Abstract
Baicalein, a natural flavonoid obtained from the rhizome of Scutellaria baicalensis Georgi, has been reported to have anticancer activities in several human cancer cell lines. However, its antimetastatic effects and associated mechanisms in melanoma cells have not been extensively studied. The current study examined the effects of baicalein on cell motility and anti-invasive activity using mouse melanoma B16F10 cells. Within the noncytotoxic concentration range, baicalein significantly inhibited the cell motility and invasiveness of B16F10 cells in a concentration-dependent manner. Baicalein also reduced the activity and expression of matrix metalloproteinase (MMP)-2 and -9; however, the levels of tissue inhibitor of metalloproteinase-1 and -2 were concomitantly increased. The inhibitory effects of baicalein on cell motility and invasiveness were found to be associated with its tightening of tight junction (TJ), which was demonstrated by an increase in transepithelial electrical resistance and downregulation of the claudin family of proteins. Additionally, treatment with baicalein markedly reduced the expression levels of lipopolysaccharide-induced phosphorylated Akt and the invasive activity in B16F10 cells. Taken together, these results suggest that baicalein inhibits B16F10 melanoma cell migration and invasion by reducing the expression of MMPs and tightening TJ through the suppression of claudin expression, possibly in association with a suppression of the phosphoinositide 3-kinase/Akt signaling pathway.
Keywords: Baicalein, Migration, Invasion, PI3K/Akt, B16F10 Mouse Melanoma Cells
INTRODUCTION
Melanoma is a cancer consisting of pigment-forming melanocytes of the skin, which are normally found in the basal layer of the epidermis (Fernandez, 2015). Melanoma is less common than other types of skin cancer; however, it causes the majority of skin-cancer-related deaths. Melanoma is an aggressive cancer with a high propensity for metastasis, and metastatic melanoma is characterized by a high mortality rate (Leiter et al., 2014; Rogiers et al., 2015). Although surgery, chemotherapy, radiotherapy, and combinations of these therapies are standard options for the treatment of melanoma (Kakavand et al., 2016), the cure rate is still unsatisfactory (Fernandez, 2015; Foth et al., 2016). Therefore, there is an urgent need to find new compounds or novel approaches that can block or inhibit metastatic capacity and thus ultimately reduce melanoma-related deaths.
Metastasis has been widely recognized as a primary cause of cancer-related mortality. Metastasis is a highly coordinated multistep process, of which degradation of the extracellular matrix (ECM) is an important component (Khasigov et al., 2003; Hanahan and Weinberg, 2011). Through degradation of the ECM, cancer cells escape the primary tumor, penetrate the basement membrane of capillary and lymphatic vessels, intravasate, and migrate to new locations in the body (Yilmaz et al., 2007; Valastyan and Weinberg, 2011). Matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases, are crucial molecules in the degradation of ECM and affect the invasion of cancer cells (Khasigov et al., 2003; Kessenbrock et al., 2010). Among the MMPs, MMP-2 (gelatinase A) and -9 (gelatinase B) play pivotal roles in the process of ECM degradation (Vu and Werb, 2000; Egeblad and Werb, 2002).
Metastasis is also regulated by the cellular signaling pathways, which have been implicated in a number of cellular functions, including cell survival, adhesion, and invasion. The phosphatidylinositide-3 kinase (PI3K)/Akt signaling pathway plays an especially important role in the invasion of cancer cells via regulation of the expression of MMP-2 and -9 by transcriptional factors, including nuclear-factor-kappaB (NF-κB) (Park et al., 2012; Li et al., 2014). Moreover, NF-κB has been implicated in many key elements of cancer development, including growth-factor-independent proliferation, prevention of apoptosis, and cancer cell metastasis (Naugler and Karin, 2008; Batra et al., 2011).
In addition, tight junction (TJ) represents one mode of cell-to-cell adhesion in endothelial cells. TJ establishes contact between adjacent cells, which serves as a physical barrier to the maintenance of cell polarity (Schneeberger and Lynch, 2004). Because TJ becomes disorganized or lost while normal epithelial cells are transforming into cancerous cells, the alteration and loss of cell polarity are hallmarks of tumorigenesis in epithelial cells (Soler et al., 1999). Claudins are major integral membrane proteins that form the backbone of TJ (Angelow and Yu, 2007; Singh et al., 2010). Recent studies have provided evidence that claudins are frequently overexpressed in various cancers and are associated with the development and progression of cancer (Tsukita and Furuse, 2000; Morin, 2005), which suggests that they have key cellular functions that are distinct from their roles in TJ complexes.
Baicalein (5,6,7-trihydroxyflavone) is a flavonoid originally isolated from the rhizome of Scutellaria baicalensis Georgi (Nagai et al., 1989; Li-Weber, 2009), which has been widely used for treating various inflammatory diseases, chronic hepatitis, bacterial and viral infections, allergies, and ischemia in traditional Oriental medicine (Huang et al., 2005; Li et al., 2011). Baicalein has been shown to inhibit cell growth and induce apoptosis in many human cancer cells including melanoma cancer (Lee et al., 2005; Kim et al., 2012; Li et al., 2013; Wang et al., 2014; Liu-Smith and Meyskens, 2016; Mu et al., 2016). Although it has also been reported that baicalein reduces prostate, lung, breast, and liver cancer as well as osteosarcoma cell metastasis in vitro and/or in vivo (Wang et al., 2010; Chen et al., 2013; Zhang et al., 2013; Gong et al., 2014; Guo et al., 2015), to the best of our knowledge, no studies have investigated the antimetastatic effect of baicalein in melanoma cells. Therefore, this study investigated the effects of baicalein on the migration and invasion of mouse melanoma B16F10 cells as well as the underlying mechanisms of this process.
MATERIALS AND METHODS
Reagents and antibodies
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotics were purchased from Welgene (Daegu, Korea). Baicalein, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium (MTT), hematoxylin and eosin (H&E), and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). Antibodies against claudin-1 (519000), -2 (516100), -3 (341700), and -4 (329400) were obtained from Calbiochem (San Diego, CA, USA). Akt (sc-8312), phosphorylated (p)-Akt (sc-101629), tissue inhibitors of metalloproteinase (TIMP)-1 (sc-5538) and TIMP-2 (sc-5539), MMP-2 (sc-10736) and MMP-9 (sc-10737), and β-actin (sc-1616) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Peroxidase-labeled donkey anti-rabbit and sheep anti-mouse immunoglobulin were purchased from Amersham Corp. (Arlington Heights, IL, USA). All other chemicals were purchased from Sigma-Aldrich Chemical Co.
