Abstract
Doxorubicin (DOX) is a highly effective chemotherapeutic agent; however, the dose-dependent cardiotoxicity associated with DOX significantly limits its clinical application. In the present study, we investigated whether Rb1 could prevent DOX-induced apoptosis in H9C2 cells via aryl hydrocarbon receptor (AhR). H9C2 cells were treated with various concentrations (− μM) of Rb1. AhR, CYP1A protein and mRNA expression were quantified with Western blot and real-time PCR analyses. We also evaluated the expression levels of caspase-3 to assess the anti-apoptotic effects of Rb1. Our results showed that Rb1 attenuated DOX-induced cardiomyocytes injury and apoptosis and reduced caspase-3 and caspase-8, but not caspase-9 activity in DOX-treated H9C2 cells. Meanwhile, pre-treatment with Rb1 decreased the expression of caspase-3 and PARP in the protein levels, with no effects on cytochrome c, Bax, and Bcl-2 in DOX-stimulated cells. Rb1 markedly decreased the CYP1A1 and CYP1A2 expression induced by DOX. Furthermore, transfection with AhR siRNA or pre-treatment with AhR antagonist CH-223191 significantly inhibited the ability of Rb1 to decrease the induction of CYP1A, as well as caspase-3 protein levels following stimulation with DOX. In conclusion, these findings indicate that AhR plays an important role in the protection of Ginsenoside Rb1 against DOX-triggered apoptosis of H9C2 cells.
Keywords: Ginsenoside Rb1, Doxorubicin, apoptosis, AhR, CYP1A
INTRODUCTION
Doxorubicin (DOX), an anthracycline chemotherapeutic agent, has been shown to be effective in treating malignant neoplasias. However, the use of DOX is limited by its dose-dependent cardiotoxicity, which may be either acute or chronic, and could ultimately lead to cardiomyopathy following severe heart failure (Wallace, 2003; Octavia et al., 2012). Thus, new drugs that can reduce DOX cardiotoxicity while maintaining and/or enhancing its antitumor activity are still needed. Sustained effort has been made to elucidate the mechanisms of cardiomyopathy induced by DOX. Among these proposed hypotheses, DOX-induced oxidative stress has been considered as the major mechanism responsible for cardiac damage (Minotti et al., 2004). Specifically, increasing evidence suggests that the activation of apoptotic pathways plays an important role in DOX-induced cardiomyopathy, which is caused by DOX-induced oxidative stress (Arola et al., 2000; Takemura and Fujiwara 2007; Yoshida et al., 2009; Zhang et al., 2009). It has also been reported that DOX treatment of cardiomyocytes causes activation of caspase 3, PARP (Xu et al., 2012) and p53 (Volkova et al., 2011) and release of cytochrome c from mitochondrion (Lv et al., 2012). Thus, prevention of cardiomyocyte apoptosis may be an effective therapy for the treatment of DOX-induced cardiotoxicity.
Cytochrome P450 (CYP) constitutes a major family of phase I xenobiotic metabolizing enzymes (XMEs) that transforms xenobiotics to either active or inactive metabolites (Guengerich, 2004). Although CYPs are expressed predominantly in the liver (Davila and Morris, 1999; Elbekai et al., 2004), many CYPs have been found in the right ventricle, coronary artery endothelial cells and cardiac fibroblasts (Elbekai and El-Kadi, 2006), all of which play a significant role in cardiovascular health. Among these CYPs, induction of CYP1A (CYP1A1 and CYP1A2) is considered cardiotoxic through disruption of endogenous substance metabolism, induction of inflammatory-mediated and hypertrophic genes and generation of reactive oxygen species (ROS) (Korashy and El-Kadi, 2006). Moreover, it has been shown that the capacity of Pb2+ and sunitinib to induce cardiac hypertrophy in in vivo and in vitro rat model was associated with induction of CYP1A1 gene (Ansari et al., 2013; Maayah et al., 2014), suggesting a possible role for the CYP1A1 in the pathogenesis of cardiovascular diseases.
Aryl hydrocarbon receptor (AhR) is a ligand-activated transcriptional factor that belongs to the basic-helix-loop-helix (bHLH) family and has the ability to regulate CYP1A. In the absence of ligand, AhR exists primarily in the cytoplasm as an inactive complex with two Hsp90 and p23. Once binding to its ligands such as PAHs, AhR dissociates from the p23 and translocates to the nucleus, where it dissociates from Hsp90 and heterodimerizes with the AhR nuclear translocator (ARNT). The AhR-ARNT complex then binds to the xenobiotic responsive element (XRE), which is located in the promoter region of the receptor regulated genes, such as CYP1A1 and CYP1A2, resulting in transcription and translation of CYP1A genes.
Due to the polycyclic aromatic structure of DOX, it has been demonstrated that DOX can activate AhR and induce the expression of CYP1A1 in ARVM and H9C2 cells (Volkova et al., 2011). Thus, the cardiotoxicity of DOX may be associated with its activation of AhR.
Ginseng has been extensively used as a traditional herbal medication in China and other Asian countries for over 2000 years. The therapeutic effects of ginseng have been mainly attributed to the various ginsenoside compounds within the herb. Among these ginsenosides, ginsenoside Rb1 has been demonstrated to possess cardiovascular benefits. Ginsenoside Rb1 has been reported to decrease cardiac contraction in adult rat ventricular myocytes (Scott et al., 2001) and reduce isoproterenol-induced cardiomyocytes apoptosis in vitro and in vivo (Wang et al., 2013). Moreover, our laboratory previously demonstrated that ginsenoside Rb1 could activate the DNA-binding capacity of AhR for the xenobiotic responsive element of CYP1A1 as assessed by an electrophoretic-mobility shift assay (EMSA) and induce CYP1A1 expression in HepG2 cells (Wang et al., 2008), suggesting that ginsenoside Rb1 may be a potential AhR ligand. Recent studies have further demonstrated the ability of ginsenoside Rb1 to stimulate gene expression in an AhR-dependent manner and confirmed its identity as AhR ligand (Hu et al., 2013). Therefore, ginsenoside Rb1 may be an agonist or (partial) antagonists of AhR, which can compete with other AhR agonists such as DOX for binding to the receptor.
Herein, we hypothesized that ginsenoside Rb1 could protect against DOX-induced apoptosis in H9C2 cells and down-regulate the expression of CYP1A genes, which may be a new strategy to reduce the cardiotoxicity of DOX.
