Abstract
Purpose
Histone deacetylase inhibitors (HDIs) are promising anticancer therapies; however, drug resistance limits their efficacy. Here, we investigated the molecular mechanisms underlying HDI resistance, focusing on the mechanism of HDI-mediated induction of insulin-like growth factor 2 (IGF2) based on our previous study.
Experimental design
The methylation status of CCCTC binding factor (CTCF)-binding sites in the IGF2/H19 imprinting control region (ICR) were determined by methylation-specific PCR and bisulfite sequencing. The effectiveness of single or combinatorial blockade of DNA methyltransferase 1 (DNMT1) and histone deacetylase (HDAC) was evaluated using cell viability assay and patient-derived tumor xenograft (PDX) model.
Results
HDAC inhibition by vorinostat increased acetylated STAT3 (K685), resulting in transcriptional upregulation of DNMT1. DNMT1-mediated hypermethylation of CTCF-binding sites in the IGF2/H19 ICR decreased CTCF insulator activity, leading to a transcriptional upregulation of IGF2 and activation of the insulin-like growth factor 1 receptor (IGF-1R) pathway in cells with acquired or de novo vorinostat resistance. Strategies targeting DNMT1 or STAT3 diminished the IGF2 expression and potentiated vorinostat sensitivity in preclinical models of lung cancer with hypermethylation in the H19/IGF2 ICR. The degree of ICR hypermethylation correlated with vorinostat resistance in patient-derived lung tumors and in patients with hematological malignancies.
Conclusions
DNMT1-mediated transcriptional upregulation of IGF2 is a novel mechanism of resistance to HDIs, highlighting the role of epigenetic deregulation of IGF2 in HDI resistance and the potential value of the H19/IGF2 ICR hypermethylation and DNMT1 expression as predictive biomarkers in HDI-based anticancer therapies.
Keywords: histone deacetylase inhibitor, insulin-like growth factor 2, DNA methyltransferase 1, drug resistance
Introduction
Epigenetics involves the regulation of gene expression by factors other than the DNA sequence, in particular, DNA methylation and post-translational modifications of chromatin, such as acetylation of histones and non-histone proteins (1). Although DNA methylation is performed by a family of enzymes called DNA methyltransferases (DNMTs), histone acetylation is controlled by two distinct enzyme families, i.e., histone acetyltransferases and histone deacetylases (HDACs) (2, 3). Over the past decades, an increasing body of evidence indicates that epigenetics plays an important role in regulating numerous biological and pathological processes. In particular, HDACs have been shown to be overexpressed, mutated, and aberrantly recruited to various oncogenic transcription factors in cancer cells (4, 5). In addition, inhibition of HDACs triggers a myriad of cellular responses, including the suppression of cell proliferation and the induction of cellular stress (6). In this regard, there have been extensive efforts to develop histone deacetylase inhibitors (HDIs) as novel anticancer drugs, culminating in the FDA approval of the first-in-class drug vorinostat (Zolinza®, suberoylanilide hydroxamic acid, SAHA) in 2006. Currently, several next-generation HDIs are under clinical development (7-9).
Vorinostat is currently approved for the treatment of patients with cutaneous T-cell lymphomas (CTCLs) (10, 11). However, the reported response rates to vorinostat therapy remain at approximately 30% in patients with CTCLs, leaving substantial room for improvement (10, 12). Moreover, the indication of vorinostat is limited to CTCLs, despite some initial evidence of its antitumor efficacy in a subset of patients with different types of hematological and solid cancers, including lung cancer during the early stages of clinical development (12-16). To fully harness the therapeutic potential of HDIs, it is imperative to investigate the molecular and genetic factors influencing the sensitivity/resistance of cancer cells to the drugs. In addition, patients who initially respond to HDIs inevitably develop resistance; thus, identification of the possible mechanisms by which cancer cells develop acquired resistance to HDI-based therapy should be necessary. Our previous study indicated that HDIs, including vorinostat, activate the insulin-like growth factor 1 receptor (IGF-1R) signaling pathway in a subset of non-small cell lung cancers (NSCLC) with primary and acquired resistance to HDIs via direct binding of STAT3 to IGF2 P3 and P4 promoters, leading to increase in IGF2 transcription (17, 18). As a ligand for IGF-1R, IGF-2R, and insulin receptor isoform A (IR-A), IGF2 is known to modulate cell proliferation, survival, and differentiation (19). In addition, previous reports also suggest the involvement of IGF2 in the reduced tumor sensitivity to anticancer drugs, including cetuximab and paclitaxel (9, 20). IGF2 expression is regulated by genomic imprinting and promoter-driven transcription (19). In our previous study, acetylated STAT3, induced by vorinostat treatment, was crucial for transcriptional upregulation of IGF2 (18); however, STAT3 acetylation was commonly observed regardless of vorinostat sensitivity, suggesting additional determinants may be associated with the HDI resistance via increase in IGF2 transcription.
Here, we report the novel mechanistic finding underlying regulation of IGF2 expression in cells displaying resistance to HDIs. We found that vorinostat increased acetylated STAT3 (K685), leading to transcriptional upregulation of DNMT1. The hypermethylation of the H19/IGF2 ICR via DNMT1 upregulation is involved in primary and acquired resistance of NSCLC cells to vorinostat. The results from NSCLC patient-derived xenograft models were consistent with the in vitro findings, in that the anticancer efficacy of vorinostat was reduced in mice harboring tumors with the hypermethylated H19/IGF2 ICR and was significantly enhanced by co-treatment with DNMT1 inhibitors. The degree of ICR hypermethylation was associated with vorinostat sensitivity in patient-derived lung tumors and in hematological malignancies. Taken together, these findings reveal a novel mechanism of cancer resistance to HDIs and provide a potential strategy that may enhance the anticancer efficacy of HDIs in NSCLC and CTCLs.
Materials and Methods
Additional or detailed methods are described in the Supplementary Materials and Methods.
Cells and reagents
Human NSCLC lines (H460, H226B, H1299, H226Br, H520, H1944, A549, H358, and H322) and human lymphoma cells (HH) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) or kindly provided by Drs. Jack A. Roth and John V. Heymach (MD Anderson Cancer Center, Houston, TX, USA). Human lymphoma cell lines (Ramos, Daudi, Hut78, and H9) were purchased from the Korean Cell Line Bank (Seoul, Republic of Korea), and mouse lymphoma EL4 cells were kindly provided by Dr. Yeonseok Chung (Seoul National University, Seoul, Republic of Korea). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics and were maintained at 37°C with 5% CO2 in a humidified atmosphere. Vorinostat was obtained from Cayman (Ann Arbor, MI, USA), and other chemicals were from Sigma-Aldrich (St. Louis, MO, USA). Human cancer cell lines were authenticated and validated at the Korean Cell Line Bank using AmplFLSTR identifier PCR Amplification Kit (Applied Biosystems, Foster, CA; cat. No. 4322288) in 2013. Cells passed for fewer than 6 months after receipt or resuscitation of validated cells were used in this study.
Methylation-specific PCR
Cell lysates were prepared with a lysis buffer (150 mM Tris, pH 8.5, 200 mM NaCl, 5 mM EDTA [pH 8.0], 0.2% SDS, and 300 μg/ml proteinase K) and subsequently incubated at 55°C overnight. DNA was extracted using isopropanol as reported previously (21) and dissolved in TE buffer. DNA (1–5 μg) was modified with sodium bisulfite, and the methylation status of DNA in the H19/IGF2 ICR locus was analyzed by a nested PCR using DNA methylation-specific primers as described previously (Supplementary Table S3) (22, 23). In addition, bisulfite-treated DNA was further cloned and sequenced using the following primers: sense 5'-TGTTGAAGGTTGGGGAGATGGGA-3' and antisense 5'-CCCAAACCATAACACTAAAACCCTC-3' as described previously (24).
