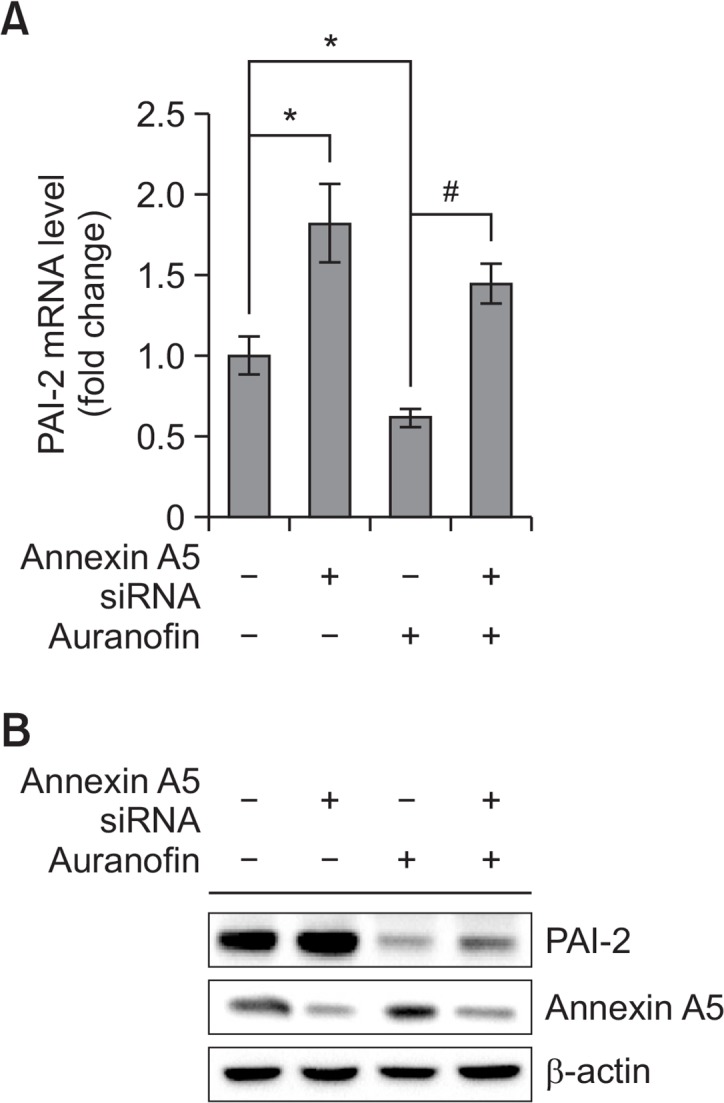
Knockdown of annexin A5 recovers auranofin mediated PAI-2 suppression in PC-3 cells. (A) qRT-PCR. PC-3 cells were treated with annexin A5 siRNA for 24 h and then treated with auranofin (1 μM) for 24 h. After treatment, total RNA was isolated and qRT-PCR was performed to determine annexin A5 and PAI-2 mRNA expression levels. 18S rRNA was used as a RNA control. The data are showed as a mean ± SD (n=3). *Significantly different from control (p<0.05); #Significantly different from auranofin-treated cells (p<0.05). (B) Western blot analysis. PC-3 cells were treated with annexin A5 siRNA for 24 h and then treated with auranofin (1 μM) for 24 h. Cells were harvested and total cellular proteins were extracted. Extracted proteins were separated by SDS-PAGE (10%) and western blot analysis was performed with specific antibodies. β-actin level was determined as loading control.