MATERIALS AND METHODS
Materials and reagents
We obtained reagents from the following vendors: ginsenoside Rb1 (HPLC≥98%) from Yuanye Biotechnology Corporation (Shanghai, China); doxorubicin (DOX) from Selleck (Boston, MA, USA); dimethyl sulfoxide (DMSO) and TRIzol Reagent from Sigma-Aldrich Chemicals (St Louis, MO, USA); Dulbecco’s modified Eagle’s medium (DMEM) and Phosphate Buffered Saline (PBS) from Gibco (Grand Island, NY, USA); fetal bovine serum (FBS) from HyClone (South Logan, UT, USA); Cell Counting kit-8 from Dojindo (Dojindo Molecular Technologies, Inc., Kumamoto, Japan); PRL-TK plasmid, Dual-Glo® Luciferase Assay System and Caspase-Glo®3/7,8,9 Assay from Promega (Madison, WI, USA); Cell Death Detection ELISA kit from Roche (Roche Diagnostics Corporation, Indianapolis, IN, USA); Hoechst 33258 from Beyotime Institute of Biotecnology (Shanghai, China); Lipofectamine® 2000 Reagent from Invitrogen (Carlsbad, CA, USA); Human pGL4.17-1A1 luciferase reporter plasmid and Human pcDNA3.1 AhR plasmid were pre-constructed by our laboratory; TransScript® First-Strand cDNA Synthesis Super-Mix and TransStart® Green q-PCR SuperMix from TransGen Biotechnology Corporation (Beijing, China); BCA protein Assay Kit from CW Biotechnology (Beijing, China); polyvinylidene diflouride (PVDF) and enhanced chemiluminescence reagent from Millipore Corporation (Billerica, MA, USA); 2-Methyl-2H-pyrazole-3-carboxylic acid-(2-methyl-4-o-tolylazophenyl)-amide (CH-223191), Ah Receptor siRNA(r) and anti-CYP1A1, anti-CYP1A2, anti-AhR from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-caspase-3, anti-Bax, anti-Bcl-2, anti-PARP and anti-cyt c from Abcam (Cambridge, MA, USA). All pairs of q-PCR primers were synthesized by Biomed Biotechnology (Beijing, China). Other chemicals and reagents were of analytical grade.
Cell culture and treatment
H9C2 cells (Cell Resource Center of Xiehe, Beijing, China) were cultured in DMEM, supplemented with 0.45% glucose, L-glutamine, 0.11% sodium pyruvate, and 10% FBS, at 37°C in a 5% CO2 humidified incubator.
Cells were treated with Rb1 at concentrations of 50, 100, 200, 400 μM or vehicle for 6 h, and then exposed to 1 μM DOX for indicated times. In separate experiments, cells were pre-incubated with CH-223191, an AhR antagonist, 2 h before addition of 200 μM Rb1 and/or 1 μM DOX. The same volumes of corresponding solvents were added to the controls.
Cytotoxicity assays
To measure cytotoxicity, H9C2 cells and MCF-7 breast cancer cells were seeded with culture medium in 96-well microplates (4000 cells/well) respectively and incubated at 37°C for 24 h before drugs exposures. After treatment with DOX or/and Rb1 for indicated times, cell viability was examined with the Cell Counting kit-8 as the manufacturer’s instructions. The absorbance (OD) values were detected at 450 nm with a plate reader. Data are reported as the percentage of cell viability in comparison to that of its respective non-treated control group (100%).
Apoptosis assay
For apoptosis analysis, DNA fragmentation was determined by a Cell Death Detection ELISA kit. H9C2 cells were seeded with culture medium in 6-well plates and cultured at about 80% confluency prior to the indicated treatments. At 24 h after DOX, Rb1 and/or vehicle treatment, the cytoplasmic monoand oligonucleosomes (histone/DNA fragments) were extracted and assayed according to the manufacturer’s instructions. Absorbance was measured at 405 nm using a microplate reader (reference wavelength approx. 490 nm). The specific enrichment of mono- and oligonucleosomes was expressed as enrichment factor and calculated using the following formula: enrichment factor=the ratio of absorbance of the sample: absorbance of the negative control group.
Hoechst 33258 staining
Hoechst 33258 staining was used to identify the morphological features of apoptosis. Briefly, H9C2 cells (5×104/well) were seeded in 6-well plates and cultured 24 h, then treated with DOX, Rb1 or/and vehicle for 24 h. The cells were fixed with 1mL of 4% paraformaldehyde for 20 min. Then, the cells were incubated in 1mL PBS containing 10 μM Hoechst 33258 at 37°C for 30 min. Cells were washed with PBS twice and dead cells and apoptotic bodies were identified by condensed or fragmented nuclei using fluorescence microscopy (Olympus, Tokyo, Japan) at ×200 magnification.
Caspase- 3/7, 8 and 9 activity assessments
The present caspase activity assay used a luminogenic substrate containing the DEVD sequence. Activity of caspase-3/7, 8, 9 was determined using a commercial kit according to the manufacturer’s instructions. Briefly, H9c2 cells were seeded in 96-well plates and cultured 24 h, after 12 h treatments with DOX in the presence or absence of Rb1, caspase reagent was added and incubated for 30 min. The production of light was measured with a luminescence spectrometer LS55 (Perkin-Elmer, CA, USA) at an excitation wavelength of 499 nm and an emission wavelength of 521 nm.
RNA isolation and quantitative real-time PCR
Total RNA of H9C2 cells was isolated after various periods of time incubation with the different treatments using TRIzol reagent according to the manufacturer’s instructions to assess the specific induction of CYP1A1, CYP1A2 and AhR expression. The quality of the RNA was confirmed by an A260/ A280 ratio of >1.8. cDNA synthesis was performed by using the TransScript® First-Strand cDNA Synthesis SuperMix and 1.5 μg of total RNA from each sample was used. Quantitative real-time PCR reactions were performed on an ABI StepOne PlusTM (Foster city, CA, USA) real time PCR instrument using SYBR Green PCR SuperMix. The amplification reactions were performed as follows: 30 s at 94°C, and 40 cycles of 94°C for 5 s and 60°C for 30 s. The primers used in the experiments are listed in Table 1. The data were analyzed as described in the instrument manual using relative gene expression. Briefly, the data are presented as the fold change in gene expression normalized to the endogenous reference gene (GAPDH) and relative to the untreated control.
Table 1.
Primer sequences used for real-time PCR
| Gene | 5′→3′ Forward primer | 5′→3′ Reverse primer | Size(bp) |
|---|---|---|---|
| CYP1A1 | CCAAACGAGTTCCGGCCT | TGCCCAAACCAAAGAGAATGA | 91 |
| CYP1A2 | CGCCCAGAGCGGTTTCTTA | TCCCAAGCCGAAGAGCATC | 81 |
| AhR | CGGCAGATGCCTTGGTCTTCTATGC | TGGAACTCAGCTCGGTCTTCTGTATGG | 124 |
| GAPDH | CAAGGTCATCCATGACAACTTTG | GGGCCATCCACAGTCTTCTG | 90 |
Western blotting
The H9C2 cells were lysed in RIPA buffer at 4–8°C and then the supernatant lysates were collected after centrifugation. The protein concentration was measured by BCA protein Assay Kit. Equal amounts of protein from each sample were loaded on and separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and then electrophoretically transferred to immobilon polyvinylidene diflouride membranes. Protein blots were then blocked with 5% skimmed milk powder in TBST at room temperature for 2 h, followed by incubation with primary antibodies overnight at 4°C. The membranes were incubated with HRP-conjugated secondary antibodies for 1 h. The bands were visualized using the enhanced chemiluminescence reagent and quantified relative to GAPDH bands using ImageJ® (National Institute of Health, MD, USA) image processing program.