Chromatin immunoprecipitation (ChIP) assay
To verify the binding of CTCF to the H19/ICF2 ICR, a ChIP assay was performed using the SimpleChIP enzymatic chromatin IP kit (Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer’s protocol. Briefly, the chromatin cross-linked to proteins was digested with micrococcal nuclease. The digested chromatin was immunoprecipitated with an anti-CTCF antibody (Cell Signaling Technology, Danvers, MA, USA) or control IgG. The DNA-protein cross-links of immunoprecipitates were reversed, and the resulting DNA was purified for PCR analyses. Primers used for PCR analyses are listed in Supplementary Table S4. The binding of CTCF was determined by the following formula: Percent Input = 2% × 2(Ct(2%Input Sample) − Ct(IP Sample)).
Quantitative PCR analyses for determining the methylation status in the H19/IGF2 ICR
The tumor samples from PDX models and the tissue specimens from CTCL patients were subjected to DNA extraction as described previously (21). For the CTCL specimens, the sections of formalin-fixed and paraffin-embedded samples, which were previously subjected to IHC analysis, were used due to limited sample availability. Due to the small size of tissue biopsy from CTCL patients, multiple sections derived from the same patient were combined for DNA extraction and digested with DNA extraction buffer (150 mM Tris-HCl [pH 8.5], 0.2% SDS, 200 mM NaCl, 5 mM EDTA, and 300 μg/ml proteinase K). After dissolving in TE buffer, DNA (100 ng) was digested with the methylation-sensitive enzyme HpaII (New England Biolabs, Ipswich, MA, USA) at 37°C for at least 8 hours. Digested and undigested DNA samples (1-2 ng) were analyzed by real-time PCR. For determining methylation status at the H19/IGF2 ICR locus, the following primer sequences were used: sense 5'-ACGCTTCCCCTTCTGTCTC-3' and antisense 5'-GGAATGTTAATGTCTGGCCACT-3' as reported previously (25). For normalization, a region with sequences not susceptible to the HpaII digestion was amplified using the following primer sequences: sense 5'-ATGGAACTGGGACACCTCATTGTT-3' and antisense 5'-AGGTATAGGAACACTCATGGGAGC-3'. The percentage of methylation in individual samples was determined by the following formula according to previous reports with modifications: [1/2[ΔCt(Digest)− ΔCt(Undigest))] × 100 (%) (25, 26).
Animal studies
All animal procedures were performed using protocols approved by the Seoul National University Institutional Animal Care and Use Committee. For xenograft models, H1944 and H1944R cells (4-10 × 106 cells/spot) were subcutaneously injected into the flanks of a 5-6-week-old nude or NOD/SCID mice. For patient-derived xenograft (PDX) models, small pieces of tumors derived from NSCLC patients were subcutaneously inoculated into the flanks of 5-6-week-old NOD/SCID mice. After the tumor volume reached 50–150 mm3, the mice were randomly assigned to different treatment groups receiving an intraperitoneal injection of either drugs or vehicle (3-6 times/week, 17-21 days). Decitabine and vorinostat were dissolved in PBS and distilled water containing 10% DMSO/45% polyethylene glycol 400, respectively. Tumor growth was determined by measuring the short and long diameters of the tumor with a caliper and using the following formula: tumor volume (mm3) = (short diameter mm)2 × (long diameter mm) × 0.5.
Statistical analyses
The data are presented as the mean ± SD unless indicated otherwise. All in vitro experiments were independently performed at least twice, and a representative result is shown unless indicated otherwise. The statistical significance was determined using two-sided Student’s t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA) or GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA), respectively. P values less than 0.05 were considered significant.
Results
Activation of the IGF-1R pathway is involved in the development of the resistance of NSCLC cell lines to vorinostat
Recently, we reported that human NSCLC cell lines vary substantially in terms of their sensitivities to vorinostat (17). To investigate the molecular mechanisms underlying cancer resistance to vorinostat, three cell lines (H1944, H358, and H322) were subjected to in vitro selection processes using gradually increasing drug concentrations for 2-6 months. The resulting sublines displayed significantly decreased sensitivity to vorinostat (Figure 1A). We confirmed that the established sublines maintained vorinostat resistance in vivo by employing xenograft models using H1944R, H322R, and their parental cells. H358 cells did not establish xenograft tumors in NOD/SCID mice. The inhibitory effects of vorinostat on tumor growth were significantly attenuated in mice bearing H1944R or H322R xenografts compared with their parental control (Supplementary Figure S1A).
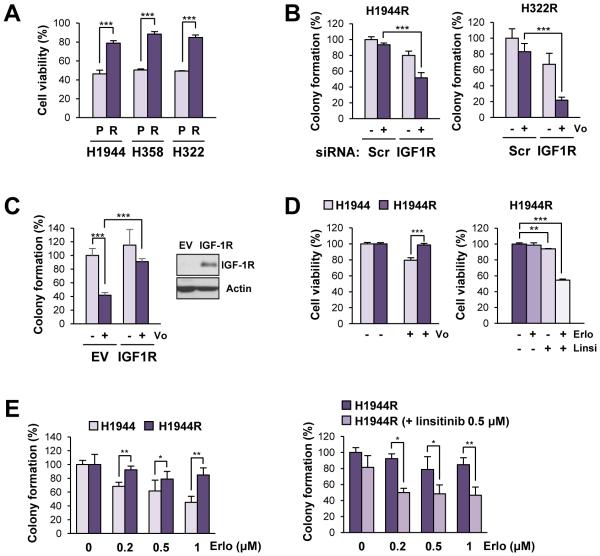
Figure 1. Association of the IGF-1R activation with acquired resistance to vorinostat in NSCLC cell lines.
(A) Viability of parental NSCLC cells vs. their sublines with acquired resistance to vorinostat in response to vorinostat treatment, determined by the MTT assay. The viability of cells treated with vorinostat (5 μM) was shown. (B) Anchorage-independent colony-forming ability of vorinostat-resistant NSCLC sublines which experienced siRNA-mediated knockdown of IGF-1R (Scr: scrambled siRNA control). Colony-forming ability of cells treated with vorinostat (1 μM) was shown. (C) Overexpression of IGF-1R led to a decreased sensitivity of H520 cells to vorinostat (assessed by the suppression of anchorage-independent colony formation following the treatment with vorinostat [1 μM for 2 weeks]) compared with the empty vector (EV) controls. (D, E) Viability (D) and anchorage-dependent colony-forming ability (E) of parental NSCLC cells vs. their sublines with acquired resistance in response to erlotinib, linsitinib, or their combination. Cells were treated with linsitinib (Linsi; 0.5 μM), alone or in combination with erlotinib (Erlo; 0.5 μM; D) or increasing concentrations of erlotinib (Erlo; 0.2, 0.5, and 1 μM; E). The data are presented as the mean ± SD (n = 3). Statistical significance was determined by Student’s t-test (*: P < 0.05; **: P < 0.01; ***: P < 0.001). Con: control; Vo: vorinostat.
