Abstract
Importance
While neoadjuvant chemoradiation for esophageal cancer improves oncologic outcomes for a broad group of patients with locally advanced and/or node-positive tumors, it is less clear which specific subset of patients derives most benefit in terms of overall survival (OS).
Objective
To determine whether neoadjuvant chemoradiation based on esophageal adenocarcinoma histology has similar oncologic outcomes for patients treated with surgery alone when stratified by clinical nodal status.
Design, Setting, and Participants
A retrospective analysis using the American College of Surgeons National Cancer Database from 1998 to 2006. Patients with esophageal adenocarcinoma histology and clinical stage T1bN1-N3 or T2-T4aN−/+M0 were divided into 2 treatment groups: (1) neoadjuvant chemoradiation followed by surgery and (2) surgery alone. Subset analysis within each treatment group was performed for clinically node-negative patients (cN−) vs node-positive patients (cN+) in conjunction with pathological nodal status. A propensity score–adjusted analysis, which included patient demographics, comorbidity status, and clinical T stage, was also performed.
Main Outcome and measures
The primary outcome was 3-year OS. Secondary outcomes included margin status, postoperative length of stay, unplanned readmission rate, and 30-day mortality.
Results
A total of 1309 patients were identified, of whom 539 received neoadjuvant chemoradiation followed by surgery and 770 received surgery alone. Of the 1309 patients, 41.2% (n = 539) received neoadjuvant chemoradiation and 47.2% (n = 618) were cN+. Median follow-up for the entire cohort was 73.3 months (interquartile range, 64.1-93.5 months). The 3-year OS was better for neoadjuvant chemoradiation followed by surgery compared with surgery alone (49% vs 38%, respectively; P < .001). Stratifying based on clinical nodal status, the propensity score–adjusted OS was significantly better for cN+ patients who received neoadjuvant chemoradiation (hazard ratio, 0.52; 95% CI, 0.42-0.66; P < .001). In contrast, there was no difference in OS for cN− patients based on treatment (hazard ratio, 0.84; 95% CI, 0.65-1.10; P = .22).
Conclusions and Relevance
Patients with cN+ esophageal adenocarcinoma benefit significantly from neoadjuvant chemoradiation. However, patients with cN− tumors treated with neoadjuvant chemoradiation plus surgery do not derive a significant OS benefit compared with surgery alone. This finding may have significant implications on the use of neoadjuvant chemoradiation in patients with cN− disease.
Neoadjuvant chemoradiation for esophageal adenocarcinoma is common practice for patients with locally advanced and/or node-positive tumors, which is reflected in the current guidelines of the National Comprehensive Cancer Network.1 There have been several randomized clinical trials that support neoadjuvant therapy for esophageal adenocarcinoma prior to surgical resection.2-5 However, many of these studies included patients with both adenocarcinoma and squamous cell carcinoma. For example, one of these trials, performed by the Chemoradiation for Oesophageal Cancer Followed by Surgery Study (CROSS) Group, included patients with either esophageal adenocarcinoma or squamous cell carcinoma who had clinical stage T1N1 or T2-3N0-1 disease without metastatic disease.3 The CROSS Trial results showed improved long-term oncologic benefits for patients treated with preoperative weekly paclitaxel and carboplatin with radiation compared with patients treated with surgery alone.3 However, when survival outcomes were analyzed by histology, neoadjuvant chemoradiation appeared to benefit adenocarcinoma to a significantly lesser degree than squamous cell carcinoma. There is also a paucity of data regarding which patient population among locally advanced and/or node-positive esophageal adenocarcinomas derive more benefit from neoadjuvant therapy.
The focus of this study was to use a large nationwide database to delineate the effects of neoadjuvant chemoradiation on patients with esophageal cancer of adenocarcinoma histology, with the specific aim to characterize the survival in patients with clinical node-positive disease (cN+) compared with clinical node-negative disease (cN−).
Methods
This retrospective database study was deemed exempt by the Roswell Park Cancer Institute institutional review board because it did not involve patient identifiers. The American College of Surgeons National Cancer Database (NCDB) from 1998-2006 was queried for patients with adenocarcinoma of the middle and lower esophagus who had clinical stage T1bN1-N3M0 or T2-T4aN−/+M0 disease. During the study, the NCDB used both the fifth and sixth editions of the American Joint Committee on Cancer Staging Manual.4,5 For esophageal staging, there are no differences between these 2 editions. All included patients had a diagnostic confirmation of esophageal adenocarcinoma. However, the methods used to characterize nodal disease (ie, computed tomographic scan, magnetic resonance imaging, or endoscopic ultrasonography) are not available as a limitation of the NCDB. The following International Classification of Diseases for Oncology, Third Revision codes were used to identify patients with adenocarcinoma: 8140-8148, 8200-8239, 8260-8263, 8480-8496, 8500-8503, and 8560-8573.
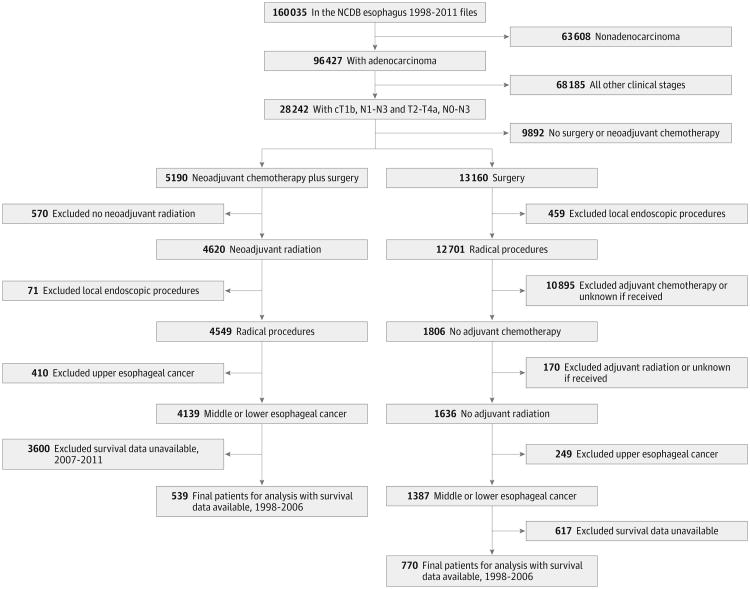
Patients were divided into 2 groups: those who received neoadjuvant chemoradiation prior to surgery (NA+S) and those who received surgery alone without any additional therapy. Additional inclusion and exclusion criteria are detailed in Figure 1. Patients in either group were excluded if the adenocarcinoma was localized to the cervical esophagus, a local endoscopic or ablative procedure was the only intervention performed, or if survival data were missing. The NCDB currently provides vital status up to December 2006 only. In addition, patients in the surgery-alone group were excluded if they received adjuvant chemotherapy or radiation.