Transient transfection and luciferase assay
Human umbilical vein endothelial cells (HUVECs) were cultured in RPMI1640, at 37°C in a 5% CO2 humidified incubator. HUVECs were plated onto 24-well cell culture plates and each well of cells was transfected with pcDNA3.1 AhR plasmid and pGL4.17-1A1 luciferase reporter plasmid plus the Renilla luciferase PRL-TK plasmid, used for normalization. Cells were transfected with 500 ng pcDNA3.1 AhR plasmid and 250 ng pGL4.17-1A1 plasmid plus 50 ng PRL-TK plasmid using Lipofectamine 2000 reagent according to the manufacturer’s instructions. After incubation with test compounds for 24 h, Luciferase assay was performed according to the manufacturer’s instructions (Promega). Briefly, initially firefly luciferase activity was measured with a proper luminometer, and then Renilla luciferase activity was measured. Luciferase activity was reported as light emitted per well, calculating ratio of firefly: Renilla luminescence for each well and normalizing the sample well ratio to the ratio from a control.
Short interfering RNA (siRNA) transfection
The AhR siRNA was a pool of three target-specific 21 nucleotide siRNAs designed to knockdown AhR gene expression. The transfection was performed in 24 wells plates. AhR siRNA and negative control oligonucleotides were transfected using the Lipofectamine 2000 according to the manufacturer’s instructions. The final concentration of the AhR siRNAs was 60 nmol/L. After 6 hours, transfected cells were washed with PBS, and then incubated in new culture media containing 10% FBS. Then cells were incubated for an additional 24 hours before drug treatment.
Statistical analysis
All data are expressed as the mean ± SD and were analyzed using GraphPad Prism 5.01 software (GraphPad software Inc., CA, USA). Comparisons among groups were made by one-way ANOVA. p<0.05 was considered significantly different between groups.
RESULTS
Rb1 increased the viability of DOX-treated H9C2 cells
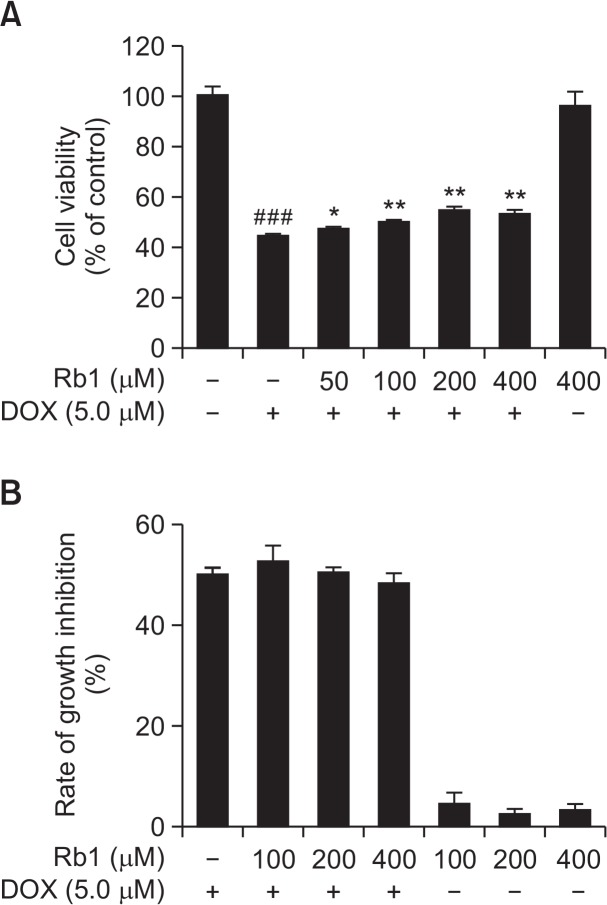
To explore the effect of ginsenoside Rb1 against the cardiotoxic effect of DOX, H9C2 cells were treated with DOX for 24 h or Rb1 (50 μM, 100 μM, 200 μM, and 400 μM) pre-treated 6 h before DOX exposure and CCK-8 assay was performed to measure cell viability. The results showed that 5 μM DOX significantly inhibited the growth of H9C2 cells. Additionally, Rb1 pre-treatment significantly increased the cell viability of DOX-induced cell death of H9C2 cells in a dose-dependent manner (Fig. 1A), with the highest effect seen at 200 μM. Rb1 alone at 400 μM was slightly toxic to cells and still could prevent DOX-induced cytotoxicity. These results suggested that Rb1 could reduce DOX-induced H9C2 cell death. In addition, various concentrations of ginsenoside Rb1 had no significant effect on the antitumor activity of DOX in MCF-7 cells (Fig. 1B).
Fig. 1.
The effect of Rb1 on H9C2 cells viability and MCF-7 cell growth inhibition after DOX treatment. (A) H9C2 cells were treated with Rb1 in the presence or absence of DOX for 24 h (n=3). (B) The effect of Rb1 on growth inhibition of MCF-7 cells treated with DOX for 12 h (n=3). ###p<0.001 compared with control group; *p<0.05, **p<0.01 compared with DOX group.
Rb1 reduced DOX-induced apoptosis in H9C2 cells by ELISA assay
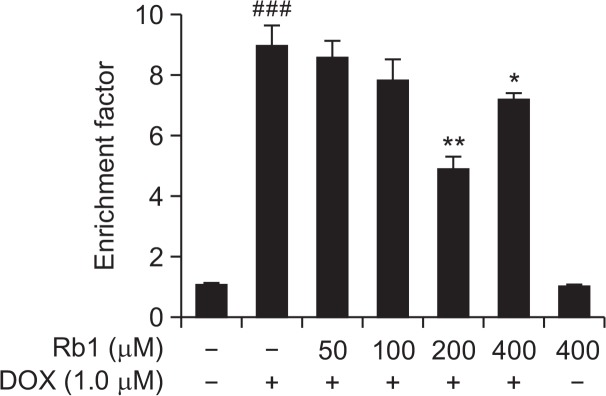
To evaluate the effect of Rb1 on H9C2 cells after DOX treatment, H9C2 cells were pre-treated with 50–400 μM for 6 h and then treated with 1 μM DOX for an additional of 24 h. The positive apoptosis was determined by an ELISA assay to quantify histone-associated DNA fragmentation generated in apoptotic H9C2 cells. We found that 1 μM DOX treatment for 24 h resulted in a noticeable increase in DNA fragmentation and Rb1 treatment reduced DOX-induced DNA fragmentation in H9C2 cells. As shown in Fig. 2, the enrichment factor was significantly higher in the DOX-treated group than in control group and was significantly lower in the Rb1-treated group than in DOX-treated group. These results suggested that Rb1 reduced DOX-induced apoptosis in H9C2 cells.
Fig. 2.
The effect of Rb1 on H9C2 cells apoptosis induced by DOX. H9C2 cells were treated with DOX in the presence or absence of Rb1 for 24 h. Apoptosis was assessed by detecting the cytoplasmic mono- and oligonucleosomes (histone-associated DNA fragments) using a Cell Death Detection ELISA kit. Data was expressed as enrichment factor (n=3). ###p<0.001 compared with control group. *p<0.05, **p<0.01 compared with DOX group.