To examine the potential involvement of kinase pathways in the development of acquired resistance to vorinostat, the phosphorylation status of 42 receptor tyrosine kinases and 43 protein kinases was profiled in H1944R and H1944 cells. The H1944R subline displayed substantially enhanced phosphorylation of various kinases, including IGF-1R and Akt (Supplementary Table S1). These results were validated by the immunoblotting analyses of IGF-1R, Akt, ERK1/2 and their phosphorylated proteins (Supplementary Figure S1B). We previously demonstrated that IGF-1R activation contributes to primary resistance to HDAC inhibitors (17). We then assessed whether IGF-1R activation contributed to the acquired resistance to vorinostat. Although the abilities of vorinostat to suppress viability (Supplementary Figure S1C) and colony-forming ability (Figure 1B) were restored in vorinostat-resistant sublines (H1944R, H358R, and H322R) following siRNA-mediated knockdown of IGF-1R, the overexpression of IGF-1R conferred resistance to vorinostat in H520 cells (Figure 1C). Therefore, the IGF-1R signaling pathway appears to play a key role in both primary and acquired resistance to vorinostat in NSCLC cells.
Considering the role of the IGF-1R pathway in the resistance to molecularly targeted anticancer drugs including EGFR TKIs (27), we reasoned that H1944R cells would be more resistant to EGFR TKIs. In support of the notion, H1944R cells showed significantly greater viability in response to erlotinib treatment, an EGFR TKI, compared with H1944 cells (Figure 1D, left). We then tested the impact of the IGF-1R pathway on NSCLC cells’ response to erlotinib and found that H1944R cells showed a significantly decreased viability when treated with erlotinib in combination with linsitinib, an IGF-1R TKI (Figure 1D, right). We further compared the anchorage-dependent colony forming ability of H1944 and H1944R cells during incubation with erlotinib alone or in combination with linsitinib. As shown in Figure 1E, H1944R cells showed a weaker sensitivity to erlotinib than H1944 cells; however, combined treatment with linsitinib significantly enhanced the efficacy of erlotinib. We calculated the combination index as described previously (28). Combination indices indicated synergism of the combinatorial treatment (Supplementary Table S2). Therefore, considering our previous findings on the association of IGF-1R activation with primary vorinostat resistance (17), activation of the IGF-1R pathway appears to play a key role in both primary and acquired resistance to vorinostat and cross-resistance to other anticancer drugs in vorinostat-resistant sublines.
Hypermethylation of the H19/IGF2 ICR in NSCLC cells with acquired and intrinsic resistance to vorinostat
To understand the mechanisms underlying IGF-1R activation in the vorinostat-resistant sublines, we attempted to identify the inducer(s) of the IGF-1R activation although there were no detectable differences in IGF-1R and IGF1 expression between the sublines and their parental cells, the protein and mRNA levels of IGF2 were markedly upregulated in the vorinostat-resistant sublines compared with their parental cells (Figure 2A). Therefore, in agreement with our recent finding (18), transcriptional increases in IGF2 expression seemed to be an important mechanism contributing to the activation of the IGF-1R pathway in vorinostat-resistant NSCLC cells. Because our recent finding suggests the possible association of additional factors with significant upregulation of IGF2 transcription in vorinostat-resistant cells upon treatment with HDIs (18), we assessed the additional mechanism underlying vorinostat-induced elevation of IGF2 levels.
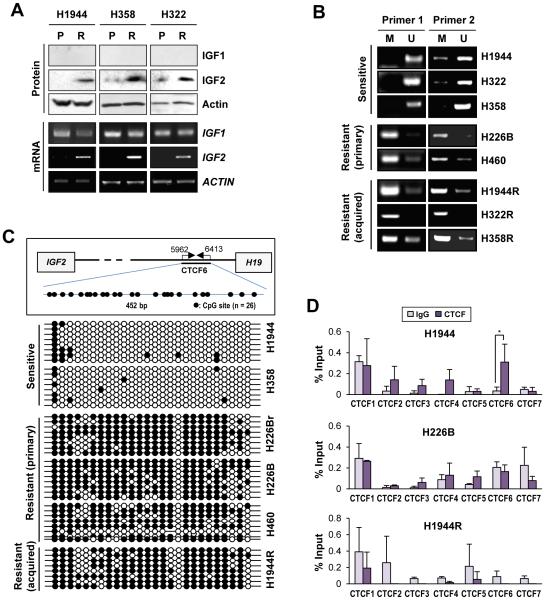
Figure 2. Transcriptional upregulation of IGF2 through hypermethylation of the H19/IGF2 ICR encompassing the CTCF-binding sites in NSCLC cell lines with acquired or primary resistance to vorinostat.
(A) Elevation in IGF2 expression at both the mRNA and protein levels in acquired vorinostat-resistant NSCLC sublines (R) compared to their parental controls (P), determined by Western blot analysis and RT-PCR. Vo: vorinostat. (B, C) The results from methylation-specific PCR (B) and bisulfite sequencing (C) indicated that the H19/IGF2 ICR encompassing the CTCF-binding sites was hypermethylated in vorinostat-resistant NSCLC cells (either acquired or intrinsic). M and U stand for methylated and unmethylated, respectively. Schematic diagram illustrating the CTCF6 in the H19/IGF2 ICR is shown in (C). (D) Changes in the binding of CTCF to seven CTCF binding sites of the H19/IGF2 ICR. The binding of CTCF was analyzed by the ChIP assay. The location of CTCF binding sites was determined according to the previous reports (36, 49). The data are presented as the mean ± SD (n = 3). Statistical significance was determined by Student’s t-test (*: P < 0.05).
IGF2 is frequently overexpressed owing to a loss of imprinting (LOI) (29-31), in which methylation of the imprinting control region (ICR)—a differentially methylated region between IGF2 and H19 containing seven CCCTC-binding factor (CTCF)-binding sites (32-34)—abrogates the binding of the zinc finger protein CTCF, which acts as an insulator that sequesters enhancers downstream of H19 to suppress IGF2 transcription (35). Hence, we postulated the involvement of differential genomic imprinting in vorinostat-resistant NSCLC cells. The sixth of seven CTCF-binding sites (CTCF6) in the human H19/IGF2 ICR has been demonstrated to have allele-specific differential methylation, and the loss of differential methylation at this site correlates with LOI (36). When methylation-specific PCR analyses were performed using primers specific for the sequence spanning CTCF6 (22, 23), the extent of DNA methylation at the CTCF-binding site was markedly higher in NSCLC cell lines with primary resistance to vorinostat (H226B and H460) than in vorinostat-sensitive cell lines (H1944, H322, and H358). Moreover, NSCLC sublines that acquired vorinostat resistance also showed markedly higher methylation at the CTCF-binding site compared with their parental controls (Figure 2B). These results were further confirmed using bisulfite sequencing interrogating the methylation status of 26 CpG sites. Extensive methylation in the sequence spanning the CTCF6 was found in the vorinostat-resistant NSCLC cells (H226Br, H226B, and H460 cells with primary resistance; H1944R with acquired resistance) (Figure 2C). We next examined the binding of CTCF to seven CTCF-binding sites in the H19/IGF2 ICR using representative cell lines (sensitive: H1944; primary resistant: H226B; acquired resistant: H1944R). The results from ChIP analyses revealed CTCF bindings to four CTCF binding sites (CTCF2, 3, 4 and 6), especially CTCF6, in H1944 cells while the CTCF bindings were obviously abolished in the vorinostat-resistant NSCLC cells (Figure 2D). A decreased CTCF binding to the CTCF6 site in the H19/IGF2 ICR was confirmed in additional vorinost-sensitive and vorinostat-resistant (both primary and acquired) NSCLC cells (Supplementary Figure S2). These results suggest that loss of CTCF binding due to the aberrant increase in DNA methylation in the CTCF6 site of the H19/IGF2 ICR is closely associated with both primary and acquired resistance to vorinostat.