Cell culture
B16F10 mouse melanoma cells were obtained from the American Type Culture Collection (Manassas, MD, USA) and maintained in DMEM supplemented with 10% heat-inactivated FBS and antibiotics (100 μg/ml streptomycin and 100 U/ml penicillin) at 37°C in a humidified incubator under an atmosphere of 5% CO2 in air. Baicalein was dissolved in dimethyl sulfoxide (DMSO) as a stock solution at 100 mM, which was then diluted with RPMI1640 medium to the desired concentration prior to use.
Cell viability assay
Cell survival was assessed by performing a standard MTT assay. Briefly, B16F10 cells were plated in 6-well culture plates at a density of 2×105 cells per well. After 24 h, the cells were treated with various concentrations of baicalein for 24 h. Next, the cells were washed twice with phosphate buffered saline (PBS) and incubated with 0.5 mg/ml MTT solution for 3 h. The cells were subsequently washed with PBS and solubilized in DMSO, and the optical density of each well was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 540 nm. The effect of baicalein on cell growth was assessed as the percentage of cell viability, in which the vehicle-treated cells were considered 100% viable.
Colony formation assay
B16F10 cells were harvested after treatment with or without baicalein for 24 h. The cells were reseeded in each new 6-well plate at a density of 2×102 with complete medium for 15 days. The medium was replaced every 3 days to maintain the cells’ growth. The colonies were then washed twice with PBS, fixed with 3.7% paraformaldehyde, and stained with 0.1% toluidine blue for 30 min at room temperature.
Wound healing assay
B16F10 cells were grown to confluence on 6-well plates coated with 20 μg/ml of rat tail collagen (BD Biosciences, Bedford, MA, USA). Confluent cells were wounded by scraping with a 200 μl pipette tip. After wounding, the cells were washed twice with PBS and incubated with 1% FBS-containing medium supplemented with various concentrations of baicalein for 24 h. Thereafter, the wounding areas were observed and photographed under an inverted microscope (Carl Zeiss, Deisenhofen, Germany) at 40x magnification.
In vitro invasion assay
The in vitro invasion assay was performed using the Transwell chamber system (10 mm diameter, 8 μm pore size with polycarbonate membrane, Corning Costar Corp., Cambridge, MA, USA). For this assay, B16F10 cells were kept for 24 h in serum-free DMEM medium and collected. The cells were placed in the upper chamber of the transwell insert (5×104 cells/well), baicalein was added to the well at the final concentrations of 0, 10, 20, and 30 μM, and DMEM containing 10% FBS was added to the lower chamber. The plates were incubated at 37°C in a humidified atmosphere with 95% air and 5% CO2 for 24 h; noninvasive cells in the upper chamber were removed, and invasive cells in the bottom were fixed with 4% formaldehyde in PBS and stained with H&E. The cells were then were counted and photographed under an inverted microscope. Finally, rates of invasion were measured at 560 nm wavelength by an ELISA reader.
Gelatin zymography assay
The activities of MMP-2 and -9 were determined by gelatin zymography. Briefly, after treatment with baicalein for 24 h, the media were collected and clarified by centrifugation at 400 g for 5 min at 4°C to remove cells and debris. The cell-free supernatant was mixed with a 2X nonreducing sample buffer (Invitrogen Co., Carlsbad, CA, USA), and electrophoresis was performed using precast gel (10% polyacrylamide and 0.1% gelatin as a protease substrate) (Invitrogen Co.). Following electrophoresis, the gel was washed twice in 2.5% Triton X-100 for 1 h to remove sodium dodecyl sulfate (SDS), subsequently washed in a buffer containing 50 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, 1 μM ZnCl2, and 0.02% NaN3 at pH7.5, and incubated in this buffer at 37°C for 24 h. Thereafter, the gel was stained for 1 h with 0.5% (w/v) Coomassie brilliant blue G-250 (Bio-Rad Laboratories, Hercules, CA, USA) and then lightly destained in methanol:acetic acid:water (3:1:6). The areas of gelatinolytic activity were then manifested as horizontal white bands on a blue background (Lee et al., 2015).
Measurement of transepithelial electrical resistance
Transepithelial electrical resistance (TER), a measure of TJ formation, was measured with an Epithelial Tissue Volt-Ohm meter (EVOM; World Precision Instruments, Sarasota, FL, USA) equipped with a pair of STX-2 chopstick electrodes. Briefly, B16F10 cells were seeded in the 8.0 μm pore size insert (upper chamber) of a Transwell® (Corning Costar Corp.) and allowed to reach full confluence, after which fresh medium was substituted for further experiments. Inserts without cells, inserts with cells in medium, and inserts with cells with baicalein were treated with baicalein for 24 h. Electrodes were placed at the upper and lower chambers, and resistance was measured with the EVOM.
RNA extraction and reverse transcription-polymerase chain reaction (PCR)
Total RNA was isolated from cells using an RNeasy kit (Qiagen, La Jolla, CA, USA) and primed with random hexamers to synthesize complementary DNA using AMV Reverse Transcriptase (Amersham Corp.). The PCR was carried out in a Mastercycler (Eppendorf, Hamburg, Germany) using primers (Table 1). Conditions for the PCR reactions were 1× (94°C for 3 min), 35× (94°C for 45 s; 58°C for 45 s; and 72°C for 1 min), and 1× (72°C for 10 min). Amplification products obtained by PCR were electrophoretically separated on a 1% agarose gel and visualized by ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA) staining.
Table 1.