Rb1 reduced H9C2 cell apoptosis by the morphological observation
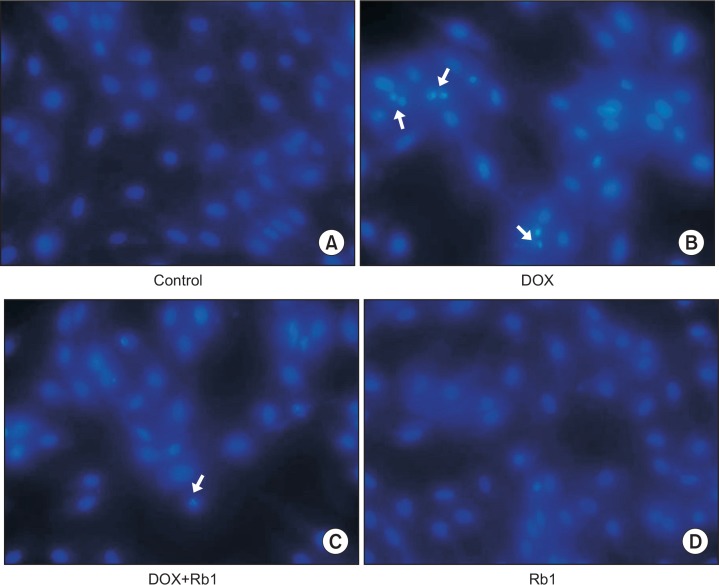
In order to determine the protective effects of Rb1 on DOX-induced apoptosis, Hoechst 33258 staining was used to further confirm the anti-apoptotic effect of Rb1. In the control group and Rb1 treated group, the nuclei were stained a less bright blue and the color was homogeneous (Fig. 3A, 3D). Compared with the control group, DOX treatment resulted in increased apoptosis with obvious changes in heterogeneous intensity, chromatin condensation, and fragmentation under fluorescent microscopy, which are the classic characteristics of apoptotic cells (Fig. 3B). However, when cells were pre-treated with Rb1 6h before DOX treatment, the morphological changes showed less apoptotic characteristics (Fig. 3C).
Fig. 3.
Morphologic changes in H9C2 cells. H9C2 cells were pretreated with Rb1 (200 μM) for 6 h and then treated with 1 μM DOX for 24 h. They were stained with Hoechst 33258 and visualized under fluorescence microscope. Condensed or fragmented nuclei were considered as apoptotic cells (magnification: 200×). (A) Control cells; (B) DOX-treated H9C2 cells; (C) DOX+Rb1-treated H9c2 cells; (D) Rb1-treated H9C2 cells. Arrows indicate apoptotic cells.
Death receptor signaling pathway is involved in inhibitory effect of Rb1 on H9C2 cells apoptosis induced by DOX
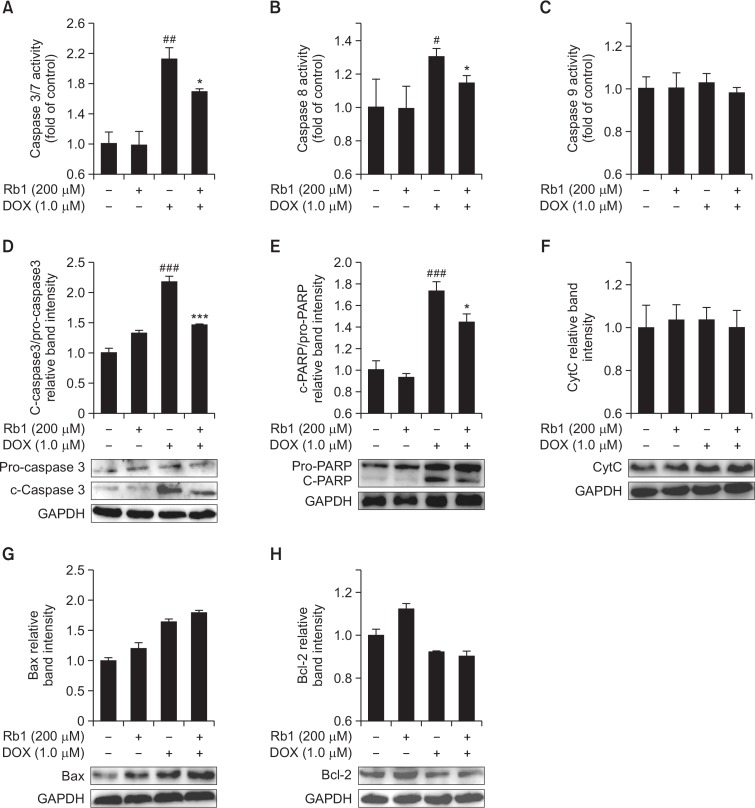
Previous study has shown that DOX-induced apoptosis is a multifactorial process (Zhang et al., 2009). Caspase-3 is an executor of apoptosis and is involved in many important events that lead to the completion of apoptosis. Cleaved caspase-3 and cleaved PARP are markers of caspase-3 activation. The activities of caspases-3/7, 8, 9 and the expression of apoptosis-related proteins including caspase-3, PARP, cytochrome c, Bcl-2 and Bax was determined in DOX-treated H9C2 cells. As shown in Fig. 4, caspase-3/7 and 8 activity were significantly higher in the DOX group than in the control group. After pre-treatment with Rb1, caspases-3/7 and 8 activity was depressed markedly in DOX+Rb1 group compared with DOX group (Fig. 4A, 4B). However, the activity of caspase-9 had no obvious change after DOX and/or Rb1 stimulation (Fig. 4C). Moreover, cleaved caspase-3 and cleaved PARP protein levels were significantly increased in DOX group compared with control, and they were attenuated by Rb1 treatment in DOX-treated H9C2 cells (Fig. 4D, 4E). However, cytochrome c level had no evident changes after DOX and/or Rb1 treatment (Fig. 4F). Meanwhile, DOX increased Bax expression and reduced Bcl-2 expression in H9C2 cells, which were not restored to normal levels by Rb1 pretreatment (Fig. 4G, 4H).
Fig. 4.
Effect of Rb1 on caspase activities, caspase 3, PARP, cytochrome c, Bax, Bcl-2 protein level in DOXtreated H9C2 cells. (A, B and C) H9C2 cells were treated with DOX (5 μM) in the presence or absence of Rb1 (200 μM) for 12 h, caspase-3/7, 8 and 9 activity were analyzed with a plate reader (n=3). (D, E, F, G and H) H9C2 cells were treated with DOX (1 μM) in the presence or absence of Rb1 (200 μM) for 12 h, levels of caspase 3, PARP, cytochrome c, Bax, Bcl-2 proteins were determined by Western blotting (n=3). #p<0.05, ##p<0.01, ###p<0.001 compared with control group. *p<0.05, ***p<0.001 compared with DOX group.