DNMT upregulation accounts for hypermethylation of the human H19/IGF2 ICR encompassing the CTCF-binding site 6 and consequent upregulation of IGF2 transcription
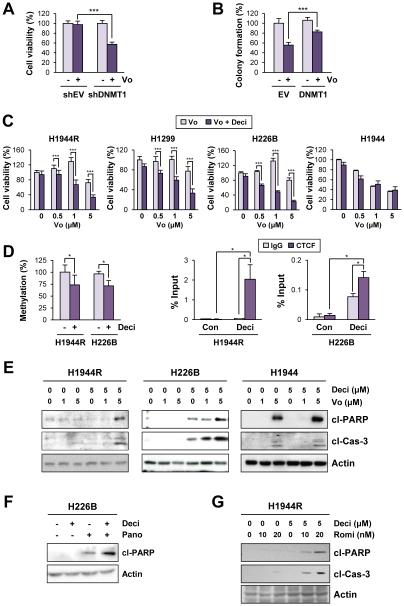
To investigate the underlying mechanism(s) for the hypermethylation of the H19/IGF2 ICR in vorinostat-resistant sublines, we compared the mRNA and protein levels of the CTCF and DNMTs. DNMT1 recognizes the hemi-methylated CpG sites established by DNMT3A and/or DNMT3B and methylates the complementary CpG, thereby converting it to a fully methylated state (37). All three sublines with acquired resistance to vorinostat showed markedly increased expression of DNMT1 compared with their parental controls (Figure 3A, top). On the other hand, the levels of CTCF, DNMT3A and DNMT3B did not show such correlations. The elevated levels of DNMT1 in vorinostat-resistant NSCLC cells were maintained in vivo as shown by the Western blot (Figure 3B) and immunohistochemical (Figure 3C) analyses of tumor tissues from mice harboring tumors derived from H1944R or H1944. Elevations in DNMT1 protein expression were also observed in primary vorinostat-resistant cell lines compared with vorinostat-sensitive cells (Figure 3D). Because DNMT1 levels were commonly increased in both primary and acquired vorinostat-resistant cells, we focused on the role of DNMT1 in vorinostat resistance. These primary and acquired vorinostat-resistant cells also showed increased DNMT1 mRNA levels compared with vorinostat-sensitive cells (Figure 3A, bottom; Figure 3E). A luciferase reporter assay showed significantly greater DNMT1 promoter activities in the vorinostat-resistant cells (H1944R, H322R, H1299, H226B, and H226Br) than in the vorinostat-sensitive cells (H1944 or H322 cells) (Figure 3F), indicating that promoter transactivation contributed to the upregulation of DNMT1 expression in vorinostat-resistant cells. We verified the role of DNMT1 in upregulation of IGF2 in NSCLC cells with vorinostat resistance. The shRNA-mediated knockdown of DNMT1 attenuated the DNA methylation near the CTCF6 site (Figure 3G, top), restored the CTCF binding to the CTCF6 site (Figure 3G, bottom), and almost completely abolished vorinostat-induced IGF2 transcription and IGF-1R phosphorylation (Figure 3H). These findings suggest that DNMT1-mediated aberrant methylation of the H19/IGF2 ICR enables vorinostat-induced IGF2 overexpression and IGF-1R activation.
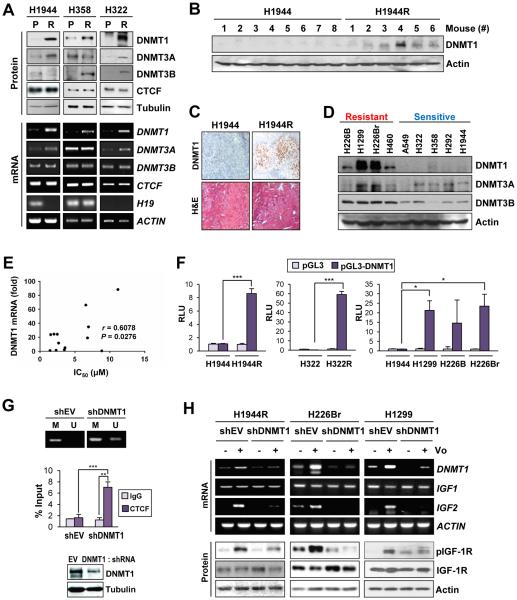
Figure 3. Upregulation of DNMT1 in NSCLC cell lines with acquired and intrinsic resistance to vorinostat and its contribution to vorinostat resistance.
(A) Western blot and RT-PCR analyses of DNMT1 protein and mRNA levels in NSCLC sublines with acquired resistance to vorinostat. DNMT1, 3A and 3B and CTCF expression levels were compared between vorinostat-resistant (R) sublines H1944, H358 and H322 and their parental controls (P). (B, C) Western blot (B) and IHC (C) analyses of DNMT1 levels in xenograft tumors of H1944R and H1944 cells. (D) Immunoblot analyses of DNMT1, DNMT3A, and DNMT3B expression in the NSCLC cell lines. (E) Correlation between the level of DNMT1 transcription and NSCLC cell lines’ resistance to vorinostat was determined by calculating the Pearson correlation coefficient using Graphpad Prism 6. DNMT1 transcription was determined by real-time PCR, and the sensitivity of NSCLC cells to vorinostat treatment (IC50 values) was previously determined and shown in our previous report (17). (F) The DNMT1 promoter activity in sensitive (H1944), primary vorinostat-resistant (H226B, H226Br), and acquired vorinostat-resistant (H1944R, H358R, and H322R) cells (RLU: relative luminescence units). (G) Methylation in the CTCF6 site of the H19/IGF2 ICR determined by Methylation-specific PCR (top) and CTCF binding to this region determined by ChIP assay (middle) in H1944R cells with shRNA-mediated knockdown of DNMT1 (bottom). (H) shRNA-mediated knockdown of DNMT1 prevented the upregulation of IGF2 and the activation of the IGF-1R pathway in response to vorinostat (5 μM for 2 days) in vorinostat-resistant NSCLC cells (either acquired or intrinsic). The data of the luciferase reporter assay and the ChIP analysis are presented as the mean ± SD. Significance was determined by Student’s t-test (F, left and middle; G) and one-way ANOVA (F, right) (*: P < 0.05; ***: P < 0.001). Vo: vorinostat.
Exposure to vorinostat activates STAT3, leading to DNMT1 expression in NSCLC cells with resistance to vorinostat
Based on previous reports on the role of the signal transducer and activator of transcription 3 (STAT3), particularly acetylated STAT3, in DNMT1 expression (manuscript submitted for publication) (38), CpG methylation (39) and vorinostat resistance in hematologic malignancies (40), we investigated whether STAT3 is involved in the upregulation of DNMT1 expression in NSCLC cells with acquired or intrinsic resistance to vorinostat. Indeed, shRNA-mediated knockdown of STAT3 decreased the DNA methylation of the CTCF6 site (Figure 4A) and DNMT and IGF2 transcription and in vorinostat-resistant H1299 cells (Figure 4B). Notably, vorinostat treatment led to an increase in transcription of DNMT1 (Figure 4C), which was suppressed by transfection with siRNA against STAT3 (Figure 4D). Moreover, combinatorial treatment with vorinostat and a STAT3 inhibitor Stattic markedly induced apoptotic cell death in vorinostat-resistant cells (Supplementary Figure S3).