Sequence of primers used for RT-PCR
| Gene name | Sequence | |
|---|---|---|
| GAPDH | Sense | 5′-CGG AGT CAA CGG ATT TGG TCG TAT-3′ |
| Antisense | 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′ | |
| MMP-2 | Sense | 5′-CTT CTT CAA GGA CCG GTT CAT-3′ |
| Antisense | 5′-GCT GGC TGA GTA GAT CCA GTA-3′ | |
| MMP-9 | Sense | 5′-TGG GCT ACG TGA CCT ATG ACC AT-3′ |
| Antisense | 5′-GCC CAG CCC ACC TCC ACT CCT C-3′ | |
| TIMP-1 | Sense | 5′-TGG GGA CAC CAG AAG TCA AC-3′ |
| Antisense | 5′-TTT TCA GAG CCT TGG AGG AG-3′ | |
| TIMP-2 | Sense | 5′-GTC AGT GAG AAG GAA GTG GAC TCT-3′ |
| Antisense | 5′-ATG TTC TTC TCT GTG ACC CAG TC-3′ | |
| Claudin-1 | Sense | 5′-TCA GCA CTG CCC TGC CCC AGT-3′ |
| Antisense | 5′-TGG TGT TGG GTA AGA GGT TGT-3′ | |
| Claudin-2 | Sense | 5′-ACA CAC AGC ACA GGC ATC AC-3′ |
| Antisense | 5′-TCT CCA ATC TCA AAT TTC ATG C-3′ | |
| Claudin-3 | Sense | 5′-AAG GCC AAG ATC ACC ATC GTG-3′ |
| Antisense | 5′-AGA CGT AGT CCT TGC GGT CGT-3′ | |
| Claudin-4 | Sense | 5′-TGG ATG AAC TGC GTG GTG CAG-3′ |
| Antisense | 5′-GAG GCG GCC CAG CCG ACG TA-3′ | |
Western blot analysis
Following treatment with various concentrations of baicalein, the cells were washed with ice-cold PBS and harvested and lysed with a lysis buffer (20 mM sucrose, 1 mM ethylendiaminetetraacetic acid, 20 μM Tris-Cl, pH 7.2, 1 mM dithiothreitol, 10 mM KCl, 1.5 mM MgCl2, 5 μg/ml pepstatin A, 10 μg/ml leupeptin, and 2 μg/ml aprotinin) for 30 min at 4°C. The supernatants were collected and protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories) according to the manufacturer’s instructions. For Western blotting, equal amounts of protein extracts were denatured by boiling at 95°C for 5 min in a sample buffer (0.5 M Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.1% bromophenol blue, and 10% β-mercaptoethanol) at a ratio of 1:1. Samples were stored at −80°C or immediately used for immunoblotting. The equal cellular proteins were separated by denaturing SDS-polyacrylamide gel electrophoresis and transferred electrophoretically to nitrocellulose membranes (Amersham Corp.). The membranes were then blocked with 5% skim milk and incubated overnight at 4°C with primary antibodies, probed with enzyme-linked secondary antibodies for 1 h at room temperature, and detected using an enhanced chemiluminescence (ECL) detection system.
Statistical analysis
All data are presented as mean ± standard deviation (SD). Significant differences among the groups were determined using the unpaired Student’s t-test. A value of p<0.05 was accepted as an indication of statistical significance. All the figures shown herein were obtained from at least three independent experiments.
RESULTS
Baicalein inhibits the cell growth of B16F10 cells
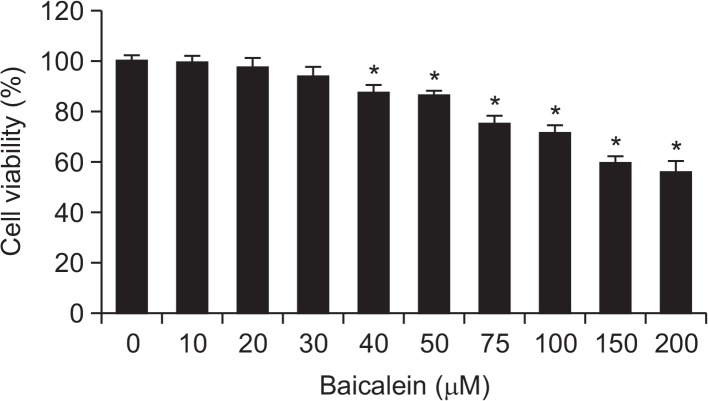
In order to investigate the effect of baicalein on the viability of B16F10 cells, cells were treated with various concentrations of baicalein for 24 h and subjected to an MTT assay. When compared with the untreated control, treatment with 40 μM baicalein resulted in approximately 87% inhibition of cell growth (Fig. 1). However, cell viabilities were not significantly changed by baicalein at concentrations below 40 μM; therefore, a concentration range of baicalein <30 μM was selected for the subsequent experiments.
Fig. 1.
Effects of baicalein on cell viability in B16F10 cells. Cells were treated with increasing concentrations of baicalein, and after 24 h their viabilities were determined using an MTT assay. Values represent the mean ± SD of three independent experiments performed in triplicate (*p<0.05 vs. control group).
Baicalein suppresses the colony formation of B16F10 cells
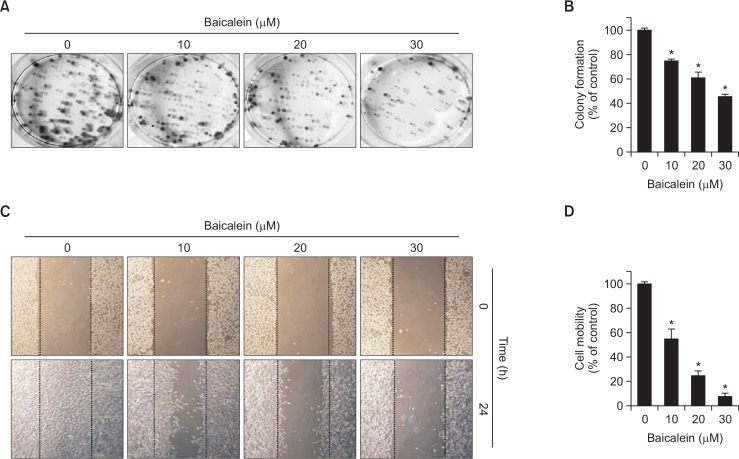
Next, we investigated whether baicalein, at noncytotoxic concentrations, influences anchorage-dependent colony formation of B16F10 cells after seeding at a low density. As shown in Fig. 2A, 2B, untreated control B16F10 cells showed rapid proliferation and formed sizable colonies from a single cell. However, baicalein treatment during incubation suppressed the colony-forming activity and reduced the number of sizable colonies and colony sizes in a dose-dependent manner. These data indicate that baicalein blocks the establishment of anchorage-dependent colonies from a single cell at the same level as growth-independent anchorage.