Effect of Rb1 on DOX-mediated induction of AhR-regulated genes in H9C2 cells
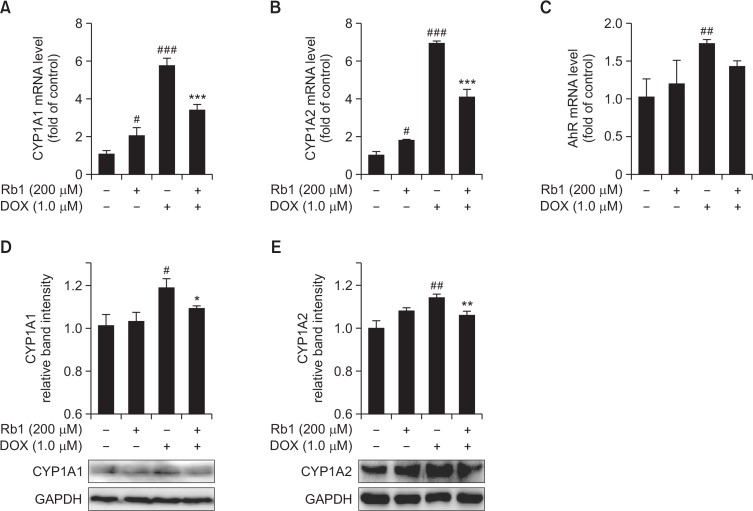
DOX is a polyaromatic hydrocarbon. It was previously reported that treatment with DOX induced AhR migration to the nucleus, increased AhR binding with its co-factor, aryl hydrocarbon receptor nuclear translocator-1 (ARNT1), and increased the expression of AhR-regulated phase I (CYP1A1) drug-metabolizing enzymes in H9C2 cells (Volkova et al., 2011), that are known to play roles in heart disease (Elbekai and El-Kadi, 2006; Korashy and El-Kadi, 2006). It was also reported that ginsenosides are novel naturally-occurring AhR ligands (Hu et al., 2013) and ginsenoside Rb1 induced CYP1A1 expression in HepG2 cells (Wang et al., 2008). In our study, we examined whether Rb1 could reduce DOX-mediated induction of AhR-regulated genes in H9C2 cells through competing with DOX for the ligation of AhR. Therefore, H9c2 cells were treated with DOX (1 μM) in the absence or presence of Rb1 (200 μM) for 12 h, and the mRNA expression levels of CYP1A1, CYP1A2, and AhR was measured by RT-PCR, and protein expression levels of CYP1A1 and CYP1A2 was measured by Western blot. As shown in Fig. 5, DOX alone significantly induced the expression of CYP1A1, CYP1A2, and AhR genes. However, pre-treatment with Rb1 significantly blocked the induction of CYP1A1, CYP1A2, AhR mRNA and CYP1A1, CYP1A2 protein in response to DOX.
Fig. 5.
Effect of Rb1 on CYP1A1, CYP1A2 and AhR mRNA and protein level in DOX-treated H9C2 cells. H9C2 cells were pretreated for 6h with 200 μM Rb1 prior to co-exposure with 1 μM DOX for additional 12 h, then CYP1A1, CYP1A2 and AhR mRNA level was determined by RT-PCR (A–C); CYP1A1 and CYP1A2 protein level was determined by Western blotting (D, E) (n=3). #p<0.05, ##p<0.01, ###p<0.001 compared with control group; *p<0.05, **p<0.01, ***p<0.001 compared with DOX group.
Effects of Rb1 on CYP1A1 luciferase reporter activity in the presence and absence of DOX
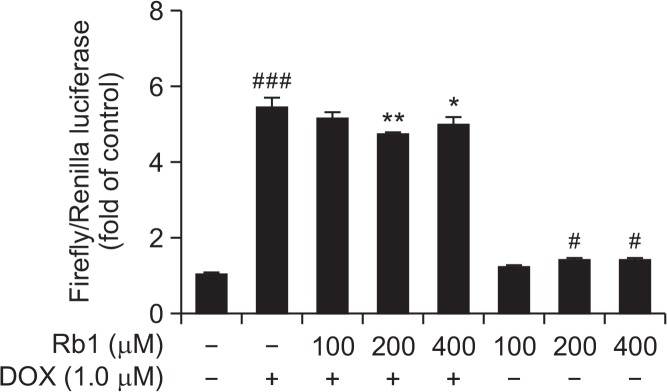
To determine whether the observed effects upon exposure to Rb1 in the presence and absence of DOX on CYP1A mRNA and protein were via an AhR-dependent mechanism, HUVECs were transiently cotransfected with AhR expression plasmid, CYP1A1 luciferase reporter plasmid and its normalizing control construct, Renilla luciferase. Luciferase activity results showed that 1 μM DOX alone significantly induced XRE-driven luciferase activity by 5.45- fold, meanwhile, 100, 200, 400 μM Rb1 alone induced luciferase activity by 1.18-, 1.36-, and 1.37-fold, respectively, compared to control. On the other hand, co-exposure to 200 or 400 μM Rb1 and DOX significantly decreased luciferase reporter activity to 4.73-, and 4.97-fold, compared to DOX group (Fig. 6).
Fig. 6.
Effects of Rb1 and DOX on CYP1A1 luciferase reporter activity. HUVECs were transiently transfected with pcDNA3.1 AhR plasmid, pGL4.17-1A1 luciferase reporter plasmid and PRL-TK plasmid for 6 h, and thereafter the cells were treated with Rb1 in the presence and absence of DOX for 24 h. Cells were lysed and luciferase activity was measured according to the manufacturer’s instructions. Luciferase activity was reported as relative light units (n=3). #p<0.05, ###p<0.001 compared with control group; *p<0.05, **p<0.01 compared with DOX group.