Figure 1. Flow Diagram of Patient Inclusion and Exclusion Criteria From the National Cancer Database (NCDB) Esophageal Cancer Participant User File.
In a subsequent a priori analysis, the patients were further divided into a subset of 2 groups based on clinical node status: those who had cN− disease and those who had cN+ disease. Additional predetermined subset survival analyses were conducted for patients who were truly node negative (defined as both clinically and pathologically node negative) and truly node positive (likewise defined as both clinically and pathologically node positive). The groups were compared by various demographic and oncologic factors, with univariate-and propensity score–adjusted comparisons performed on treatment groups that were subcategorized based on clinical node status. Only patients with complete data regarding demographics and tumor characteristics were included within the propensity score–adjusted comparisons, the percentages of which are presented in the Results section.
Patient characteristics were reported by group using the mean, median, SD, and interquartile range for continuous variables and using frequencies for categorical variables. Comparisons were made using the Wilcoxon rank sum and Fisher exact tests for continuous and categorical variables, respectively. Overall survival (OS) was summarized by group using standard Kaplan-Meier methods, with comparisons made using the Tarone-Ware log-rank test. Regardless of significance, a preplanned propensity score–adjusted analysis was performed. A logistic regression model was used to generate propensity scores based on the predetermined factors: age, sex, race/ethnicity, facility type, insurance, income, education, margins, nodes examined, clinical T stage, and primary site. Owing to limited sample size, interaction terms and a formal assessment of collinearity were not considered. A Cox regression model, stratified by propensity score quintile, was then used to evaluate the association between OS and treatment group within each clinical N stage group (ie, cN− and cN+). The model was fit using the Firth penalized function, and patients missing demographics for the propensity score were excluded. From the estimated model coefficients, propensity score–adjusted hazard ratios (HRs) comparing the 2 treatment options (neoadjuvant therapy vs surgery alone) were obtained. No power analysis was performed because this was an observational study based on a preset national database.
All analyses were conducted in SAS version 9.4 (SAS Institute) at a nominal significance level of α = .05.
Results
A total of 1309 patients were identified who met the inclusion criteria. Of these, 41.2% (n = 539) received neoadjuvant chemoradiation and 58.8% (n = 770) were treated with surgery alone. The baseline unadjusted comparison of patient demographics and short-term oncologic outcomes by treatment groups (NA+S vs surgery alone) are shown in Table 1. As expected, a higher pathologic N stage correlated with a higher clinical or pathologic T stage (eTable 1 in the Supplement). Patients who received neoadjuvant chemoradiation tended to be younger, healthier (as estimated by the Charlson-Deyo Comorbidity Index score), and had more cT3N+ tumors of the lower esophagus. None of the patients who received neoadjuvant chemoradiation received adjuvant chemotherapy. Short-term outcomes showed that patients treated with NA+S had higher rates of negative margins and pathologic complete response, fewer nodes examined and fewer positive nodes, and decreased inpatient postoperative stay and 30-day mortality. Conversely, patients in the surgery-alone group were older, had more comorbid conditions, and had a longer inpatient length of stay compared with patients in the NA+S group, which likely reflected the worse 30-day mortality. Univariate and multivariate analyses of 30-day mortality within the surgery-alone group are shown in eTable 2 and eTable 3 in the Supplement, respectively. Factors associated with patients who died within 30 days included female sex, treatment at a community cancer program, and having insurance other than private insurance.
Table 1. Comparison of Patient Demographics and Tumor Characteristics in the NA+S Group vs the Surgery-Alone Group.
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| NA+S | Surgery | Overall | ||
| Overall | 539 (41.2) | 770 (58.8) | 1309 (100) | |
| Age, mean (SD), y | 61.18 (9.45) | 67.45 (10.55) | 64.87 (10.57) | <.001 |
| Q1-Q3, median (IQR) | 62.00 (55.00-68.00) | 69.00 (60.00-76.00) | 65.00 (58.00-73.00) | |
| Sex | ||||
| Male | 477 (88.5) | 668 (86.8) | 1145 (87.5) | .35 |
| Female | 62 (11.5) | 102 (13.2) | 164 (12.5) | |
| Race/ethnicity | ||||
| White | 523 (98.3) | 739 (97.5) | 1262 (97.8) | .57 |