Fig. 2.
Baicalein inhibits colony formation suppresses motility of B16F10 cells. (A, B) For the colony-forming assay, control cells and baicalein-treated cells were seeded in 6-well plates at a density of 2×102 and cultured at 37°C in a CO2 incubator. (A) After 15 days, cells were fixed with 3.7% paraformaldehyde for 30 min at room temperature and stained with 0.1% toluidine blue. (B) Rates of colony formation were measured at 650 nm wavelength by an ELISA reader. (C, D) Cells were kept on 6-well plates for 24 h before a wound was created by a yellow pipette tip to scrape the confluent cell layers. Baicalein at various concentrations was added to the wells, and cells were then incubated for 24 h. (C) Some of the representative photographs of invading treated and untreated cells are presented. (D) Percentage of inhibition of cell migration was quantified by manual counting, and six randomly chosen fields were analyzed for each well. Each point represents the mean ± SD of three independent experiments (*p<0.05 vs. control group).
Baicalein inhibits the cell migration of B16F10 cells
To investigate the inhibitory effect of baicalein on the migration of B16F10 cells, the confluent monolayer was scraped to create a scratch wound, and the cells migrated to the denuded zone and the levels of wound closure area were analyzed after 24 h incubation with baicalein. As shown in Fig. 2C, 2D, the results indicate that treatment with baicalein significantly reduces migration of B16F10 cells to the denuded zone by comparison with control cells in a concentration-dependent fashion, indicating that baicalein inhibits the motility of B16F10 cells.
Baicalein inhibits the cell invasion of B16F10 cells
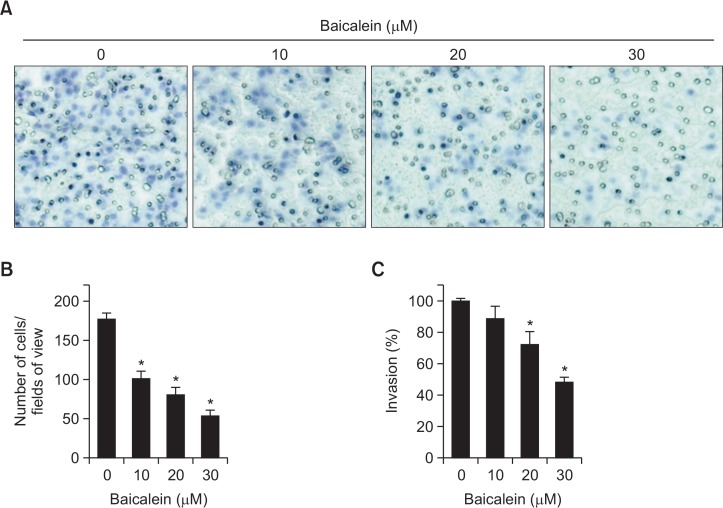
In addition, we attempted to determine whether the inhibitory effects of baicalein on cell migration are connected to the decreases in B16F10 cell invasion using a Matrigel invasion assay. The results, which are consistent with those of the wound-healing assay, demonstrate that baicalein significantly inhibits the invasion of B16F10 cells and that these effects are dose dependent (Fig. 3).
Fig. 3.
Baicalein inhibits invasion of B16F10 cells. Cells pretreated with the indicated concentrations of baicalein for 24 h were plated onto the apical side of Matrigel-coated filters in serum-free medium containing either vehicle or baicalein. Medium containing 10% FBS was placed in the basolateral chamber to act as a chemoattractant. After 24 h, cells on the apical side were wiped off using a Q-tip. Next, (A) cells on the bottom of the filter were stained using H&E, and then (B) counted. (C) Rates of invasion were measured at 560 nm wavelength by an ELISA reader. Results are in mean ± SD values obtained from three independent experiments (*p<0.05 vs. control group).
Baicalein suppresses the activity and expression of MMP-2 and -9 in B16F10 cells
Because the activation of MMP-2 and -9 is crucial for ECM degradation, which is required for tumor invasion and metastasis (Vu and Werb, 2000; Egeblad and Werb, 2002), gelatin zymography, RT-PCR and Western blot analyses were conducted to assess whether baicalein regulates activation and expression of MMP-2 and -9 in B16F10 cells. As indicated in Fig. 4A, baicalein inhibited the activities of MMP-2 and -9, and that the effects occur in a dose-dependent manner (Fig. 4B, 4C). In contrast, baicalein treatment concentration-dependently increased the levels of TIMP-1 and -2 mRNA and protein compared with the control group (Fig. 4B, 4C). These results indicate that the anti-invasive effect of baicalein is associated with increased TIMPs levels as well as inhibition of both enzyme activities and expressions of MMP-2 and -9 in B16F10 cells.
Fig. 4.