DISCUSSION
PAI-2 is previously known to protect TNF-α-induced apoptosis in HeLa and HT-1080 cells (Kumar and Baglioni, 1991; Dickinson et al., 1995). But, the role of PAI-2 as an antiapoptotic factor is still unclear. To explore the role of PAI-2 as an antiapoptotic factor, we transfected PAI-2 siRNA to elucidate the effect of PAI-2 in apoptosis. We observed that decrease of cell viability and increase of caspase 3 and PARP cleavage, the indicative of cells undergoing apoptosis relative to control by PAI-2 knockdown. Thus, PAI-2 in PC-3 cells was assumed to have an antiapoptotic role. Recently, we have studied that annexin A5 may be directly associated with apoptotic signaling (Kwon et al., 2013; Hong et al., 2014; Jeong et al., 2014). According to the results, we analyzed several annexin A5-controlled genes in annexin A5 siRNA-transfected PC-3 cells by using microarray data analysis. Of them, PAI-2 was increased in annexin A5 knockdown cells and was decreased in annexin A5-overexpressing cells. Previous study revealed that auranofin increases annexin A5 expression and translocation into mitochondria to induce apoptosis through oligomerization of VDAC in PC-3 cells (Park and Chun, 2014). Hence, we treated auranofin to induce annexin A5-mediated apoptosis and observed the PAI-2 level resulting in reduction of PAI-2 expression in a concentration-dependent manner. Interestingly, when annexin A5 siRNA was treated into cells, auranofin-induced apoptosis was significantly recovered. However, PAI-2 siRNA promoted apoptosis by auranofin. Furthermore, annexin A5 siRNA restored auranofin-mediated PAI-2 suppression. Thus, our results suggested that induction of annexin A5 by auranofin may augment apoptosis through suppression of PAI-2 expression.
Previous reports suggested that annexin A5 may suppress PKC activity in mesangial cells (Rothhut et al., 1995). Auranofin also inhibits the catalytic activity of PKC (Mahoney et al., 1989). Phorbol 12-myristate 13-acetate (PMA), which is the activator of PKC, induces PAI-2 expression by controlling PAI-2 promoter activity in many cells (Stringer et al., 2012). Therefore, there is a possibility that annexin A5 induction during auranofin-mediated apoptosis inhibits PKC pathway resulting in PAI-2 suppression. To clarify this mechanism, further studies will be required.
In summary, our results reveal that increased annexin A5 during auranofin-mediated apoptosis prevents PAI-2 expression in PC-3 cells, and this mechanism could be a novel pathway to understand annexin A5-mediated apoptosis as well as mitochondria-dependent annexin A5 pathway. Lastly, we suggest that annexin A5 could be considered as an apoptotic factor and targeted for new anticancer therapy.
Acknowledgments
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2016 and National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (NRF-2015R1A2A2A01003865).
REFERENCES
- Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, Heidendal GA, Reutelingsperger CP. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med. 2005;46:2035–2050. [PubMed] [Google Scholar]
- Bohn H, Kraus W. Isolation and characterization of a new placenta specific protein (PP10) (author’s transl) Arch Gynecol. 1979;227:125–134. doi: 10.1007/BF02103286. [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Cardó-Vila M, Arap W, Pasqualini R. ανβ5 integrin-dependent programmed cell death triggered by a peptide mimic of annexin V. Mol. Cell. 2003;11:1151–1162. doi: 10.1016/S1097-2765(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Clinicaltrials.gov. Phase I and II study of auranofin in chronic lymphocytic leukemia (CLL) 2016 Available from: https://clinicaltrials.gov/ct2/show/NCT01419691/.