| Black | 6 (1.1) | 14 (1.8) | 20 (1.6) | |
| Other | 3 (0.6) | 5 (0.7) | 8 (0.6) | |
| Facility type | ||||
| Community CP | 29 (5.4) | 49 (6.5) | 78 (6.1) | .12 |
| Comprehensive community CP | 216 (40.5) | 263 (35.0) | 479 (37.3) | |
| Academic/research program | 288 (54.0) | 439 (58.5) | 727 (56.6) | |
| Urban/rural | ||||
| Metro | 398 (79.4) | 563 (79.2) | 961 (79.3) | .93 |
| Urban | 88 (17.6) | 124 (17.4) | 212 (17.5) | |
| Rural | 15 (3.0) | 24 (3.4) | 39 (3.2) | |
| Hospital distance, mi | ||||
| Mean (SD) [No.] | 42.81 (87.22) [517] | 52.76 (108.13) [719] | 48.60 (100.00) [1236] | .81 |
| Q1-Q3, median (IQR) | 17.60 (7.30-41.50) | 17.30 (6.00-54.10) | 17.50 (6.40-49.15) | |
| Insurance | ||||
| Not insured | 8 (1.5) | 15 (2.0) | 23 (1.8) | <.001 |
| Private | 309 (57.5) | 259 (35.3) | 568 (44.7) | |
| Medicaid | 16 (3.0) | 23 (3.1) | 39 (3.1) | |
| Medicare | 200 (37.2) | 433 (59.1) | 633 (49.8) | |
| Other | 4 (0.7) | 3 (0.4) | 7 (0.6) | |
| Income, $a | ||||
| <30 000 | 43 (8.4) | 98 (13.5) | 141 (11.4) | .04 |
| 30 000-34 999 | 95 (18.5) | 123 (16.9) | 218 (17.6) | |
| 35 000-45 999 | 141 (27.4) | 201 (27.7) | 342 (27.6) | |
| >46 000 | 235 (45.7) | 304 (41.9) | 539 (43.5) | |
| Education, %b | ||||
| ≥29 | 57 (11.1) | 101 (13.9) | 158 (12.7) | .42 |
| 20-28.9 | 119 (23.2) | 168 (23.1) | 287 (23.1) | |
| 14-19.9 | 149 (29.0) | 189 (26.0) | 338 (27.3) | |
| <14 | 189 (36.8) | 268 (36.9) | 457 (36.9) | |
| Charlson-Deyo Comorbidity Index scorec | ||||
| 0 | 410 (76.1) | 269 (66.1) | 679 (71.8) | .003 |
| 1 | 101 (18.7) | 106 (26.0) | 207 (21.9) | |
| 2 | 28 (5.2) | 32 (7.9) | 60 (6.3) | |
| Grade | ||||
| I | 16 (3.7) | 45 (6.3) | 61 (5.3) | .02 |
| II | 177 (40.4) | 292 (40.6) | 469 (40.5) | |
| III | 234 (53.4) | 377 (52.4) | 611 (52.8) | |
| IV | 11 (2.5) | 5 (0.7) | 16 (1.4) | |
| Disease site | ||||
| Middle | 28 (5.2) | 79 (10.3) | 107 (8.2) | <.001 |
| Lower | 511 (94.8) | 691 (89.7) | 1202 (91.8) | |
| Clinical T stage | ||||
| 2 | 105 (19.5) | 411 (53.4) | 516 (39.4) | <.001 |
| 3 | 415 (77.0) | 329 (42.7) | 744 (56.8) | |
| 4 | 19 (3.5) | 30 (3.9) | 49 (3.7) | |
| Clinical N stage | ||||
| 0 | 195 (36.2) | 496 (64.4) | 691 (52.8) | <.001 |
| 1 | 344 (63.8) | 274 (35.6) | 618 (47.2) | |
| Tumor size, mm | ||||
| Mean (SD) [No.] | 45.58 (50.91) [320] | 39.82 (26.72) [647] | 41.73 (36.62) [967] | .048 |
| Q1-Q3, median (IQR) | 40.00 (25.00-50.00) | 35.00 (25.00-50.00) | 37.00 (25.00-50.00) | |
| Inpatient stay, d | ||||
| Mean (SD) [No.] | 12.17 (9.83) [452] | 17.34 (16.49) [358] | 14.45 (13.43) [810] | <.001 |
| Q1-Q3, median (IQR) | 9.00 (8.00-14.00) | 11.00 (9.00-21.00) | 10.00 (8.00-16.00) | |
| Margins | ||||
| Negative | 486 (92.9) | 617 (87.5) | 1103 (89.8) | .002 |
| Positive | 37 (7.1) | 88 (12.5) | 125 (10.2) | |
| Path T stage | ||||
| 0 | 61 (17.6) | 1 (0.1) | 62 (6.1) | <.001 |
| 1 | 47 (13.5) | 112 (16.7) | 159 (15.6) | |
| 2 | 83 (23.9) | 203 (30.2) | 286 (28.1) | |
| 3 | 142 (40.9) | 314 (46.7) | 456 (44.7) | |
| 4 | 8 (2.3) | 33 (4.9) | 41 (4.0) | |
| IS | 6 (1.7) | 9 (1.3) | 15 (1.5) | |
| Path N stage | ||||
| 0 | 239 (65.7) | 359 (54.9) | 598 (58.7) | <.001 |
| 1 | 125 (34.3) | 295 (45.1) | 420 (41.3) | |
| No. of nodes examined | ||||
| Mean (SD) No. | 10.36 (11.19) [518] | 11.89 (10.70) [747] | 11.26 (10.93) [12.65] | .002 |
| Q1-Q3, median (IQR) | 8.00 (3.00-14.00) | 10.00 (5.00-17.00) | 9.00 (4.00-15.00) | |
| No. of positive nodes | ||||
| Mean (SD) [No.] | 1.57 (7.95) [455] | 2.21 (5.20) [682] | 1.96 (6.4) [1137] | <.001 |
| Q1-Q3, median (IQR) | 0.00 (0.00-1.00) | 0.00 (0.00-3.00) | 0.00 (0.00-2.00) | |
| Readmission | ||||
| None | 473 (95.0) | 353 (91.2) | 826 (93.3) | .08 |
| Planned | 5 (1.0) | 6 (1.6) | 11 (1.2) | |
| Unplanned | 20 (4.0) | 28 (7.2) | 48 (5.4) | |
| 30-d Mortality | ||||
| No | 526 (97.6) | 698 (91.0) | 1224 (93.7) | <.001 |
| Yes | 13 (2.4) | 69 (9.0) | 82 (6.3) | |
Abbreviations: CP, cancer program; IQR, interquartile range; IS, in situ; NA+S, neoadjuvant chemoradiation plus surgery; Q, quintile.
Income as reported by the National Cancer Database (NCDB) is the median household income for the area of residence of a given patient based on zip code derived from the 2000 US Census.
Education as reported by the NCDB is the percentage of adults in the area of residence of a given patient (based on zip code derived from the 2000 US Census) who did not graduate from high school.
Charlson-Deyo Comorbidity Index score is an estimate of comorbid conditions based on International Classification of Diseases, Ninth Revision diagnosis codes. A score of 0 indicates no comorbidities. Point values are assigned to comorbid conditions based on severity. The NCDB truncates possible scores to 0, 1, and 2 owing to the small proportion of cases exceeding a score of 2.