The role of AhR in protection of Rb1 against DOX-induced apoptosis
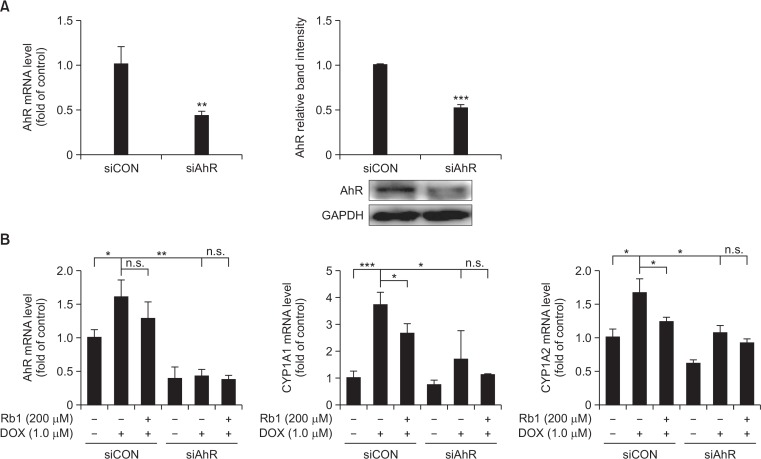
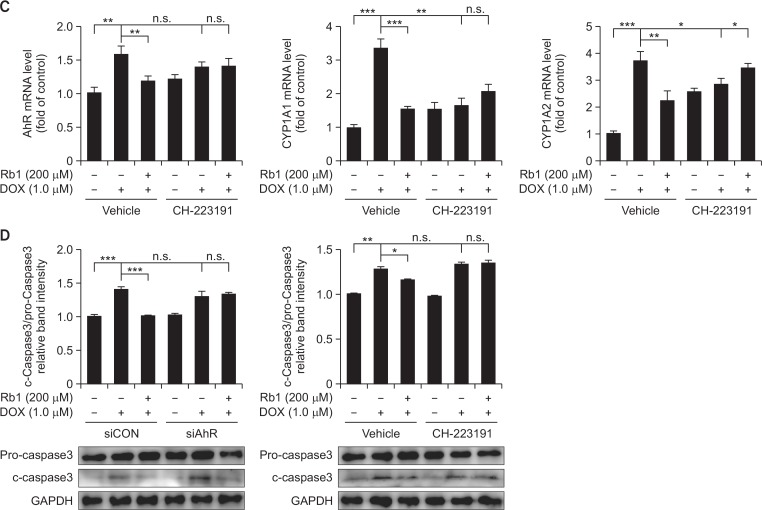
To further examine whether AhR was directly involved in the effect of Rb1 on DOX-mediated induction of AhR downstream target genes in H9C2 cells, we knocked down AhR expression with siRNA. H9C2 cells were transfected with a control siRNA (siCON) or a siRNA against AhR (siAhR), and then cultured 24 h before drug treatment. RT-PCR and western blot analysis showed that siAhR dramatically reduced AhR mRNA and protein levels (Fig. 7A), indicating the effectiveness of the siRNA-mediated AhR gene silencing. Knockdown of AhR resulted in a decrease in the expression of CYP1A1 and CYP1A2 under basal conditions. Furthermore, the inhibition of AhR expression blocked DOX-induced up regulation of CYP1A1, CYP1A2 and AhR mRNA, and the effect of Rb1 on DOX-mediated induction of CYP1A1, CYP1A2 and AhR mRNA was significantly blocked compared with siCON group (Fig. 7B). In addition, pretreatment with CH-223191 (10 μM) for 2 h, an AhR antagonist, increased the basal expression of CYP1A1, CYP1A2 and AhR, an effect that has been previously described in ARVM that may be due to ligand-dependent effects of CH-223191 (Volkova et al., 2011). However, addition of CH-223191 inhibited the expression of CYP1A1, CYP1A2 and AhR in response to DOX treatment compared with vehicle group, and the addition of Rb1 failed to exert an additive effect (Fig. 7C). We next determined whether the effect of Rb1 on DOX-induced apoptosis in H9C2 cells was an AhR-dependent mechanism. It was noteworthy that after AhR knockdown, DOX-induced apoptosis was not additionally reduced by the treatment with Rb1 as evidenced by c-caspase 3 levels. Similarly, pre-treatment of H9C2 cells with CH-223191 abolished the effect of Rb1 (Fig. 7D). These results raise the possibility that the cardioprotective effects of Rb1 may be mediated through AhR/CYP1A inhibition.
Fig. 7.
The role of AhR in protection of Rb1 against DOX-induced apoptosis. H9C2 cells were transfected with a control siRNA or a siRNA against AhR, the expression of AhR was determined by RT-PCR and Western blot (A). Transfected cells were treated with DOX in the presence or absence of Rb1 and the mRNA expression of CYP1A1, CYP1A2 and AhR were determined (B). H9C2 cells were pre-treated with vehicle or CH-223191, and then treated with DOX in the presence or absence of Rb1, the mRNA expression of CYP1A1, CYP1A2 and AhR were determined (C). Caspase-3 protein level was determined after AhR knockdown and CH-223191 pre-treatment (D). *p<0.05; **p<0.01; ***p<0.001, n.s., not significant.
DISCUSSION
DOX-induced cardiomyopathy is a major obstacle that limits the clinical application of doxorubicin in cancer chemotherapy. To mitigate this hurdle, recent studies have focused on identifying agents that can protect against cardiac dysfunction. In the present study, using H9C2 cells, which is the most popular cell model used to carry out cardiovascular research (Shi et al., 2009), we demonstrated that ginsenoside Rb1 protects against DOX-induced cardiomyocyte apoptosis.
Ginseng, as a natural product, has been shown to exert potential benefits on cardiovascular diseases. Ginsenoside Rb1, as a compound of ginseng, has been reported to reduce isoproterenol-induced cardiomyocytes apoptosis in vitro and in vivo (Wang et al., 2013) and protect against ischaemia/reperfusion injury (Pasupathy and Homer-Vanniasinkam, 2005). In the present study, we examined the effect of Rb1 on cultured H9C2 cells injury caused by DOX and found that Rb1 significantly reduced cell damage in cells exposed to DOX in a dose-dependent manner as evidenced by cytotoxic activity assay (Fig. 1A). Interestingly, Rb1 at doses of 100, 200 and 400 μM did not significantly affect the viability of breast cancer MCF-7 cells treated with 5 μM DOX. These findings suggest that Rb1 could protect against cardiotoxicity without impairing the anti-tumor efficiency produced by DOX. The DNA fragmentation assay also showed the effect of Rb1 against DOX-induced apoptosis and Rb1 at 200 μM was the most efficacious (Fig. 2). It has demonstrated that the intrinsic and extrinsic pathways are involved in DOX-induced cardiomyocyte apoptosis (Zhang et al., 2009). Thus, we examined the effects of Rb1 on the activities of caspase-3/7, caspase-8 and caspase-9 in H9C2 cells treated with DOX. The results showed that Rb1 at 200 μM significantly decreased caspase-3/7 and caspase-8 activities, but not caspase-9 activity in cells stimulated with DOX (Fig. 4A, 4B, 4C). The results indicated that Rb1 attenuated H9C2 cells apoptosis caused by DOX might be associated with the death receptor signaling pathway. We also examined the protein expression of caspase-3, PARP, cytochrome c, Bax and Bcl-2. Rb1 treatment attenuated the increase in cleaved caspase-3 and cleaved PARP levels in H9C2 cells stimulated by DOX (Fig. 4D, 4E). PARP plays an important role in the repair of DNA damage but loses its activity once it was cleaved by active caspase-3, accelerating the process of apoptosis. These results were consistent with the previous enzyme activity assay. It has been demonstrated that Bax can promote cytochrome c release from the mitochondria to induce apoptosis; however, Bcl-2 can suppress apoptosis progression through inhibiting mitochondria cytochrome c release (Green and Reed, 1998; Childs et al., 2002). The release of cytochrome c results in the activation of caspase-9, which in turn further increases activation of caspase-3. In this study, we found that DOX markedly increased Bax and decreased Bcl-2 expression while Rb1 treatment had no effects on these molecules. Cytochrome c expression also did not change in the presence of Rb1after DOX treatment (Fig. 4F, 4G, 4H). These results were consistent with the activity of caspase-9 and further highlighted the possibility that mechanisms underlying Rb1 attenuated DOX-induced H9C2 cells apoptosis might be associated with the death receptor signaling pathway.