- Croucher DR, Saunders DN, Lobov S, Ranson M. Revisiting the biological roles of PAI2 (SERPINB2) in cancer. Nat. Rev. Cancer. 2008;8:535–545. doi: 10.1038/nrc2400. [DOI] [PubMed] [Google Scholar]
- Demange P, Voges D, Benz J, Liemann S, Göttig P, Berendes R, Burger A, Huber R. Annexin V: the key to understanding ion selectivity and voltage regulation? Trends Biochem Sci. 1994;19:272–276. doi: 10.1016/0968-0004(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Dickinson JL, Bates EJ, Ferrante A, Antalis TM. Plasminogen activator inhibitor type 2 inhibits tumor necrosis factor α-induced apoptosis. J Biol Chem. 1995;270:27894–27904. doi: 10.1074/jbc.270.46.27894. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh S, Sier CF, Griffioen G, Vloedgraven HJ, de Boer A, Welvaart K, van de Velde CJ, van Krieken JH, Verheijen JH, Lamers CBHW, Verspaget HW. Prognostic relevance of plasminogen activators and their inhibitors in colorectal cancer. Cancer Res. 1994;54:4065–4071. [PubMed] [Google Scholar]
- Han S, Kim K, Kim H, Kwon J, Lee YH, Lee CK, Song Y, Lee SJ, Ha N, Kim K. Auranofin inhibits overproduction of pro-inflammatory cytokines, cyclooxygenase expression and PGE2 production in macrophages. Arch Pharm Res. 2008;31:67–74. doi: 10.1007/s12272-008-1122-9. [DOI] [PubMed] [Google Scholar]
- Hong M, Park N, Chun YJ. Role of annexin a5 on mitochondria-dependent apoptosis induced by tetramethoxystilbene in human breast cancer cells. Biomol. Ther. (Seoul) 2014;22:519–524. doi: 10.4062/biomolther.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.GR-1478R. [DOI] [PubMed] [Google Scholar]
- Jeon KI, Jeong JY, Jue DM. Thiol-reactive metal compounds inhibit NF-κB activation by blocking IκB kinase. J Immunol. 2000;164:5981–5989. doi: 10.4049/jimmunol.164.11.5981. [DOI] [PubMed] [Google Scholar]
- Jeong JJ, Park N, Kwon YJ, Ye DJ, Moon A, Chun YJ. Role of annexin A5 in cisplatin-induced toxicity in renal cells: molecular mechanism of apoptosis. J Biol Chem. 2014;289:2469–2481. doi: 10.1074/jbc.M113.450163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Chen L, Fu HY, Fan K, Yao Q, Ge YF, Lu JC, Yao B. Annexin V-induced rat Leydig cell proliferation involves Ect2 via RhoA/ROCK signaling pathway. Sci Rep. 2015;5:9437. doi: 10.1038/srep09437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean WF, Hart L, Buchanan WW. Auranofin. Br J Rheumatol. 1997;36:560–572. doi: 10.1093/rheumatology/36.5.560. [DOI] [PubMed] [Google Scholar]
- Kim NH, Lee MY, Park SJ, Choi JS, Oh MK, Kim IS. Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology. 2007;122:607–614. doi: 10.1111/j.1365-2567.2007.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- Kruithof EK, Baker MS, Bunn CL. Biological and clinical aspects of plasminogen activator inhibitor type 2. Blood. 1995;86:4007–4024. [PubMed] [Google Scholar]
- Kumar S, Baglioni C. Protection from tumor necrosis factor-mediated cytolysis by overexpression of plasminogen activator inhibitor type-2. J Biol Chem. 1991;266:20960–20964. [PubMed] [Google Scholar]
- Kwon YJ, Jung JJ, Park NH, Ye DJ, Kim D, Moon A, Chun YJ. Annexin A5 as a new potential biomarker for cisplatin-induced toxicity in human kidney epithelial cells. Biomol. Ther. (Seoul) 2013;21:190–195. doi: 10.4062/biomolther.2013.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Hu J, Wu S, Wang L, Cao X, Zhang X, Dai B, Cao M, Shao R, Zhang R, Majidi M, Ji L, Heymach JV, Wang M, Pan S, Minna J, Mehran RJ, Swisher SG, Roth JA, Fang B. Auranofin-mediated inhibition of PI3K/AKT/mTOR axis and anticancer activity in non-small cell lung cancer cells. Oncotarget. 2016;7:3548–3558. doi: 10.18632/oncotarget.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Li X, Huang H, Zhao C, Liao S, Yang C, Liu S, Song W, Lu X, Lan X, Chen X, Yi S, Xu L, Jiang L, Zhao C, Dong X, Zhou P, Li S, Wang S, Shi X, Dou PQ, Wang X, Liu J. Clinically used antirheumatic agent auranofin is a proteasomal deubiquitinase inhibitor and inhibits tumor growth. Oncotarget. 2014;5:5453–5471. doi: 10.18632/oncotarget.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CW, Hensey CE, Azzi A. Auranofin, gold thiomalate, and gold thioglucose inhibit protein kinase C. Biochem Pharmacol. 1989;38:3383–3386. doi: 10.1016/0006-2952(89)90638-2. [DOI] [PubMed] [Google Scholar]