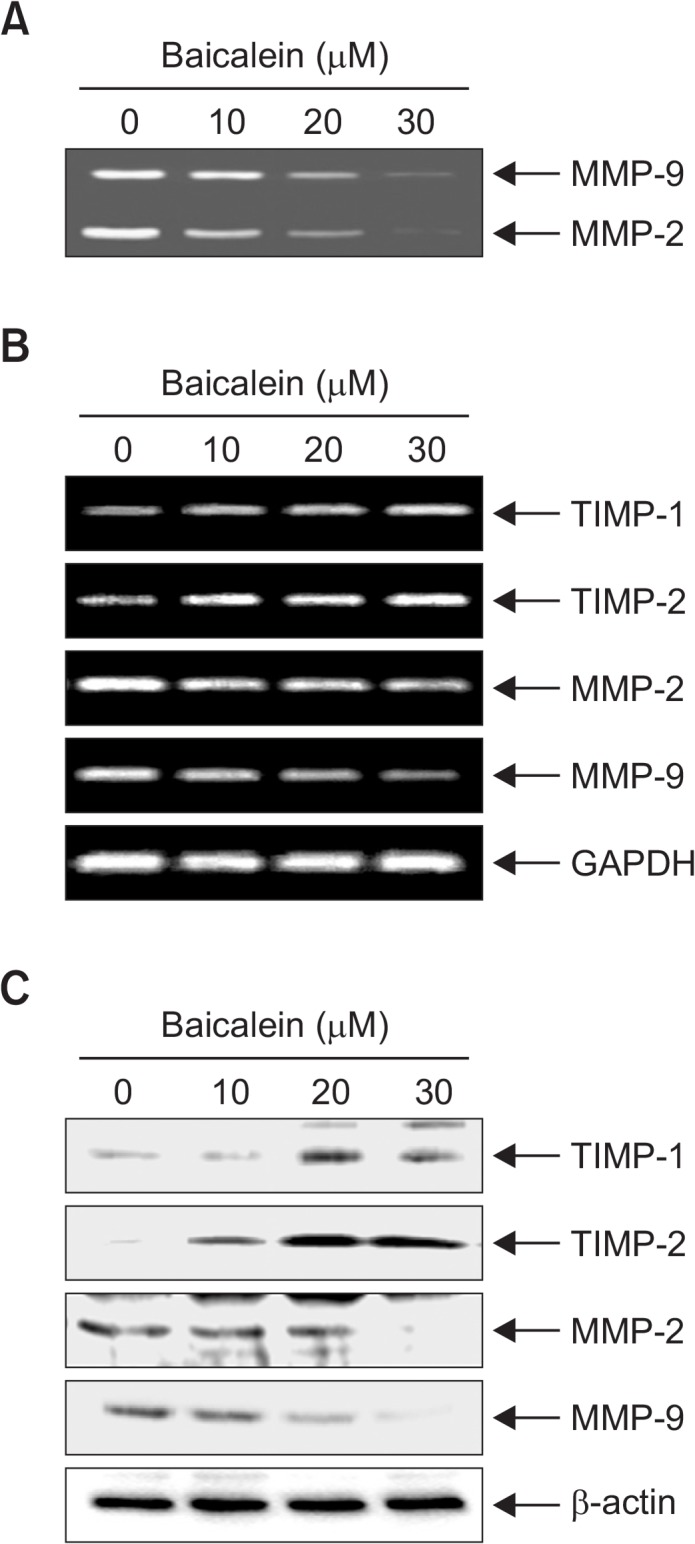
Baicalein suppresses activities and levels of MMP-2 and -9 and increase levels of TIMP-1 and -2 in B16F10 cells. (A) Cells were treated with the indicated concentrations of baicalein for 24 h. The medium was collected, and activities of MMP-2 and -9 were measured by zymography. (B) Total RNA was isolated from cells grown under the same conditions as (A) and reverse-transcribed. Resulting cDNAs were then subjected to the polymerase chain reaction. The reaction products were run on 1% agarose gel electrophoresis and visualized by ethidium bromide staining. GAPDH was used as the internal control. (C) Cells were sampled and lysed, and 30 μg of proteins were separated by electrophoresis on SDS-polyacrylamide gels. Western blotting was then performed using the indicated antibodies and an ECL detection system. β-actin was used as an internal control.
Baicalein increases TER values in B16F10 cells
Because altered TJ leads to a decrease in resistance to electrical current (as measured by TER) and an increase in paracellular permeability (Soler et al., 2004; Utech et al., 2006), the TER values were measured to determine the interaction between the tightening of TJ and the anti-invasive activity of baicalein. The results shown in Fig. 5A indicate that TER values were substantially increased in response to increasing concentrations of baicalein, suggesting that baicalein increases the tightening of TJs in B16F10 cells. To elucidate the mechanism by which baicalein enhances TJ activity and reduces invasive activity, we determined the levels of claudins, which are TJ regulators, using RT-PCR and Western blot analyses. As shown in Fig. 5B, 5C, the levels of claudins (claudin-1, -2, -3, and -4) were dose-dependently downregulated in baicalein-treated cells, suggesting that this modulation contributes to TJ tightening.
Fig. 5.
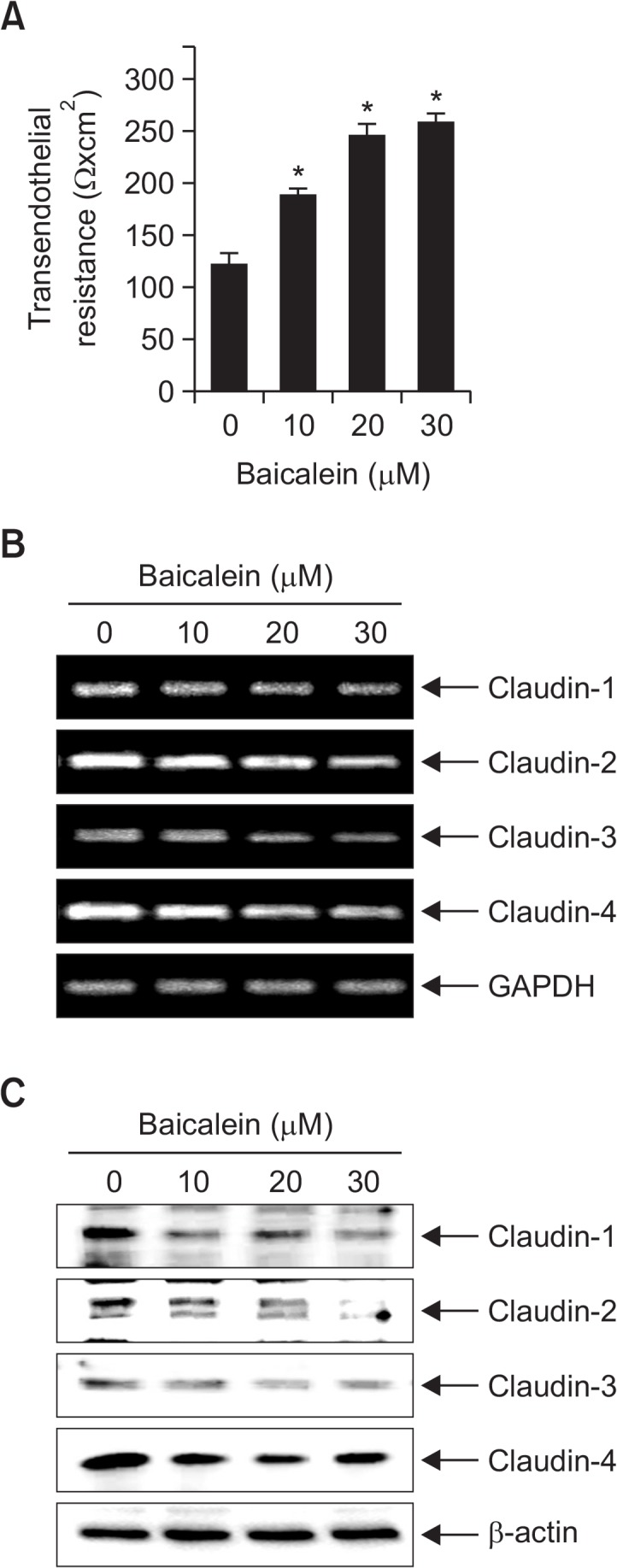
Effects of baicalein on TER values and expression of claudins in B16F10 cells. (A) Cells were plated onto transwells and grown in media, and baicalein was then added to both the apical and basolateral compartments in triplicate. TER values were measured using an EVOM. Each point represents the mean ± SD of three independent experiments (*p<0.05 vs. control group). (B) Total RNA was isolated from cells grown under the same conditions as (A) and reverse-transcribed. Resulting cDNAs were then subjected to the polymerase chain reaction. The reaction products were run on 1% agarose gel electrophoresis and visualized by ethidium bromide staining. GAPDH was used as the internal control. (C) Cells were sampled and lysed, and 30 μg of proteins were subjected to Western blot analyses using the indicated antibodies and an ECL detection system. β-actin was used as an internal control.