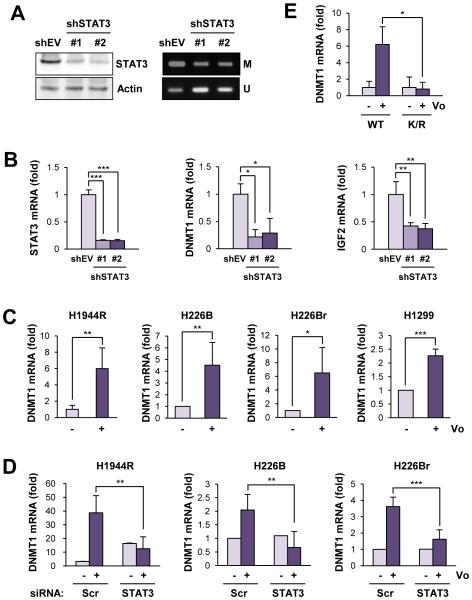
Figure 4. Involvement of acetylated STAT3 in upregulation of DNMT1 in NSCLC cell lines with acquired and intrinsic reistance to vorinostat.
(A) Decreases in the DNA methylation of the CTCF6 site in H1299 cells with shRNA-based knockdown of STAT3 expression. Left. The decreased level of STAT3 expression in H1299 cells stably transfected with STAT3 shRNAs. Right. The DNA methylation status was examined by methylation-specific PCR. (B) Downregulation of the transcription of STAT3, DNMT1, and IGF2 by H1299 cells with shRNA-based stable ablation of STAT3 expression, determined by real-time PCR. (C) DNMT1 transcription in the indicated NSCLC cells after treatment with vorinostat. Cells were treated with vorinostat (5 μM) for 2 days. The mRNA expression was analyzed by real-time PCR. (D) The siRNA-mediated knockdown of STAT3 attenuated the vorinostat-induced mRNA expression of DNMT1 in vorinostat-resistant NSCLC cells (either acquired or intrinsic). (E) Mutation of the lysine acetylation site of STAT3 (K/R) led to a decrease in vorinostat-induced DNMT1 expression in H1299 cells. The data are presented as the mean ± SD (n = 3). Significance was determined by Student’s t-test (*: P < 0.05; **: P < 0.01; ***: P < 0.001). Vo: vorinostat.
We then assessed the impact of STAT3 acetylation on DNMT1 expression by employing H1299 cells, where either wild-type STAT3 (WT) or acetylation-null mutant STAT3 (K685R) were introduced after stably depleting endogenous STAT3 by shRNA transfection. Vorinostat treatment no longer led to the upregulation of DNMT1 in the presence of the acetylation-null STAT3 mutant (Figure 4E). These results suggested that vorinostat-mediated acetylation of STAT3 led to transcription of the DNMT1 gene, thereby inducing methylation of the CTCF6 site and transcriptional increase in IGF2 expression in vorinostat-resistant NSCLC cells.
Co-treatment with the DNMT inhibitor decitabine reverses vorinostat resistance in NSCLC cell lines and PDXs with a hypermethylated human H19/IGF2 ICR
We investigated whether DNMT1 was involved in acquired and intrinsic resistance of NSCLC cells to vorinostat and whether DNMT1 inhibition is a potential therapeutic strategy to overcome vorinostat resistance. We found that shRNA-mediated DNMT1 knockdown significantly enhanced the cytotoxic effects of vorinostat in H226B cells (Figure 5A). Conversely, DNMT1 overexpression in H1944 cells significantly attenuated the response to vorinostat (Figure 5B). The co-treatment of decitabine (5-aza-2′-deoxycytidine), an orally available small-molecule DNMT inhibitor, with vorinostat universally sensitized both primary (H1299 and H226B) and acquired (H1944R) resistant cells, but not sensitive (H1944) cells, to vorinostat treatment (Figure 5C). Consistently, treatment with decitabine significantly decreased the methylation status in the CTCF6 site of the H19/IGF2 ICR and consequently restored the binding of CTCF to this region (Figure 5D). Moreover, blockade of both HDAC and DNMT revealed enhanced apoptotic activities in vorinostat-resistant H1944R, H1299 and H226B cells but not in vorinostat-sensitive H1944 cells (Figure 5E). Similar findings were obtained using co-treatment with decitabine and other HDIs, such as panobinostat (Figure 5F) or romidepsin (Figure 5G), in vorinostat-resistant H1944R and H226B cells.
Figure 5. Restoration of vorinostat sensitivity by the inhibition of DNMT in NSCLC cell lines with acquired and intrinsic resistance to vorinostat.
(A) Viability of H226B cells with stable transfection with DNMT1 shRNA determined by the MTT assay. (B) Overexpression of DNMT1 led to a decreased sensitivity of H1944 cells to vorinostat (assessed by the suppression of colony formation following the treatment with vorinostat [1 μM for 2 weeks]) compared to the empty vector (EV) controls. (C) The results from the MTT assay indicated that co-treatment of decitabine (Deci) with vorinostat sensitized vorinostat-resistant NSCLC cells (either acquired or intrinsic) to vorinostat. (D) DNA methylation in the CTCF6 site of the H19/IGF2 ICR determined by methylation-specific PCR, and the CTCF binding to the CTCF6 site was determined by the ChIP assay. H1944R and H226B cells were daily treated with decitabine (5 μM) for 4 days. (E-G) Immunoblotting analyses indicating enhanced apoptotic cell death by the co-treatment of decitabine with HDIs (E: vorinostat; F: panobinostat [Pano]; G: romidepsin [Romi]) in vorinostat-resistant NSCLC cells (either acquired or intrinsic) compared with single treatments of vorinostat or decitabine. The data are presented as the mean ± SD (n = 3). Significance was determined by Student’s t-test (*: P < 0.05; ***: P < 0.001). Vo: vorinostat.
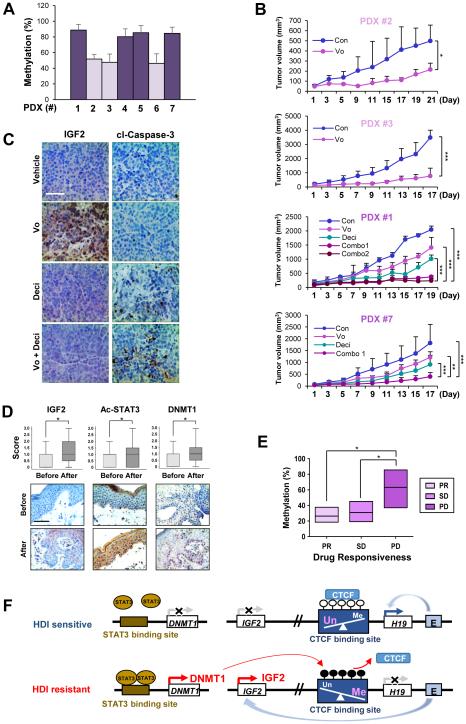
To further examine the clinical relevance of combining vorinostat and decitabine, we utilized PDX models from NSCLC patients. Four of seven PDX models were considered hypermethylated in the H19/IGF2 ICR (Figure 6A). PDX models with the H19/IGF2 ICR hypermethylated (PDX #1 and #7) were less responsive to vorinostat than those with the H19/IGF2 ICR hypomethylated (PDX #2 and #3) (Figure 6B). For PDX #1 and #7, the co-treatment of vorinostat and decitabine effectively enhanced tumor growth suppression in vivo. The effectiveness of decitabine to enhance tumor growth suppression was comparable between the concurrent (combo 1) and sequential (combo 2) treatment of decitabine and vorinostat. The results from IHC analyses indicated an increased apoptosis and decreased IGF2 expression in the mice treated with the combination of decitabine and vorinostat compared with those treated with individual drugs (Figure 6C). These findings suggest that DNMT1 antagonism may sensitize HDI-resistant tumors to HDIs by inducing apoptosis. Collectively, these data suggest that the methylation of the H19/IGF2 ICR due to the overexpression of DNMT may function not only as a predictable marker for the response to HDIs but also as a poor prognostic marker.