In the current study, the inhibition of DOX-induced H9C2 cells apoptosis by ginsenoside Rb1 was associated with proportional decrease in the mRNA and protein levels of the CYP1A1 and CYP1A2 genes regulated by AhR (Fig. 5), indicating an AhR-dependent mechanism. These results were consistent with previous studies, which showed that TCDD-induced CYP1A1 expression, an index of dioxin toxicity, was suppressed by flavonoids permeating the human intestinal Caco-2 cell monolayers (Hamada et al., 2006). The role of AhR and its regulated genes in the pathogenesis of cardiovascular diseases has been previously reported. For example, the mortality analysis of workers routinely exposed to TCDD showed a significant trend for the development of heart disease, cancer, and diabetes (Steenland et al., 1999). In addition, studies on TCDD exposure to medaka fish embryos showed that TCDD induced vascular hemorrhage and blood clotting. However, the vascular damage could be suppressed by AhR antagonist or CYP inhibitor, further underscoring the role of AhR and CYPs in cardiovascular diseases (Kawamura and Yamashita, 2002). Although there are many studies which have examined the effects of AhR ligands, particularly TCDD, on the cardiovascular system, the mechanisms underlying the cardiotoxic effect of TCDD has not yet been explained definitively. Generally, three main mechanisms are postulated, including disruption of the metabolism of endogenous substances, modulation of genes expression and oxidative stress, which result in endothelial cell dysfunction and apoptosis of the myocytes (Korashy and El-Kadi, 2006). Many reports have suggested that TCDD can induce apoptosis. It was particularly noteworthy that mice deficient for Fas expression were less sensitive to TCDD-mediated thymic atrophy than Fas-positive counterparts. Likewise, Fas ligand-defective mice were refractory to TCDD-induced thymic atrophy (Rhile et al., 1996; Kamath et al., 1999). These observations suggested that the AhR plays a role in Fas-mediated apoptosis.
Benzo(a)pyrene and 3-methylcholanthrene, as an AhR ligand, induced CYP1A1 and CYP1B1 expression and caused cardiac hypertrophy in SD rats (Aboutabl et al., 2009), whereas benzo(e)pyrene, an isomer of benzo(a)pyrene, was a poor ligand for the AhR and failed to induce cardiac hypertrophy. Furthermore, resveratrol which is a naturally occurring polyphenolic compound significantly restored the hypertrophic genes mRNA expression induced by sunitinib by blocking of the AhR, suggesting a cardioprotective effect (Maayah et al., 2014). Therefore, we hypothesized that Rb1 was a weaker AhR agonist than DOX and it inhibited DOX-induced H9C2 cells apoptosis through competing with DOX for AhR affinity to decrease the expression of CYP1A genes. To test our hypothesis, we examined the capacity of Rb1 to decrease the CYP1A genes expression after DOX treatment in rat cardiomyocyte cell line. We found that treatment of H9C2 cells with DOX caused serious cell damage while significantly induced the expression of mRNA and protein of AhR-regulated CYP1A1 and CYP1A2 genes, whereas the induction was markedly restored by Rb1 pretreatment. These results may indicate the involvement of the AhR/CYP1A in Rb1 prevention of DOX-induced cardiotoxicity.
The activity of CYP1A is an index of dioxin toxicity. There is published evidence supporting the role of CYP1A1 in TCDD toxicities that TCDD-induced weight loss, liver pathology, and wasting syndrome were alleviated in CYP1A1–/– mice (Uno et al., 2004). CYP1A2 also plays a major role in enhancing uroporphyrinogen oxidation consistent with TCDD-induced uroporphyria. New considerations suggest that CYP1A functions via metabolism of endogenous chemicals. For example, the membrane lipid Arachidonic Acid (AA) is known as an endogenous substrate for CYP1A enzymes and the main HETEs metabolites, such as 20-HETEs, were associated with responses to hyperoxia and vasoconstriction that may result in hypertension and adverse effects in cardiac ischemia and cerebral (Su et al., 1998; Kehl et al., 2002; Roman, 2002). Eicosapentanoic acids contained in fish oils is an endogenous CYP1A substrate and the products have similar effects on vasoreactivity and ion channels compared with AA CYP products (Schwarz et al., 2004). In addition, human CYP1A1 and CYP1A2 preferentially metabolize 17-β estradiol to 2-hydroxyestradiol (Aoyama et al., 1990; Tsuchiya et al., 2005) and CYP1B1 primarily generated 4-Hydroxy-estradiol (Hayes et al., 1996). It is considered that both 4 and 2-hydroxy estradiol can induce ROS generation and contribute to DNA damage and Carcinogenesis (Lavigne et al., 2001; Chen et al., 2004). These highlighted observations further demonstrate that inhibition of DOX-induced apoptosis by Rb1 may be associated with decreasing the induction of CYP1A.
In order to verify whether the effects of ginsenoside Rb1 were mediated by the AhR, we examined the effects of Rb1 and DOX on CYP1A1 luciferase reporter activity. The results showed that both DOX and Rb1 were capable of enhancing the interaction between AhR and CYP1A1 promoter region and DOX induction was more significant than Rb1; however, different concentrations of Rb1 could attenuate the effects of different levels of DOX (Fig. 6). It further confirms our conjecture that Rb1 is a weaker AhR agonist than DOX, and Rb1 can inhibit AhR agonist activity of DOX. Interestingly, Rb1 at 200 μM was the most efficacious concentration and this was consistent with the results of cytotoxic activity and DNA fragmentation assays. This may be due to Rb1 being a partial agonist; full agonist in combination could produce antagonism by competing for binding and depending on both drug doses and achieving the efficacy of partial agonists, thus Rb1 at 200 μM may be the most appropriate dose. In addition, ginsenoside Rb1 can also induce CYP1A1 expression in a dose-dependent manner and 400 μM Rb1 may have slight toxicity. The direct evidence for the involvement of AhR in the capability of Rb1 decreasing the induction of CYP1A induced by DOX was supported by knockdown of AhR using AhR siRNA or blocking of AhR using CH-223191, a novel AhR antagonist, both of which significantly abolished the previous effects of Rb1 (Fig. 7B, 7C). Importantly, the abolished effects of Rb1 on CYP1A expression were accompanied with the same effect on the protein expression of caspase-3 (Fig. 7D). These results suggested that the mechanism was AhR dependent. Based on data Fig. 7D, DOX-mediated caspase3 activation is independent of AhR because DOX induced caspase3 activation even in siAhR or AhR antagonist treated cells. But in the condition of siAhR or AhR antagonist, the increased caspase3 activation by DOX was not reversed with Rg1 treated. Such results indicated that the toxicity of DOX is only partly related with AhR. Ginsenoside Rb1 inhibited DOX-induced cells apoptosis may through, at least in part, competing with DOX for AhR to decrease the CYP1A induction.
In conclusion, to the best of our knowledge, the present study is the first report that clearly demonstrates that ginsenoside Rb1 inhibits doxorubicin-mediated H9C2 cells apoptosis is associated with the aryl hydrocarbon receptor signaling pathway. However, these findings remain to be confirmed in vivo pre-clinical and clinical studies.
Acknowledgments
This study was supported by National Natural Science Foundation of China (grant 81073149), and National Basic Research Program of China (“973 Program”) (grant 2012CB518402).