Baicalein inhibits phosphorylation of Akt in B16F10 cells
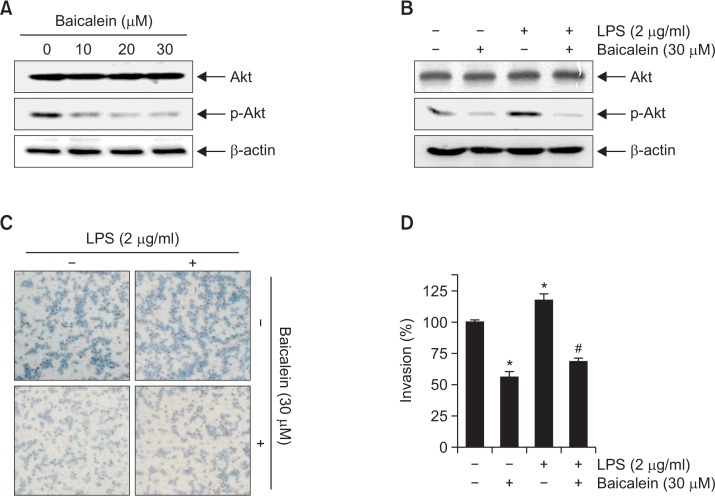
Because several lines of evidence have implicated the PI3K/Akt signaling pathway in the expression of MMPs and induction of cancer cell metastasis (Park et al., 2012; Li et al., 2014), we assessed the changes in the phosphorylation of Akt, a downstream of PI3K, after baicalein treatment. Western blotting indicated that treatment with baicalein significantly reduced the relative expression levels of p-Akt in a concentration-dependent manner, but the levels of total Akt protein remained unchanged (Fig. 6A).
Fig. 6.
Baicalein attenuates LPS-induced Akt phosphorylation and cell invasion in B16F10 cells. Cells were treated with the indicated concentrations of baicalein for 24 h (A) or incubated with 30 μM baicalein for 1 h before treatment with 2 μg/ml LPS for 24 h (B). Cellular proteins were separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. Immunoblotting analyses were performed with anti-Akt and anti-p-Akt antibodies and an ECL detection system. β-actin was used as an internal control. (C, D) Cells treated with 30 μM baicalein for 1 h before being challenged with LPS (2 μg/ml) for 24 h were plated onto the apical side of Matrigel-coated filters in serum-free medium containing either vehicle or baicalein. Medium containing 10% FBS was placed in the basolateral chamber to act as a chemoattractant. After 24 h, cells on the apical side were wiped off using a Q-tip. Next, cells on the bottom of the filter were stained using H&E, and rates of invasion were then measured at 560 nm wavelength by an ELISA reader. The results are in mean ± SD values obtained from three independent experiments (*p<0.05 vs. control group; #p<0.05 vs. baicalein-treated group).
The endotoxin LPS, a major component of the outer membrane in gram negative bacteria, is one of the most potent activators of the PI3K/Akt signaling pathway for the stimulation of cancer cell metastasis, and its activation mechanism is relatively well established (Hsu et al., 2011; O’Leary et al., 2012). Accordingly, we evaluated whether baicalein regulates LPS-induced activation of the PI3K/Akt signaling pathway. As indicated in Fig. 6B, stimulation with LPS significantly induced Akt phosphorylation; however, pretreatment with baicalein abolished the phosphorylation of Akt. The results of parallel experiments show that incubation with baicalein pretreatment inhibits LPS-induced invasion of B16F10 cells (Fig. 6C, 6D).
DISCUSSION
Baicalein is known to exhibit anticancer effects in various types of cancer cells (Lee et al., 2005; Kim et al., 2012; Li et al., 2013; Wang et al., 2014; Liu-Smith and Meyskens, 2016; Mu et al., 2016). Although it has been reported that this flavonoid inhibits migration and invasion in several cancer cell lines in vitro (Wang et al., 2010; Chen et al., 2013; Zhang et al., 2013; Gong et al., 2014; Guo et al., 2015), its antimetastatic activity and associated mechanisms in melanoma cells remains unclear. Therefore, we investigated the underlying mechanisms of this phenomenon, and we found that baicalein significantly reduces the invasive and metastatic ability of murine melanoma B16F10 cells.