Figure 6. Restoration of vorinostat sensitivity by the inhibition of DNMT in PDX models with hypermethylation of the H19/IGF2 ICR and association of IGF2, acetylated STAT, DNMT1 and the methylation levels at the H19/IGF2 ICR with vorinostat resistance in CTCL patients.
(A) Real-time PCR analysis revealed varying degrees of methylation in the H19/IGF2 ICR in tumors from seven PDX models. (B) Co-treatment with decitabine (0.4 mg/kg) and vorinostat (50 mg/kg) suppressed tumor growth in PDX models harboring xenografts with hypermethylation of the H19/IGF2 ICR compared to single treatment of vorinostat (combo 1: concurrently treated with decitabine and vorinostat; combo 2: pretreated decitabine [four times] and then co-treated with vorinostat; combo [for #7]: sequential treatment with the two drugs). The tumor volumes of each group at day 0 (start of vorinostat treatment) and during 17-21 days of drug treatment are presented. (C) Representative immunohistochemical images showing that co-treatment of decitabine and vorinostat leads to decreased IGF2 expression and increased apoptosis in PDX #1 model compared to single treatment with vorinostat. Scale bar: 50 μm. (D) IHC analysis indicating increased expression levels of IGF2, acetylated STAT3, and DNMT1 in the paired tissue samples from patients with CTCL after oral vorinostat treatment (6 weeks, n = 12). Scale bar: 50 μm. (E) Real-time PCR results showing the extent of DNA methylation in the H19/IGF2 ICR in CTCL patient groups showing differing clinical outcomes to vorinostat therapy (PR: partial response; SD: stable disease; PD: progressive disease). The data are presented as the mean ± SEM (A; n = 3-6) and the mean ± SD (B: n = 6-8; D: n =12). Significance was determined by one-way ANOVA (B, E) and Wilcoxon signed-rank test (two-tailed) (D) (*: P < 0.05; **: P < 0.01; ***: P < 0.001). Vo: vorinostat. (F) A schematic diagram illustrating a mechanism underlying cancer resistance to vorinostat. In vorinostat-sensitive cancer cells, the hypomethylated CTCF-binding sites at the IGF2/H19 ICR allow for the binding of CTCF and the transactivation of the H19 promoter by the downstream enhancers, thereby preferentially expressing H19 and silencing IGF2. On the other hand, in cancer cells with vorinostat resistance, the accumulation of acetylated STAT3 induces the transactivation of the DNMT1 promoter and subsequently increases the methylation of the CTCF-binding site in the IGF2/H19 ICR. These changes prevent CTCF from binding to the CTCF-binding sites, and in turn, the downstream enhancers gain access to the IGF2 promoter, leading to the transactivation of IGF2 and subsequent activation of the IGF-1R signaling pathway.
Association of IGF2, acetylated STAT, DNMT1 and methylation levels of the H19/IGF2 ICR with vorinostat resistance in CTCL
Because vorinostat has been used to treat CTCL (10, 11), we assessed whether the mechanisms of vorinostat resistance involving DNA hypermethylation via DNMT1 upregulation are applicable to lymphoma. To this end, we assessed the correlation between sensitivities to vorinostat and DNMT1 expression in a panel of lymphoma cell lines. The MTT assay revealed that vorinostat treatment induced a dose-dependent decrease in the viability of two human lymphoma cell lines (HH, Ramos) with low levels of DNMT1 expression compared with two human lymphoma cell lines (Hut 78, H9) and one mouse lymphoma cell line (EL4) with high expression levels of DNMT1 (Supplementary Figure S4, A and B). Moreover, combined treatment with decitabine and vorinostat showed enhanced apoptosis in vorinostat-resistant Hut 78, H9, and EL4 cells (Supplementary Figure S4C). Next, we analyzed a subset of clinical tissue samples available from previously published clinical trial in patients with CTCL (12, 41). We assessed the expression of DNMT1, IGF2 and acetylated STAT3 in paired clinical tissue samples before and after vorinostat treatment from 12 patients with CTCL. We observed that IGF2, DNMT1, and acetylated STAT3 levels were significantly increased in CTCL tissues after vorinostat treatment (Figure 6D). More importantly, the methylation levels of H19/IGF2 ICR from progressive disease (PD) samples were significantly higher than those from partial response (PR) and stable disease (SD) samples (Figure 6E). These findings suggest that methylation of the H19/IGF2 ICR may be predictive of clinical response to vorinostat treatment in patients with CTCL.
Discussion
Drug resistance is generally thought to be the most significant obstacle to improving outcomes in cancer patients. The diverse heterogeneity of genetic and molecular alterations observed in cancer cells and their ability to compensate the perturbed pathways in response to drug treatment likely account for drug resistance, either de novo or acquired (42, 43). In this work, we have provided evidence that activation of the IGF-1R pathway through transcriptional upregulation of IGF2 via DNMT1-mediated hypermethylation of the H19/IGF2 ICR play a key role in both de novo and acquired resistance to HDIs.
In our recent report (18), we demonstrated that HDIs induce IGF2 expression through a novel mechanism that involves STAT3. We showed HDI-mediated stabilization of STAT3 protein through acetylation (K685) and STAT3-mediated transcriptional upregulation of IGF2 in both primary and acquired HDI-resistant cells. Further, genomic and pharmacologic approaches targeting STAT3 effectively suppressed the HDI-induced IGF2 transcription and restored drug sensitivity in both primary and acquired HDI-resistant cells in vitro and in vivo. These findings indicated the universal role of STAT3 in HDI-induced IGF2 expression and NSCLC cells’ resistance to HDIs. However, in the study, the basal level of acetylated STAT3 was not correlated with vorinostat resistance. Upon vorinostat treatment, increased STAT3 activity was commonly observed in both vorinostat-sensitive and -resistant cells, implying the existence of additional factors associated with the HDIs-induced events. In the current study, we show that NSCLC cells initially sensitive to vorinostat achieve STAT3-mediated DNMT1 overexpression and DNMT1-mediated hypermethylation of the IGF2/H19 ICR upon prolonged drug exposure. NSCLC cells with primary resistance to vorinostat were found to have an endogenous DNMT1 overexpression. Hypermethylation in a CTCF-binding site of the IGF2/H19 ICR eliminates enhancer blocking, augmenting IGF2 transcription and resistance to HDIs. We show that blockade of DNMT effectively suppresses HDI-induced IGF2 transcription and potentiates the therapeutic efficacy of HDIs in various human NSCLC cell lines and PDXs. We also show the potential value of the H19/IGF2 ICR methylation as a predictive biomarker for both acquired and primary resistance to HDIs. A model of how HDAC inhibition by vorinostat treatment leads to IGF2 transcription and drug resistance is shown in Figure 6F. To our knowledge, this study is the first to demonstrate the increased expression of a cancer-promoting gene via epigenetic mechanisms as a determinant of HDI resistance.