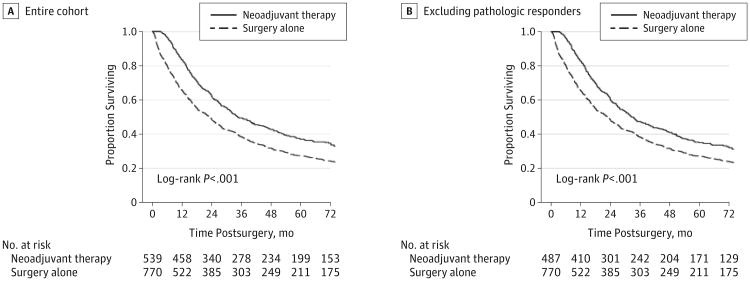
The median follow-up for the entire cohort was 73.3 months (interquartile range, 64.1-93.5 months). Patients with NA+S had better OS compared with surgery-alone patients (3-year OS, 49% vs 38%, respectively; P < .001) as shown in Figure 2A. This survival advantage was significant even after the complete pathologic responders were excluded from the NA+S group as shown in Figure 2B (3-year OS, 47% for NA+S vs 38% for surgery alone; P < .001).
Figure 2. Overall Survival Between Treatment Groups For the Entire Cohort (A) and Excluding Complete Pathologic Responders (B).
Table 2 and Table 3 show the comparison of patients within each treatment group based on clinical nodal status. For cN− patients, patients in the NA+S group were younger, healthier, and tended to have private insurance (Table 2 and Table 3). They also had more advanced T stage, a higher proportion of tumors of the lower esophagus, and a shorter inpatient stay. For cN+ patients, patients in the NA+S group were also younger, more privately insured with higher incomes, and had lower in-patient stay and 30-day mortality (Table 2 and Table 3).
Table 2. Comparison of Patient Demographics and Tumor Characteristics in the NA+S Group vs the Surgery-Alone Group for the Clinical Node-Negative Patients.
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| NA+S | Surgery | Overall | ||
| Overall, No. | 195 (28.2) | 496 (71.8) | 691 (100) | |
| Age, mean (SD), y | 61.70 (9.47) | 67.21 (10.54) | 65.65 (10.54) | <.001 |
| Q1-Q3, median (IQR) | 63.00 (56.00-69.00) | 68.00 (60.00-75.00) | 67.00 (58.00-74.00) | |
| Sex | ||||
| Male | 171 (87.7) | 430 (86.7) | 601 (87.0) | .73 |
| Female | 24 (12.3) | 66 (13.3) | 90 (13.0) | |
| Race/ethnicity | ||||
| White | 188 (97.9) | 473 (96.7) | 661 (97.1) | .49 |
| Black | 2 (1.0) | 12 (2.5) | 14 (2.1) | |
| Other | 2 (1.0) | 4 (0.8) | 6 (0.9) | |
| Facility type | ||||
| Community CP | 8 (4.2) | 29 (6.0) | 37 (5.5) | .34 |
| Comprehensive community CP | 81 (42.4) | 179 (37.0) | 260 (38.5) | |
| Academic/research program | 102 (53.4) | 276 (57.0) | 378 (56.0) | |
| Urban/rural | ||||
| Metro | 145 (79.7) | 378 (81.6) | 523 (81.1) | .32 |
| Urban | 34 (18.7) | 70 (15.1) | 104 (16.1) | |
| Rural | 3 (1.6) | 15 (3.2) | 18 (2.8) | |
| Hospital distance, mi | ||||
| Mean (SD) [No.] | 44.25 (104.18) [186] | 52.86 (111.83) [470] | 50.42 (109.71) [656] | .68 |
| Q1-Q3, median (IQR) | 15.85 (6.20-32.70) | 15.55 (5.60-53.10) | 15.60 (5.80-48.90) | |
| Insurance | ||||
| Not insured | 4 (2.1) | 11 (2.3) | 15 (2.2) | .003 |
| Private | 102 (52.3) | 172 (36.4) | 274 (41.0) | |
| Medicaid | 7 (3.6) | 14 (3.0) | 21 (3.1) | |
| Medicare | 81 (41.5) | 275 (58.1) | 356 (53.3) | |
| Other | 1 (0.5) | 1 (0.2) | 2 (0.3) | |
| Income, $ | ||||
| <30 000 | 17 (9.2) | 55 (11.7) | 72 (11.0) | .35 |
| 30 000-34 999 | 27 (14.6) | 87 (18.4) | 114 (17.4) | |
| 35 000-45 999 | 52 (28.1) | 135 (28.6) | 187 (28.5) | |
| >46 000 | 89 (48.1) | 195 (41.3) | 284 (43.2) | |
| Education, % | ||||
| ≥29 | 14 (7.6) | 67 (14.2) | 81 (12.3) | .11 |
| 20-28.9 | 40 (21.6) | 106 (22.5) | 146 (22.2) | |
| 14-19.9 | 58 (31.4) | 129 (27.3) | 187 (28.5) | |
| <14 | 73 (39.5) | 170 (36.0) | 243 (37.0) | |
| Charlson-Deyo Comorbidity Index score | ||||
| 0 | 150 (76.9) | 169 (65.5) | 319 (70.4) | .03 |
| 1 | 36 (18.5) | 70 (27.1) | 106 (23.4) | |
| 2 | 9 (4.6) | 19 (7.4) | 28 (6.2) | |
| Grade | ||||
| I | 5 (3.3) | 40 (8.7) | 45 (7.4) | .15 |
| II | 66 (44.0) | 193 (41.9) | 259 (42.4) | |
| III | 76 (50.7) | 223 (48.4) | 299 (48.9) | |
| IV | 3 (2.0) | 5 (1.1) | 8 (1.3) | |
| Disease site | ||||
| Middle | 9 (4.6) | 55 (11.1) | 64 (9.3) | .008 |
| Lower | 186 (95.4) | 441 (88.9) | 627 (90.7) | |