REFERENCES
- Aboutabl ME, Zordoky BN, El-Kadi AO. 3-methylcholanthrene and benzo(A)pyrene modulate cardiac cytochrome P450 gene expression and arachidonic acid metabolism in male Sprague Dawley rats. Br J Pharmacol. 2009;158:1808–1819. doi: 10.1111/j.1476-5381.2009.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Maayah ZH, Bakheet SA, El-Kadi AO, Korashy HM. The role of aryl hydrocarbon receptor signaling pathway in cardiotoxicity of acute lead intoxication in vivo and in vitro rat model. Toxicology. 2013;306:40–49. doi: 10.1016/j.tox.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Korzekwa K, Nagata K, Gillette J, Gelboin HV, Gonzalez FJ. Estradiol metabolism by complementary deoxyribonucleic acid-expressed human cytochrome P450s. Endocrinology. 1990;126:3101–3106. doi: 10.1210/endo-126-6-3101. [DOI] [PubMed] [Google Scholar]
- Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. [PubMed] [Google Scholar]
- Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, Kang KS, Cho MH, Surh YJ. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25:2005–2013. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- Davila JC, Morris DL. Analysis of cytochrome P450 and phase II conjugating enzyme expression in adult male rat hepatocytes. In Vitro Cell Dev Biol Anim. 1999;35:120–130. doi: 10.1007/s11626-999-0013-9. [DOI] [PubMed] [Google Scholar]
- Elbekai RH, El-Kadi AO. Cytochrome P450 enzymes: Central players in cardiovascular health and disease. Pharmacol Ther. 2006;112:564–587. doi: 10.1016/j.pharmthera.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Elbekai RH, Korashy HM, El-Kadi AO. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab. 2004;2:157–167. doi: 10.2174/1389200043489054. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome P450: what have we learned and what are the future issues? Drug Metab Rev. 2004;36:159–197. doi: 10.1081/DMR-120033996. [DOI] [PubMed] [Google Scholar]
- Hamada M, Satsu H, Natsume Y, Nishiumi S, Fukuda I, Ashida H, Shimizu M. TCDD-induced CYP1A1 expression, an index of dioxin toxicity, is suppressed by flavonoids permeating the human intestinal Caco-2 cell monolayers. J Agric Food Chem. 2006;54:8891–8898. doi: 10.1021/jf060944t. [DOI] [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, He G, Zhao J, Soshilov A, Denison MS, Zhang A, Yin H, Fraccalvieri D, Bonati L, Xie Q, Zhao B. Ginsenosides are novel naturally-occurring aryl hydrocarbon receptor ligands. PLoS ONE. 2013;8:e66258. doi: 10.1371/journal.pone.0066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AB, Camacho I, Nagarkatti PS, Nagarkatti M. Role of Fas-Fas ligand interactions in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced immunotoxicity: increased resistance of thymocytes from Fas-deficient (lpr) and Fas ligand-defective (gld) mice to TCDD-induced toxicity. Toxicol Appl Pharmacol. 1999;160:141–155. doi: 10.1006/taap.1999.8753. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Yamashita I. Aryl hydrocarbon receptor is required for prevention of blood clotting and for the development of vasculature and bone in the embryos of medaka fish, Oryzias latipes. Zool Sci. 2002;19:309–319. doi: 10.2108/zsj.19.309. [DOI] [PubMed] [Google Scholar]
- Kehl F, Cambj-Sapunar L, Maier KG, Miyata N, Kametani S, Okamoto H, Hudetz AG, Schulte ML, Zagorac D, Harder DR, Roman RJ. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H1556–H1565. doi: 10.1152/ajpheart.00924.2001. [DOI] [PubMed] [Google Scholar]
- Korashy HM, El-Kadi AO. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev. 2006;38:411–450. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- Lavigne JA, Goodman JE, Fonong T, Odwin S, He P, Roberts DW, Yager JD. The effects of catechol-O-methyltransferase inhibition on estrogen metabolite and oxidative DNA damage levels in estradiol-treated MCF-7 cells. Cancer Res. 2001;61:7488–7494. [PubMed] [Google Scholar]
- Lv X, Yu X, Wang Y, Wang F, Li H, Wang Y, Lu D, Qi R, Wang H. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS ONE. 2012;7:e47351. doi: 10.1371/journal.pone.0047351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayah ZH, Ansari MA, El Gendy MA, Al-Arifi MN, Korashy HM. Development of cardiac hypertrophy by sunitinib in vivo and in vitro rat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway. Arch Toxicol. 2014;88:725–738. doi: 10.1007/s00204-013-1159-5. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Pasupathy S, Homer-Vanniasinkam S. Ischaemic preconditioning protects against ischaemia/reperfusion injury: emerging concepts. Eur J Vasc Endovasc Surg. 2005;29:106–115. doi: 10.1016/j.ejvs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Rhile MJ, Nagarkatti M, Nagarkatti PS. Role of Fas apoptosis and MHC genes in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced immunotoxicity of T cells. Toxicology. 1996;110:153–167. doi: 10.1016/0300-483X(96)83962-X. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Ericksen SS, Szklarz GD, Chernogolov A, Honeck H, Schunck WH, Roots I. Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol. 2004;67:1445–1457. doi: 10.1016/j.bcp.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Scott GI, Colligan PB, Ren BH, Ren J. Ginsenosides Rb-1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmacol. 2001;134:1159–1165. doi: 10.1038/sj.bjp.0704377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Huang CC, Aronstam RS, Ercal N, Martin A, Huang YW. N-acetylcysteine amide decreases oxidative stress but not cell death induced by doxorubicin in H9c2 cardiomyocytes. BMC Pharmacol. 2009;9:7. doi: 10.1186/1471-2210-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Piacitelli L, Deddens J, Fingerhut M, Chang LI. Cancer, heart disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Natl Cancer Inst. 1999;91:779–786. doi: 10.1093/jnci/91.9.779. [DOI] [PubMed] [Google Scholar]
- Su P, Kaushal KM, Kroetz DL. Inhibition of renal arachidonic acid omega-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol. 1998;275:R426–R438. doi: 10.1152/ajpregu.1998.275.2.R426. [DOI] [PubMed] [Google Scholar]
- Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Sinclair PR, Gorman N, Wang B, Smith AG, Miller ML, Shertzer HG, Nebert DW. Cyp1a1(−/−) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196:410–421. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Volkova M, Palmeri M, Russell KS, Russell RR. Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovasc Res. 2011;90:305–314. doi: 10.1093/cvr/cvr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KB. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol Toxicol. 2003;93:105–115. doi: 10.1034/j.1600-0773.2003.930301.x. [DOI] [PubMed] [Google Scholar]
- Wang XF, Liu XJ, Zhou QM, Du J, Zhang TL, Lu YY, Su SB. Ginsenoside rb1 reduces isoproterenol-induced cardiomyocytes apoptosis in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2013;2013:454389. doi: 10.1155/2013/454389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ye X, Ma Z, Liang Q, Lu B, Tan H, Xiao C, Zhang B, Gao Y. Induction of cytochrome P450 1A1 expression by ginsenoside Rg1 and Rb1 in HepG2 cells. Eur J Pharmacol. 2008;601:73–78. doi: 10.1016/j.ejphar.2008.10.057. [DOI] [PubMed] [Google Scholar]
- Xu X, Chen K, Kobayashi S, Timm D, Liang Q. Resveratrol attenuates doxorubicin-induced cardiomyocyte death via inhibition of p70 S6 kinase 1-mediated autophagy. J Pharmacol Exp Ther. 2012;341:183–195. doi: 10.1124/jpet.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Shiojima I, Ikeda H, Komuro I. Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J Mol Cell Cardiol. 2009;47:698–705. doi: 10.1016/j.yjmcc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. Ther. Exp. (Warsz.) 2009;57:435–445. doi: 10.1007/s00005-009-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]