Metastasis, the main cause of death in patients with cancer, is a complex multistep process involving cell adhesion, invasion, and migration. MMPs that are highly expressed in various malignant tumors have been recognized as playing an important role in cell motility and invasion via the degradation of ECM components of blood or lymph vessels (Khasigov et al., 2003; Hanahan and Weinberg, 2011). Gelatinase types of MMPs such as MMP-2 and -9 promote tumor cell invasion in various cancer cell lines because of their ability to degrade various types of collagens (Vu and Werb, 2000; Egeblad and Werb, 2002). It has also been reported that MMP activity is tightly controlled by TIMPs, which form complexes with MMPs for inhibiting the active form of enzymes (Khasigov et al., 2003; Kessenbrock et al., 2010). To elucidate the mechanism of the antimetastatic effect of baicalein, we identified the activities of MMP-2 and -9 in B16F10 cells treated with baicalein. The results indicate that baicalein significantly inhibits the activities of both MMP-2 and -9, as determined by a gelatin zymography assay (Fig. 4A). Our results also show that baicalein markedly inhibits the expression of MMP-2 and -9 mRNA and protein; however, the those levels of both TIMP-1 and -2 exhibit concentration-dependent upregulation in response to baicalein treatment (Fig. 4B, 4C). Therefore, the results indicate that baicalein promotes an increase in the TIMPs/MMPs ratio as a key factor in the regulation of the antimetastatic process, which may subsequently block the breakdown of the ECM and lead to the inhibition of cell invasion.
On the other hand, there is an association between the loss of cell-cell adhesion structures and metastasis in many cancers. In precancerous lesions, the tissue remodeling performed by the disassembled and disorganized TJs as determined by decreased resistance to TER causes a loss of cell polarity and in turn promotes cancer cell motility and invasiveness (Soler et al., 1999; Utech et al., 2006). The components of TJs have been well identified, in particular those of the claudin family, which include transmembrane proteins and their extracellular domains. The claudin family of proteins forms the backbone of TJs, which are directly implicated in the barrier and adhesive functions of cells to regulate paracellular permeability (Tsukita and Furuse, 2000; Morin, 2005). Several reports have shown that the TER in some tumor tissues is significantly lower than that of normal tissue and concurrently that the transepithelial paracellular permeability is higher than that in normal tissue, confirming the loss of TJ function (Soler et al., 1999; Tsukita and Furuse, 2000; Morin, 2005). In particular, recent studies have provided evidence that claudins are aberrantly overexpressed in various types of human cancers, including melanoma, and are associated with the development and progression of cancer metastasis (Saeki et al., 2010; Izraely et al., 2015). These observations suggest that claudin proteins may be useful biomarkers for the detection and diagnosis of certain cancers. Therefore, we measured TER values to examine the relationship between TJ remodeling and the anti-invasive activity of baicalein, and we found that incubating B16F10 cells with baicalein concentration-dependently increases TER (Fig. 5A). In addition, baicalein treatment markedly suppressed the levels of claudins (-1, -2, -3 and -4) (Fig. 5B, 5C), indicating that downregulation of claudin expression by baicalein is associated with increased tightening of TJ in B16F10 cells.
By regulating the transcriptional activity of MMPs, numerous cell-signaling pathways play a critical role in the regulation of cancer cell migration and invasion (Park et al., 2012; Li et al., 2014). For example, activation of the PI3K/Akt signaling pathway is associated with the expression of MMP-2 and -9 in cancerous tissues, by which cancer cells promote neovascularization for invasion and metastasis (Dilly et al., 2013; Liu et al., 2015). To this end, we investigated whether the anti-invasive effects of baicalein are associated with inactivation of the PI3K/Akt signaling pathway, and we found that baicalein itself significantly reduces the phosphrylation of Akt (Fig. 6A). In addition, stimulation with LPS significantly induced Akt phosphorylation; however, pretreatment with baicalein downregulated the phosphorylation of Akt (Fig. 6B). A Matrigel assay showed that baicalein significantly suppresses LPS-induced B16F10 cell invasion. These results indicate that baicalein inhibits LPS-induced activation of the PI3K/Akt signaling pathway and may subsequently lead to the suppression of B16F10 cell migration and invasion.
In summary, the study found that baicalein significantly inhibits migration and invasion in B16F10 melanoma cells by suppressing MMP-2 and -9 expression and activity. The study’s data also show that baicalein increases TJ tightening associated with the downregulation of claudin expression, which is associated with inactivation of the PI3K/Akt signaling pathway. Thus, the findings of the present study indicate that baicalein is a potential candidate for the development of chemotherapeutic treatments for melanoma.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (2015R1A2A2A01004633) and the Functional Districts of the Science Belt support program, Ministry of Science, ICT and Future Planning.
REFERENCES
- Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens. 2007;16:459–464. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- Batra S, Balamayooran G, Sahoo MK. Nuclear factor-κB: a key regulator in health and disease of lungs. Arch. Immunol. Ther. Exp. (Warsz.) 2011;59:335–351. doi: 10.1007/s00005-011-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Zhang S, Ji Y, Li J, An P, Ren H, Liang R, Yang J, Li Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS ONE. 2013;8:e72927. doi: 10.1371/journal.pone.0072927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilly AK, Ekambaram P, Guo Y, Cai Y, Tucker SC, Fridman R, Kandouz M, Honn KV. Platelet-type 12-lipoxygenase induces MMP9 expression and cellular invasion via activation of PI3K/Akt/NF-κB. Int. J. Cancer. 2013;133:1784–1791. doi: 10.1002/ijc.28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Fernandez A. Dermatology update: The dawn of targeted treatment. Cleve Clin J Med. 2015;82:309–320. doi: 10.3949/ccjm.82gr.15002. [DOI] [PubMed] [Google Scholar]
- Foth M, Wouters J, de Chaumont C, Dynoodt P, Gallagher WM. Prognostic and predictive biomarkers in melanoma: an update. Expert Rev Mol Diagn. 2016;16:223–237. doi: 10.1586/14737159.2016.1126511. [DOI] [PubMed] [Google Scholar]