HDIs have been intensively studied in preclinical models and in clinical trials for solid and hematological malignancies. However, intrinsic mechanisms of drug resistance and acquired drug resistance due to stable genetic/epigenetic alterations after chronic drug exposure hinder the mode of action of HDIs. We have previously reported that activation of the IGF-1R pathway is involved in the primary resistance of NSCLC cells to HDIs, including vorinostat (17). We also used an IGF-1R monoclonal antibody to demonstrate that targeting the IGF-1R pathway can restore drug sensitivity of the NSCLC cells (17). In our current study, we found that activation of the IGF-1R pathway is involved in the acquired resistance of NSCLC cells to HDIs. Hence, we continued our interrogation of the molecular and genetic events responsible for the IGF-1R activation upon HDAC inhibition. We observed that persistent hypermethylation of the H19/IGF2 ICR encompassing the CTCF-binding sites represents a unique mechanism for the IGF2 upregulation and subsequent IGF-1R activation in a subset of NSCLC cells that are intrinsically resistant or become resistant to vorinostat. The NSCLC cells with such changes appeared to be resistant to multiple anticancer drugs, such as TKIs inhibiting EGFR, and inactivation of IGF-1R enhanced the antiproliferative activities of the drug. As such, activation of the IGF2-IGF-1R signaling cascade seemed to enhance the capacity of NSCLC cells to survive. Therefore, identification of the molecular determinants responsible for IGF2 overexpression may provide strategies for combined therapies for patients with resistance to HDIs.
Recently, activation of STAT3 has been suggested as a factor for HDI resistance in CTCL (40). However, the molecular mechanisms that link STAT3 activation to vorinostat resistance have been elusive. Most previous studies have focused on phosphorylated STAT3 as a mediator of vorinostat resistance. Localization of phosphorylated STAT3 protein changes from nuclear to cytoplasmic in response to vorinostat treatment in CTCL patients (12). However, our recent study revealed that STAT3 acetylation, but not phosphorylation, was induced upon HDAC inhibition, resulting in protection of STAT3 from the proteasome-mediated degradation (18). In that study, the antitumor effects of vorinostat were significantly enhanced when STAT3 was inactivated. These data suggest that the increased STAT3 activity via acetylation plays a key role in vorinostat resistance. These results are consistent with the established role of STAT3 in IGF2 gene expression (44, 45). However, vorinostat treatment uniformly increased acetylation of STAT3 in both vorinostat-sensitive and vorinostat-resistant cells, indicating that other genetic/epigenetic alterations in cancer cells are required for HDI-induced IGF2 transcription.
Our study reveals that overexpression of DNMT1, either endogenous or induced upon vorinostat treatment, causes hypermethylation of the H19/IGF2 ICR in a CTCF-binding site and decreased CTCF binding to the site and thus plays an essential role in transcriptional increase in IGF2 expression in both primary and acquired vorinostat-resistant NSCLC cells. Our results show that increased STAT3 activity through acetylation plays an additional role as an important transcription factor for DNMT expression. These findings are in line with the previous report showing increases in STAT3 acetylation along with DNMT1-mediated promoter methylation of several tumor suppressor genes in human cancer cells (39, 46). More importantly, inactivation of DNMT1 universally suppressed the vorinostat-induced IGF2 gene expression and IGF-1R activation and significantly restored HDI sensitivity in both primary and acquired vorinostat-resistant NSCLC cells by inducing apoptosis. Similar inhibitory effects were also observed in a small number of NSCLC PDXs with hypermethylation of the H19/IGF2 ICR. These findings are consistent with recent preclinical studies demonstrating synergistic antitumor activities of combined treatment with vorinostat and decitabine (47, 48). Finally, our initial analyses of clinical CTCL samples available from a previously conducted vorinostat-based clinical trial showed apparent correlations between hypermethylation of the H19/IGF2 ICR and clinical response. These results suggest that targeting DNMT1 may be a potential option for overcoming cancer resistance to vorinostat. Indeed, positive outcomes were reported from a phase I clinical trial which investigated the combination regimen of vorinostat and decitabine in patients with untreated and relapsed/refractory acute myeloid leukemia (48). Together, these results indicate the essential role of the DNMT1-dependent methylation of the H19/IGF2 ICR locus in HDI-induced IGF2 transcription and the clinical utility of the DNMT inhibitors to overcome resistance to HDIs.
In summary, the present study demonstrates the involvement of the IGF2-IGF-1R signaling cascade via the upregulation of DNMT1 and subsequent loss of imprinting in the IGF2 gene in vorinostat resistance. These results suggest that targeting DNMT1 could be a potential strategy to overcome vorinostat resistance in patients with NSCLC and CTCL. Given that epigenetic mechanisms often involve an active interplay between DNA methylation and histone modifications, the combination strategy of vorinostat and DNMT1 inhibitors would have a solid rationale. Further studies are required to understand the role of DNMT3B in the H19/IGF2 ICR hypermethylation and IGF2 expression along with HDI resistance. In addition, clinical investigations are warranted to assess whether therapeutic strategies combining HDIs with inhibitors of DNA methylation could overcome HDI resistance in additional types of hematological and nonhematological malignancies. Further, the potential value of the H19/IGF2 ICR hypermethylation and DNMT1 expression as predictive biomarkers needs to be evaluated. Given that decitabine likely affects other subtypes of DNMTs in addition to DNMT1, it may be necessary to determine whether specific inhibitors of DNMT1 would be more effective than broad-spectrum inhibitors of DNMTs in reversing cancer resistance to vorinostat.
Supplementary Material
Statement of translational relevance.
Histone deacetylase inhibitors (HDIs) have been considered as an effective anticancer therapy, but their clinical uses have been limited to hematological malignancy due to ineffectiveness to solid tumors. Drug resistance mediates these limitations, yet the underlying mechanisms are largely unknown. Here, we show epigenetically induced IGF2 expression is a common mechanism underlying both intrinsic and acquired resistance to HDIs. We found that a representative HDI vorinostat increased acetylated STAT3, leading to transcriptional upregulation of DNMT1. Increased IGF2 expression caused by DNMT1-induced hypermethylation of the H19/IGF2 ICR is involved in primary and acquired resistance of NSCLC cells to vorinostat. Co-targeting DNMT1 improved antitumor efficacy of vorinostat and other HDIs. Further, the degree of ICR hypermethylation was closely associated with vorinostat sensitivity in patient-derived lung tumors and in hematological malignancies. These results suggest DNMT1 as a potential therapeutic target to overcome HDI resistance in patients with NSCLC and CTCL.
Acknowledgments
Financial support: This work was supported by grants from the National Research Foundation of Korea (NRF), the Ministry of Science, ICT & Future Planning (MSIP), Republic of Korea (No. NRF-2016R1A3B1908631) and the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (No. 1520250).
Footnotes
Potential conflicts of Interest: There are no potential conflicts of interest to declare.