| Clinical T stage | ||||
| 2 | 51 (26.2) | 326 (65.7) | 377 (54.6) | <.001 |
| 3 | 138 (70.8) | 154 (31.0) | 292 (42.3) | |
| 4 | 6 (3.1) | 16 (3.2) | 22 (3.2) | |
| Tumor size, mm | ||||
| Mean (SD) [No.] | 46.65 (72.12) [124] | 37.49 (27.48) [407] | 39.63 (42.43) [531] | .06 |
| Q1-Q3, median (IQR) | 38.00 (25.00-50.00) | 32.00 (23.00-45.00) | 34.00 (23.00-(50.00) | |
| Inpatient stay, d | ||||
| Mean (SD) [No.] | 12.19 (8.81) [161] | 17.74 (17.1) [226] | 15.43 (14.56) [387] | <.001 |
| Q1-Q3, median (IQR) | 10.00 (8.00-15.00) | 12.00 (9.00-21.00) | 11.00 (8.00-18.00) | |
| Margins | ||||
| Negative | 174 (91.1) | 407 (89.5) | 581 (89.9) | .53 |
| Positive | 17 (8.9) | 48 (10.5) | 65 (10.1) | |
| Path T stage | ||||
| 0 | 17 (13.9) | 17 (3.1) | <.001 | |
| 1 | 19 (15.6) | 84 (19.6) | 103 (18.7) | |
| 2 | 29 (23.8) | 148 (34.5) | 177 (32.1) | |
| 3 | 49 (40.2) | 176 (41.0) | 225 (40.8) | |
| 4 | 5 (4.1) | 14 (3.3) | 19 (3.4) | |
| IS | 3 (2.5) | 7 (1.6) | 10 (1.8) | |
| Path N stage | ||||
| 0 | 92 (70.8) | 312 (75.5) | 404 (74.4) | .28 |
| 1 | 38 (29.2) | 101 (24.5) | 139 (25.6) | |
| No. of nodes examined | ||||
| Mean (SD) [No.] | 9.14 (10.50) [189] | 10.63 (9.60) [484] | 10.21 (9.88) [673] | .03 |
| Q1-Q3, median (IQR) | 7.00 (2.00-13.00) | 8.50 (3.00-15.00) | 8.00 (3.00-15.00) | |
| No. of positive nodes | ||||
| Mean (SD) [No.] | 0.83 (2.56) [161] | 1.02 (2.59) [424] | 0.97 (2.58) [585] | .39 |
| Q1-Q3, median (IQR) | 0.00 (0.00-0.00) | 0.00 (0.00-1.00) | 0.00 (0.00-1.00) | |
| Readmission | ||||
| None | 171 (95.5) | 227 (91.9) | 398 (93.4) | .32 |
| Planned | 2 (1.1) | 4 (1.6) | 6 (1.4) | |
| Unplanned | 6 (3.4) | 16 (6.5) | 22 (5.2) | |
| 30-d Mortality | ||||
| No | 189 (96.9) | 464 (93.5) | 653 (94.5) | .08 |
| Yes | 6 (3.1) | 32 (6.5) | 38 (5.5) | |
Abbreviations: CP, cancer program; IQR, interquartile range;
NA+S, neoadjuvant chemoradiation plus surgery; Q, quintile.
Table 3. Comparison of Patient Demographics and Tumor Characteristics in the NA+S Group vs the Surgery-Alone Group for the Clinical Node-Positive Patients.
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| NA+S | Surgery | Overall | ||
| Overall, No. | 344 (55.7) | 274 (44.3) | 618 (100) | |
| Age, mean (SD), y | 60.89 (9.44) | 67.88 (10.56) | 63.99 (10.54) | <.001 |
| Q1-Q3, median (IQR) | 61.00 (54.50-68.00) | 69.50 (60.00-76.00) | 64.00 (57.00-72.00) | |
| Sex | ||||
| Male | 306 (89.0) | 238 (86.9) | 544 (88.0) | .43 |
| Female | 38 (11.0) | 36 (13.1) | 74 (12.0) | |
| Race/ethnicity | ||||
| White | 335 (98.5) | 266 (98.9) | 601 (98.7) | .85 |
| Black | 4 (1.2) | 2 (0.7) | 6 (1.0) | |
| Other | 1 (0.3) | 1 (0.4) | 2 (0.3) | |
| Facility type | ||||
| Community CP | 21 (6.1) | 20 (7.5) | 41 (6.7) | .12 |
| Comprehensive community CP | 135 (39.5) | 84 (31.5) | 219 (36.0) | |
| Academic/research program | 186 (54.4) | 163 (61.0) | 349 (57.3) | |
| Urban/rural | ||||
| Metro | 253 (79.3) | 185 (74.6) | 438 (77.2) | .35 |
| Urban | 54 (16.9) | 54 (21.8) | 108 (19.0) | |
| Rural | 12 (3.8) | 9 (3.6) | 21 (3.7) | |
| Hospital distance, mi | ||||
| Mean (SD) [No.] | 42.00 (76.21) [331] | 52.58 (100.99) [249] | 46.54 (87.79) [580] | .42 |
| Q1-Q3, median (IQR) | 18.70 (7.70-44.50) | 23.30 (7.20-54.70) | 19.50 (7.50-49.90) | |
| Insurance | ||||
| Not insured | 4 (1.2) | 4 (1.5) | 8 (1.3) | <.001 |
| Private | 207 (60.5) | 87 (33.5) | 294 (48.8) | |
| Medicaid | 9 (2.6) | 9 (3.5) | 18 (3.0) | |
| Medicare | 119 (34.8) | 158 (60.8) | 277 (46.0) | |
| Other | 3 (0.9) | 2 (0.8) | 5 (0.8) | |
| Income, $ | ||||
| <30 000 | 26 (7.9) | 43 (16.9) | 69 (11.8) | .004 |
| 30 000-34 999 | 68 (20.7) | 36 (14.2) | 104 (17.8) | |
| 35 000-45 999 | 89 (27.1) | 66 (26.0) | 155 (26.6) | |