- Gong WY, Wu JF, Liu BJ, Zhang HY, Cao YX, Sun J, Lv YB, Wu X, Dong JC. Flavonoid components in Scutellaria baicalensis inhibit nicotine-induced proliferation, metastasis and lung cancer-associated inflammation in vitro. Int J Oncol. 2014;44:1561–1570. doi: 10.3892/ijo.2014.2320. [DOI] [PubMed] [Google Scholar]
- Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng X, Su J, Zhou Z, Xu Z, Nilsson S, Liu Z. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 2015;406:111–119. doi: 10.1007/s11010-015-2429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE. LPS-induced TLR4 signaling in human colorectal cancer cells increases β1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71:1989–1998. doi: 10.1158/0008-5472.CAN-10-2833. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tsang SY, Yao X, Chen ZY. Biological properties of baicalein in cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:177–184. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- Izraely S, Sagi-Assif O, Klein A, Meshel T, Ben-Menachem S, Zaritsky A, Ehrlich M, Prieto VG, Bar-Eli M, Pirker C, Berger W, Nahmias C, Couraud PO, Hoon DS, Witz IP. The metastatic microenvironment: Claudin-1 suppresses the malignant phenotype of melanoma brain metastasis. Int. J. Cancer. 2015;136:1296–1307. doi: 10.1002/ijc.29090. [DOI] [PubMed] [Google Scholar]
- Kakavand H, Wilmott JS, Long GV, Scolyer RA. Targeted therapies and immune checkpoint inhibitors in the treatment of metastatic melanoma patients: a guide and update for pathologists. Pathology. 2016;48:194–202. doi: 10.1016/j.pathol.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasigov PZ, Podobed OV, Gracheva TS, Salbiev KD, Grachev SV, Berezov TT. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochemistry Mosc. 2003;68:711–717. doi: 10.1023/A:1025051214001. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD, Choi CS, Nam JS, Jung JY. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol Med Rep. 2012;6:1443–1449. doi: 10.3892/mmr.2012.1085. [DOI] [PubMed] [Google Scholar]
- Lee HZ, Leung HW, Lai MY, Wu CH. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res. 2005;25:959–964. [PubMed] [Google Scholar]
- Lee H, Pyo MJ, Bae SK, Heo Y, Kim CG, Kang C, Kim E. Improved therapeutic profiles of PLA2-free bee venom prepared by ultrafiltration method. Toxicol Res. 2015;31:33–40. doi: 10.5487/TR.2015.31.1.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol. 2014;810:120–140. doi: 10.1007/978-1-4939-0437-2_7. [DOI] [PubMed] [Google Scholar]
- Li C, Li F, Zhao K, Yao J, Cheng Y, Zhao L, Li Z, Lu N, Guo Q. LFG-500 inhibits the invasion of cancer cells via down-regulation of PI3K/AKT/NF-κB signaling pathway. PLoS ONE. 2014;9:e91332. doi: 10.1371/journal.pone.0091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lin G, Zuo Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos. 2011;32:427–445. doi: 10.1002/bdd.771. [DOI] [PubMed] [Google Scholar]
- Li HL, Zhang S, Wang Y, Liang RR, Li J, An P, Wang ZM, Yang J, Li ZF. Baicalein induces apoptosis via a mitochondrial-dependent caspase activation pathway in T24 bladder cancer cells. Mol Med Rep. 2013;7:266–270. doi: 10.3892/mmr.2012.1123. [DOI] [PubMed] [Google Scholar]
- Liu G, Xu S, Jiao F, Ren T, Li Q. Vascular endothelial growth factor B coordinates metastasis of non-small cell lung cancer. Tumour Biol. 2015;36:2185–2191. doi: 10.1007/s13277-014-2829-5. [DOI] [PubMed] [Google Scholar]
- Liu-Smith F, Meyskens FL. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol Nutr Food Res. 2016;60:1264–1274. doi: 10.1002/mnfr.201500822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Morin PJ. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M, Dong Q, Liu Y, Xu H. The traditional Chinese medicine baicalein potently inhibits gastric cancer cells. J. Cancer. 2016;7:453–461. doi: 10.7150/jca.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamada H, Otsuka Y. Inhibition of mouse liver sialidase by the root of Scutellaria baicalensis. Planta Med. 1989;55:27–29. doi: 10.1055/s-2006-961769. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M. NF-κB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DP, Bhatt L, Woolley JF, Gough DR, Wang JH, Cotter TG, Redmond HP. TLR-4 signalling accelerates colon cancer cell adhesion via NF-κB mediated transcriptional up-regulation of Nox-1. PLoS ONE. 2012;7:e44176. doi: 10.1371/journal.pone.0044176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kim YH, Kim Y, Lee SJ. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J Cell Biochem. 2012;113:3653–3662. doi: 10.1002/jcb.24238. [DOI] [PubMed] [Google Scholar]
- Rogiers A, van den Oord JJ, Garmyn M, Stas M, Kenis C, Wildiers H, Marine JC, Wolter P. Novel therapies for metastatic melanoma: An update on their use in older patients. Drugs Aging. 2015;32:821–834. doi: 10.1007/s40266-015-0304-7. [DOI] [PubMed] [Google Scholar]
- Saeki R, Kondoh M, Kakutani H, Matsuhisa K, Takahashi A, Suzuki H, Kakamu Y, Watari A, Yagi K. A claudin-targeting molecule as an inhibitor of tumor metastasis. J Pharmacol Exp Ther. 2010;334:576–582. doi: 10.1124/jpet.110.168070. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: A multifunctional complex. Am J Physiol, Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010;2010:541957. doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Pores in the wall: Claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 2000;149:13–16. doi: 10.1083/jcb.149.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utech M, Brüer M, Nusrat A. Tight junctions and cell-cell interactions. Methods Mol Biol. 2006;341:185–195. doi: 10.1385/1-59745-113-4:185. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Wang L, Ling Y, Chen Y, Li CL, Feng F, You QD, Lu N, Guo QL. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42–48. doi: 10.1016/j.canlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang C, Chen W, Zhang G, Luo D, Cao Y, Wu J, Ding Y, Liu B. Baicalein induces apoptosis and autophagy via endoplasmic reticulum stress in hepatocellular carcinoma cells. Biomed Res Int. 2014;2014:732516. doi: 10.1155/2014/732516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M, Christofori G, Lehembre F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol Med. 2007;13:535–541. doi: 10.1016/j.molmed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Song L, Cai L, Wei R, Hu H, Jin W. Effects of baicalein on apoptosis, cell cycle arrest, migration and invasion of osteosarcoma cells. Food Chem Toxicol. 2013;53:325–333. doi: 10.1016/j.fct.2012.12.019. [DOI] [PubMed] [Google Scholar]