- Marzano C, Gandin V, Folda A, Scutari G, Bindoli A, Rigobello MP. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic Biol Med. 2007;42:872–881. doi: 10.1016/j.freeradbiomed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Medcalf RL, Stasinopoulos SJ. The undecided serpin. The ins and outs of plasminogen activator inhibitor type 2. FEBS J. 2005;272:4858–4867. doi: 10.1111/j.1742-4658.2005.04879.x. [DOI] [PubMed] [Google Scholar]
- Nakaya A, Sagawa M, Muto A, Uchida H, Ikeda Y, Kizaki M. The gold compound auranofin induces apoptosis of human multiple myeloma cells through both down-regulation of STAT3 and inhibition of NF-κB activity. Leuk Res. 2011;35:243–249. doi: 10.1016/j.leukres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Nordengren J, Fredstorp Lidebring M, Bendahl PO, Brünner N, Fernö M, Högberg T, Stephens RW, Wilén R, Casslén B. High tumor tissue concentration of plasminogen activator inhibitor 2 (PAI-2) is an independent marker for shorter progression-free survival in patients with early stage endometrial cancer. Int. J. Cancer. 2002;97:379–385. doi: 10.1002/ijc.1611. [DOI] [PubMed] [Google Scholar]
- Park N, Chun YJ. Auranofin promotes mitochondrial apoptosis by inducing annexin A5 expression and translocation in human prostate cancer cells. J. Toxicol. Environ. Health Part A. 2014;77:1467–1476. doi: 10.1080/15287394.2014.955834. [DOI] [PubMed] [Google Scholar]
- Park SH, Lee JH, Berek JS, Hu MC. Auranofin displays anticancer activity against ovarian cancer cells through FOXO3 activation independent of p53. Int J Oncol. 2014;45:1691–1698. doi: 10.3892/ijo.2014.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutelingsperger CP, Hornstra G, Hemker HC. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur J Biochem. 1985;151:625–629. doi: 10.1111/j.1432-1033.1985.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Rothhut B, Dubois T, Feliers D, Russo-Marie F, Oudinet JP. Inhibitory effect of annexin V on protein kinase C activity in mesangial cell lysates. Eur J Biochem. 1995;232:865–872. doi: 10.1111/j.1432-1033.1995.tb20885.x. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Jockel JI, Domer PH, Mohr RM, Taillon-Miller P, Korsmeyer SJ. Yeast artificial chromosome cloning of a two-megabase-size contig within chromosomal band 18q21 establishes physical linkage between BCL2 and plasminogen activator inhibitor type-2. Genomics. 1991;9:219–228. doi: 10.1016/0888-7543(91)90245-A. [DOI] [PubMed] [Google Scholar]
- Stern I, Wataha JC, Lewis JB, Messer RLW, Lockwood PE, Tseng WY. Anti-rheumatic gold compounds as sub-lethal modulators of monocytic LPS-induced cytokine secretion. Toxicol. In Vitro. 2005;19:365–371. doi: 10.1016/j.tiv.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Stringer B, Udofa EA, Antalis TM. Regulation of the human plasminogen activator inhibitor type 2 gene: cooperation of an upstream silencer and transactivator. J Biol Chem. 2012;287:10579–10589. doi: 10.1074/jbc.M111.318758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- Zhou HM, Bolon I, Nichols A, Wohlwend A, Vassalli JD. Overexpression of plasminogen activator inhibitor type 2 in basal keratinocytes enhances papilloma formation in transgenic mice. Cancer Res. 2001;61:970–976. [PubMed] [Google Scholar]