| >46 000 | 146 (44.4) | 109 (42.9) | 255 (43.7) | |
| Education % | ||||
| ≥29 | 43 (13.1) | 34 (13.4) | 77 (13.2) | .72 |
| 20-28.9 | 79 (24.0) | 62 (24.4) | 141 (24.2) | |
| 14-9.9 | 91 (27.7) | 60 (23.6) | 151 (25.9) | |
| <14 | 116 (35.3) | 98 (38.6) | 214 (36.7) | |
| Charlson-Deyo Comorbidity Index score | ||||
| 0 | 260 (75.6) | 100 (67.1) | 360 (73.0) | .13 |
| 1 | 65 (18.9) | 36 (24.2) | 101 (20.5) | |
| 2 | 19 (5.5) | 13 (8.7) | 32 (6.5) | |
| Grade | ||||
| I | 11 (3.8) | 5 (1.9) | 16 (2.9) | .03 |
| II | 111 (38.5) | 99 (38.4) | 210 (38.5) | |
| III | 158 (54.9) | 154 (59.7) | 312 (57.1) | |
| IV | 8 (2.8) | 8 (1.5) | ||
| Disease site | ||||
| Middle | 19 (5.5) | 24 (8.8) | 43 (7.0) | .12 |
| Lower | 325 (94.5) | 250 (91.2) | 575 (93.0) | |
| Clinical T stage | ||||
| 2 | 54 (15.7) | 85 (31.0) | 139 (22.5) | <.001 |
| 3 | 277 (80.5) | 175 (63.9) | 452 (73.1) | |
| 4 | 13 (3.8) | 14 (5.1) | 27 (4.4) | |
| Tumor size, mm | ||||
| Mean (SD) [No.] | 44.91 (30.96) [196] | 43.76 (24.95) [240] | 44.28 (27.79) [436] | .78 |
| Q1-Q3, median (IQR) | 40.00 (27.00-57.50) | 40.00 (30.00-54.00) | 40.00 (28.00-55.00) | |
| Inpatient stay, d | ||||
| Mean (SD) [No.] | 12.16 (10.37) [291] | 16.64 (15.25) [132] | 13.56 (12.26) [423] | .002 |
| Q1-Q3, median (IQR) | 9.00 (8.00-14.00) | 11.00 (8.00-18.00) | 10.00 (8.00-15.00) | |
| Margins | ||||
| Negative | 312 (94.0) | 210 (84.0) | 522 (89.7) | <.001 |
| Positive | 20 (6.0) | 40 (16.0) | 60 (10.3) | |
| Path T stage | ||||
| 0 | 44 (19.6) | 1 (0.4) | 45 (9.6) | <.001 |
| 1 | 28 (12.4) | 28 (11.5) | 56 (12.0) | |
| 2 | 54 (24.0) | 55 (22.6) | 109 (23.3) | |
| 3 | 93 (41.3) | 138 (56.8) | 231 (49.4) | |
| 4 | 3 (1.3) | 19 (7.8) | 22 (4.7) | |
| IS | 3 (1.3) | 2 (0.8) | 5 (1.1) | |
| Path N stage | ||||
| 0 | 147 (62.8) | 47 (19.5) | 194 (40.8) | <.001 |
| 1 | 87 (37.2) | 194 (80.5) | 281 (59.2) | |
| No. of nodes examined | ||||
| Mean (SD) [No.] | 11.06 (11.53) [329] | 14.20 (12.16) [263] | 12.46 (11.90) [592] | <.001 |
| Q1-Q3, median (IQR) | 9.00 (5.00-15.00) | 12.00 (6.00-18.00) | 11.00 (5.00-16.00) | |
| No. of positive nodes | ||||
| Mean (SD) [No.] | 1.98 (9.69) [294] | 4.17 (7.39) [258] | 3.00 (8.75) [552] | <.001 |
| Q1-Q3, median (IQR) | 0.00 (0.00-1.00) | 2.00 (1.00-5.00) | 1.00 (0.00-3.00) | |
| Readmission | ||||
| None | 302 (94.7) | 126 (90.0) | 428 (93.2) | .18 |
| Planned | 3 (0.9) | 2 (1.4) | 5 (1.1) | |
| Unplanned | 14 (4.4) | 12 (8.6) | 26 (5.7) | |
| 30-d Mortality | ||||
| No | 337 (98.0) | 234 (86.3) | 571 (92.8) | <.001 |
| Yes | 7 (2.0) | 37 (13.7) | 44 (7.2) | |
Abbreviations: CP, cancer program; IQR, interquartile range;
NA+S, neoadjuvant chemoradiation plus surgery; Q, quintile.
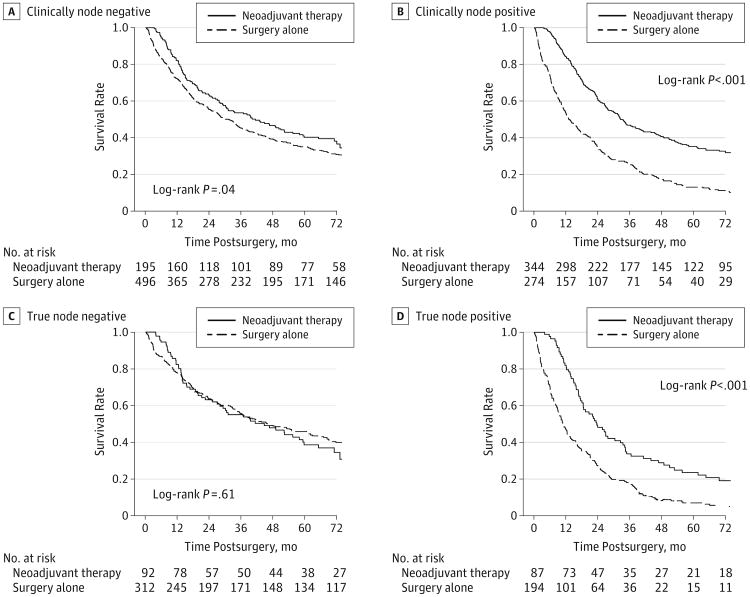
For cN− patients, OS was better for the NA+S group vs the surgery-alone group (3-year OS, 54% vs 45%, respectively; P = .03) (Figure 3A). A propensity score–adjusted analysis was performed as described here using only patients with complete data, which included 170 of the 195 patients (87.2%) in the NA+S group and 402 of the 496 (81.3%) in the surgery-alone group. When adjusted for other variables using this propensity score–adjusted analysis, there was no significant difference in OS (HR, 0.84; 95% CI, 0.65-1.10; P = .22). For cN+ patients, OS was significantly better for the NA+S group on both the unadjusted (3-year OS, 47% vs 25%, respectively; P < .001) and propensity score–adjusted (HR, 0.52; 95% CI, 0.42-0.66; P < .001) analyses (Figure 3B). In this propensity score–adjusted analysis, complete patient data were available for 297 of the 344 patients (86.3%) in the NA+S group and 209 of the 274 patients (85.7%) in the surgery-alone group.
Figure 3. Overall Survival Among the Clinical Node-Negative Patients (A) and Clinically Node-Positive Patients (B) and the True Node-Negative Patients (C) and True Node-Positive Patients (D) Based on Treatment Group.
To account for potential confounding effects of tumor downstaging and clinical misclassification, the clinical nodal status was matched to pathologic node status to define truly node-negative and truly node-positive patients. For truly node-negative patients, 3-year OS was identical in the NA+S and surgery-alone groups (55% vs 55%; P = .61) (Figure 3C). For truly node-positive patients, 3-year OS was significantly better for the NA+S group (34% vs 18%; P < .001) (Figure 3D). Only patients with complete data were included in the propensity score–adjusted analysis, which included 76 of the 87 patients (87.4%) in the NA+S group and 151 of the 194 (77.8%) in the surgery-alone group. The benefits of neoadjuvant chemoradiation remained significant when adjusted for other variables using this propensity score analysis (HR, 0.65; 95% CI, 0.46-0.92; P = .02).
Discussion
Neoadjuvant chemoradiation for esophageal cancer has been tested in several large clinical trials and has shown benefit for oncologic outcomes, thus establishing this approach as the standard of care for T1bN1-N3 or T2-T4aN−/+M0 patients prior to surgery.1 These studies have included patients with squamous cell carcinoma2,3 or adenocarcinoma of the eosphagogastric junction or proximal stomach.6,7 Several reviews and a meta-analysis also support neoadjuvant chemoradiation.8-10 Unique to these studies that included squamous cell histology, our analysis of the NCDB specifically characterized the OS benefit of neoadjuvant chemoradiation on patients with adenocarcinoma histology (Figure 2A). This benefit was still observed for patients who did not achieve pathologic complete response of the primary tumor (Figure 2B).
Of significant importance, our study calls into question the long-term benefits of neoadjuvant chemoradiation for patients who are cN− in terms of OS. As shown in Figure 3A, patients who were cN− and received neoadjuvant therapy had very similar OS to cN− patients who were treated with surgery alone. Conversely, we showed that patients who were cN+ did derive a significant OS benefit, which persisted even on propensity score–adjusted analysis (Figure 3B).
There is the potential for misclassification or tumor downstaging as a result of neoadjuvant therapy. Indeed, as reflected in Table 1, clinical staging did not correlate with pathologic staging in up to 25% of cases in this study. A possible way to address clinical misclassification would be to perform image-guided (ie, endoscopic ultrasonography) needle biopsy of periesophagealor perigastric lymph nodes to determine whether patients are truly node positive prior to surgical intervention. Although biopsy is not part of routine practice, image-guided biopsy may be particularly relevant in the case of 1 or 2 suspicious periesophageal or perigastric nodes to help definitively establish nodal staging. On the other hand, the presence of multiple suspicious nodes observed on imaging would more likely obviate the need for biopsy, given the stronger clinical suspicion for nodal disease. In any event, the ability to perform these biopsies may not always be feasible owing to technical challenges or availability of resources in a given treating facility.
Nonetheless, to account for the potential misclassification of clinical nodal categories, as well as tumor downstaging effect of neoadjuvant therapy, we matched the clinical stage to pathologic stage and performed the analysis on patients who were truly node negative and truly node positive. Although this predetermined subset analysis resulted in decreased numbers for comparison, this allowed for a more stringent comparison. We showed that patients with esophageal adenocarcinoma who were truly node positive derived a significant OS benefit with the neoadjuvant approach even when propensity score adjusted for other demographic and pathological data (Figure 3D). In contrast, our study showed no OS benefit of neoadjuvant chemoradiation among patients who were truly node negative when compared with patients treated with surgery alone (Figure 3C). Although it is possible to generate comparisons among patients who were clinically node negative and pathologically node positive or who were clinically node positive and pathologically node negative, the usefulness of such an analysis is limited owing to the influence of clinical misclassification and/or tumor downstaging.
Other retrospective studies have investigated the benefits of neoadjuvant chemoradiation for specific categories, particularly clinical T2N0M0. There are reports that do not support the use of neoadjuvant chemoradiation in this subset of patients,11 although other studies have shown a benefit perhaps as a result of stage migration.12,13 In addition, the high rate of T stage misclassification makes the result clinically less relevant. Studies have reported benefits of neoadjuvant therapy with histologic response rates, although the sample sizes were small.14-16 A study using the NCDB has shown survival benefits of neoadjuvant chemoradiation for patients with clinical T2-3N0 and cT1-3N+ patients,17 although this study also included patients with squamous cell carcinoma, corroborating the findings of other randomized trials. In contrast with this study, we have shown that patients with clinical node-negative esophageal adenocarcinoma did not benefit from neoadjuvant chemoradiation with regard to OS. Neoadjuvant therapy primarily benefits clinical node-positive patients even when adjusted for other variables.
There are several well-characterized advantages of using the NCDB. It is a large nationwide database that captures 70% of cancer cases through its participating hospitals. The NCDB provides a multitude of useful patient demographic and oncologic variables. Its large size allows for a robust statistical analysis. As more current survival data are verified and subsequently released, there will be opportunities to perform updated analyses of many studies, including this one.
Conversely, we recognize that there are several limitations of the NCDB. The NCDB does not provide specific information regarding disease recurrence and, therefore, survival analysis is limited to OS. Disease-free survival cannot be readily analyzed. As previously mentioned, the accuracy of clinical staging for both T and N stages is difficult, and information on how staging was derived is not available. However, to minimize the effects of this limitation, we used a propensity score–adjusted analysis that included stage matching. Another limitation inherent to the database was the amount of missing data. Patient vital status is available through December 2006. Within the cohort of patients with survival data, there is still a substantial amount of other missing data pertaining to patient demographics or tumor characteristics, up to 22% in 1 subset. We make the assumption that the data are missing from the NCDB at random. Although imputational analysis can be performed, this technique has its own set of limitations. Restricting our analysis to those patients with complete data provided for a more stringent comparison, albeit with the result of smaller sample sizes. In addition, the NCDB does not provide data on other factors that may influence OS, including the experience of the operating surgeon or inpatient care team, which may contribute to a list of unknown confounders affecting outcomes.
The database also does not specify the types of chemotherapy used or dosage of radiation delivered. Each of the large trials that investigated neoadjuvant therapy for esophageal cancer used different regimens. For example, the 2002 trial conducted by the Medical Research Council Oesophageal Cancer Working Group used 5-fluorouracil and cisplatin,2 whereas the 2012 CROSS Trial used paclitaxel and carboplatin.3 Moreover, there are smaller phase 2 clinical trials, which have tested other regimens.18-20 Changes in neoadjuvant chemoradiation regimens over time have been observed, and these trends may have impacts on oncologic outcomes.17 Information on performance status and comorbidities is limited to the Charlson-Deyo Comorbidity Index. While patients who received neo-adjuvant chemoradiation tended to be healthier (as noted by a lower Charlson-Deyo score), this influence on OS was accounted for through a propensity score–adjusted analysis.
Despite these limitations, the NCDB allows for a robust analysis of patients with esophageal adenocarcinoma and the benefits of neoadjuvant chemoradiation when stratified by clinical and pathologic nodal status.
Conclusions
Our study provides further evidence that patients who are clinically node positive gain a significant OS benefit from neoadjuvant chemoradiation plus surgery compared with surgery alone. However, patients who are clinically node negative may not derive an OS benefit from neoadjuvant therapy. These conclusions stress the importance for accurate clinical staging with respect to nodal status as this may have implications on treatment algorithms. Methods to accurately diagnosis or even predict truly positive nodal disease warrant clinical application and further study.
Supplementary Material
Acknowledgments
Additional Contributions: We acknowledge and thank the American College of Surgeons Committee on Cancer for providing access to the Participant User File from the National Cancer Database.
Footnotes
Author Contributions: Drs Gabriel and Kukar had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gabriel, Tuttle, Nurkin, Hochwald, Kukar.
Acquisition, analysis, or interpretation of data: Gabriel, Attwood, Du, Alnaji, Nurkin, Malhotra, Hochwald, Kukar.
Drafting of the manuscript: Gabriel, Du, Alnaji, Kukar.
Critical revision of the manuscript for important intellectual content: Gabriel, Attwood, Tuttle, Nurkin, Malhotra, Hochwald, Kukar.
Statistical analysis: Attwood, Du, Kukar.
Administrative, technical, or material support: Gabriel, Du, Tuttle.
Study supervision: Tuttle, Nurkin, Malhotra, Hochwald.
Conflict of Interest Disclosures: None reported.
Disclaimers: The American College of Surgeons Committee on Cancer provided the Participant User File from the National Cancer Database but did not review or validate the results or conclusions of our study.
References
- 1.National Comprehensive Cancer Network. [Accessed January 5, 2015];Esophagus: version 4. http://www.nccn.org/professionals/physician_gls/pdf/esophagus.pdf Published 2014.
- 2.Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359(9319):1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, et al. CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.American Joint Committee on Cancer. Manual for Staging of Cancer. 5th. Philadelphia, PA: Lippincott Raven Publishers; 1997. [Google Scholar]
- 5.American Joint Committee on Cancer. Manual for Staging of Cancer. 6th. Philadelphia, PA: Lippincott Raven Publishers; 2002. [Google Scholar]
- 6.Cunningham D, Allum WH, Stenning SP, et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 7.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 8.Sjoquist KM, Burmeister BH, Smithers BM, et al. Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 9.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer. 2013;49(15):3149–3158. doi: 10.1016/j.ejca.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. GE Adenocarcinoma Meta-analysis Group. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev. 2013;5:CD008107. doi: 10.1002/14651858.CD008107.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speicher PJ, Ganapathi AM, Englum BR, et al. Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. J Thorac Oncol. 2014;9(8):1195–1201. doi: 10.1097/JTO.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JQ, Hooker CM, Brock MV, et al. Neoadjuvant chemoradiation therapy is beneficial for clinical stage T2 N0 esophageal cancer patients due to inaccurate preoperative staging. Ann Thorac Surg. 2012;93(2):429–435. doi: 10.1016/j.athoracsur.2011.10.061. discussion 436-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kountourakis P, Correa AM, Hofstetter WL, et al. Combined modality therapy of cT2N0M0 esophageal cancer: the University of Texas MD Anderson Cancer Center experience. Cancer. 2011;117(5):925–930. doi: 10.1002/cncr.25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? a randomised phase II trial. Eur J Cancer. 2011;47(3):354–360. doi: 10.1016/j.ejca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Rivera F, Galán M, Tabernero J, et al. Spanish Cooperative Group for Digestive Tumor Therapy. Phase II trial of preoperative irinotecan-cisplatin followed by concurrent irinotecan-cisplatin and radiotherapy for resectable locally advanced gastric and esophagogastric junction adenocarcinoma. Int J Radiat Oncol Biol Phys. 2009;75(5):1430–1436. doi: 10.1016/j.ijrobp.2008.12.087. [DOI] [PubMed] [Google Scholar]
- 16.Zemanova M, Petruzelka L, Pazdro A, et al. Prospective non-randomized study of preoperative concurrent platinum plus 5-fluorouracil-based chemoradiotherapy with or without paclitaxel in esophageal cancer patients: long-term follow-up. Dis Esophagus. 2010;23(2):160–167. doi: 10.1111/j.1442-2050.2009.00984.x. [DOI] [PubMed] [Google Scholar]
- 17.Speicher PJ, Wang X, Englum BR, et al. Induction chemoradiation therapy prior to esophagectomy is associated with superior long-term survival for esophageal cancer. Dis Esophagus. doi: 10.1111/dote.12285. published online September 12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilson DH. Cancer of the gastroesophageal junction: combined modality therapy. Surg Oncol Clin N Am. 2006;15(4):803–824. doi: 10.1016/j.soc.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Knox JJ, Wong R, Visbal AL, et al. Phase 2 trial of preoperative irinotecan plus cisplatin and conformal radiotherapy, followed by surgery for esophageal cancer. Cancer. 2010;116(17):4023–4032. doi: 10.1002/cncr.25349. [DOI] [PubMed] [Google Scholar]
- 20.Hamai Y, Hihara J, Taomoto J, Yamakita I, Ibuki Y, Okada M. Effects of neoadjuvant chemoradiation on postoperative morbidity and mortality associated with esophageal cancer. Dis Esophagus. 2014;28(4):358–364. doi: 10.1111/dote.12207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.