References
- 1.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 2.Hamidi T, Singh AK, Chen T. Genetic alterations of DNA methylation machinery in human diseases. Epigenomics. 2015;7:247–65. doi: 10.2217/epi.14.80. [DOI] [PubMed] [Google Scholar]
- 3.Peserico A, Simone C. Physical and functional HAT/HDAC interplay regulates protein acetylation balance. Journal of biomedicine & biotechnology. 2011;2011:371832. doi: 10.1155/2011/371832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richon V. Cancer biology: mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor. British journal of cancer. 2006;95:S2–S6. [Google Scholar]
- 5.Fantin VR, Richon VM. Mechanisms of resistance to histone deacetylase inhibitors and their therapeutic implications. Clin Cancer Res. 2007;13:7237–42. doi: 10.1158/1078-0432.CCR-07-2114. [DOI] [PubMed] [Google Scholar]
- 6.Marks PA, Xu WS. Histone deacetylase inhibitors: Potential in cancer therapy. Journal of cellular biochemistry. 2009;107:600–8. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Zhong Q. Histone deacetylase inhibitors and cell death. Cellular and molecular life sciences : CMLS. 2014;71:3885–901. doi: 10.1007/s00018-014-1656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner JM, Hackanson B, Lubbert M, Jung M. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clinical epigenetics. 2010;1:117–36. doi: 10.1007/s13148-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanella ER, Galimi F, Sassi F, Migliardi G, Cottino F, Leto SM, et al. IGF2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci Transl Med. 2015;7:272ra12. doi: 10.1126/scitranslmed.3010445. [DOI] [PubMed] [Google Scholar]
- 10.Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–15. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 11.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. The oncologist. 2007;12:1247–52. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 12.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–9. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly WK, O'Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–31. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traynor AM, Dubey S, Eickhoff JC, Kolesar JM, Schell K, Huie MS, et al. Vorinostat (NSC # 701852) in patients with relapsed non-small cell lung cancer: a Wisconsin Oncology Network phase II study. J Thorac Oncol. 2009;4:522–6. doi: 10.1097/jto.0b013e3181952478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara Y, Yamamoto N, Yamada Y, Yamada K, Otsuki T, Kanazu S, et al. Phase I and pharmacokinetic study of vorinostat (suberoylanilide hydroxamic acid) in Japanese patients with solid tumors. Cancer science. 2009;100:1728–34. doi: 10.1111/j.1349-7006.2009.01237.x. [DOI] [PubMed] [Google Scholar]
- 16.Bradley D, Rathkopf D, Dunn R, Stadler WM, Liu G, Smith DC, et al. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer. 2009;115:5541–9. doi: 10.1002/cncr.24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JS, Lee SC, Min HY, Park KH, Hyun SY, Kwon SJ, et al. Activation of insulin-like growth factor receptor signaling mediates resistance to histone deacetylase inhibitors. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Lee SC, Min HY, Jung HJ, Park KH, Hyun SY, Cho J, et al. Essential role of insulin-like growth factor 2 in resistance to histone deacetylase inhibitors. Oncogene. 2016 doi: 10.1038/onc.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livingstone C. IGF2 and cancer. Endocrine-related cancer. 2013;20:R321–39. doi: 10.1530/ERC-13-0231. [DOI] [PubMed] [Google Scholar]
- 20.Huang GS, Brouwer-Visser J, Ramirez MJ, Kim CH, Hebert TM, Lin J, et al. Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin Cancer Res. 2010;16:2999–3010. doi: 10.1158/1078-0432.CCR-09-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic acids research. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Xie J, Zhang M, Wang S. Homocysteine harasses the imprinting expression of IGF2 and H19 by demethylation of differentially methylated region between IGF2/H19 genes. Acta biochimica et biophysica Sinica. 2009;41:464–71. doi: 10.1093/abbs/gmp033. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Gao D, Liu Y, Huang J, Lessnick S, Tanaka S. IGF2 is critical for tumorigenesis by synovial sarcoma oncoprotein SYT-SSX1. Oncogene. 2006;25:1042–52. doi: 10.1038/sj.onc.1209143. [DOI] [PubMed] [Google Scholar]
- 24.Ulaner GA, Yang YW, Hu JF, Li T, Vu TH, Hoffman AR. CTCF binding at the insulin-like growth factor-II (IGF2)/H19 imprinting control region is insufficient to regulate IGF2/H19 expression in human tissues. Endocrinology. 2003;144:4420–6. doi: 10.1210/en.2003-0681. [DOI] [PubMed] [Google Scholar]
- 25.Bruce S, Hannula-Jouppi K, Lindgren CM, Lipsanen-Nyman M, Kere J. Restriction site-specific methylation studies of imprinted genes with quantitative real-time PCR. Clinical chemistry. 2008;54:491–9. doi: 10.1373/clinchem.2007.098491. [DOI] [PubMed] [Google Scholar]
- 26.Oakes CC, La Salle S, Robaire B, Trasler JM. Evaluation of a quantitative DNA methylation analysis technique using methylation-sensitive/dependent restriction enzymes and real-time PCR. Epigenetics : official journal of the DNA Methylation Society. 2006;1:146–52. doi: 10.4161/epi.1.3.3392. [DOI] [PubMed] [Google Scholar]
- 27.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer research. 2006;66:10100–11. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 28.Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- 29.Kohda M, Hoshiya H, Katoh M, Tanaka I, Masuda R, Takemura T, et al. Frequent loss of imprinting of IGF2 and MEST in lung adenocarcinoma. Mol Carcinog. 2001;31:184–91. doi: 10.1002/mc.1053. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Ueda R, Takahashi T. Altered imprinting in lung cancer. Nat Genet. 1994;6:332–3. doi: 10.1038/ng0494-332. [DOI] [PubMed] [Google Scholar]
- 31.Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, de Boom Witzel S, Okano H, et al. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307:1976–8. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- 32.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–9. doi: 10.1038/35013106. [DOI] [PubMed] [Google Scholar]
- 34.Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482–5. doi: 10.1038/35013100. [DOI] [PubMed] [Google Scholar]
- 35.Schoenherr CJ, Levorse JM, Tilghman SM. CTCF maintains differential methylation at the Igf2/H19 locus. Nat Genet. 2003;33:66–9. doi: 10.1038/ng1057. [DOI] [PubMed] [Google Scholar]
- 36.Takai D, Gonzales FA, Tsai YC, Thayer MJ, Jones PA. Large scale mapping of methylcytosines in CTCF-binding sites in the human H19 promoter and aberrant hypomethylation in human bladder cancer. Human molecular genetics. 2001;10:2619–26. doi: 10.1093/hmg/10.23.2619. [DOI] [PubMed] [Google Scholar]
- 37.Chen ZX, Riggs AD. DNA methylation and demethylation in mammals. J Biol Chem. 2011;286:18347–53. doi: 10.1074/jbc.R110.205286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Wang HY, Woetmann A, Raghunath PN, Odum N, Wasik MA. STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood. 2006;108:1058–64. doi: 10.1182/blood-2005-08-007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee H, Zhang P, Herrmann A, Yang C, Xin H, Wang Z, et al. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7765–9. doi: 10.1073/pnas.1205132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fantin VR, Loboda A, Paweletz CP, Hendrickson RC, Pierce JW, Roth JA, et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer research. 2008;68:3785–94. doi: 10.1158/0008-5472.CAN-07-6091. [DOI] [PubMed] [Google Scholar]
- 41.Choudhury KR, Yagle KJ, Swanson PE, Krohn KA, Rajendran JG. A robust automated measure of average antibody staining in immunohistochemistry images. J Histochem Cytochem. 2010;58:95–107. doi: 10.1369/jhc.2009.953554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavi O. Redundancy: a critical obstacle to improving cancer therapy. Cancer research. 2015;75:808–12. doi: 10.1158/0008-5472.CAN-14-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musso L, Dallavalle S, Zunino F. Perspectives in the development of hybrid bifunctional antitumour agents. Biochem Pharmacol. 2015;96:297–305. doi: 10.1016/j.bcp.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Wang K, Wang C, Xiao F, Wang H, Wu Z. JAK2/STAT2/STAT3 are required for myogenic differentiation. J Biol Chem. 2008;283:34029–36. doi: 10.1074/jbc.M803012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JS, Kang JH, Boo HJ, Hwang SJ, Hong S, Lee SC, et al. STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody. Nat Commun. 2015;6:8499. doi: 10.1038/ncomms9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Cui G, Sun L, Wang SJ, Li YL, Meng YG, et al. STAT3 acetylation-induced promoter methylation is associated with downregulation of the ARHI tumor-suppressor gene in ovarian cancer. Oncology reports. 2013;30:165–70. doi: 10.3892/or.2013.2414. [DOI] [PubMed] [Google Scholar]
- 47.Stathis A, Hotte SJ, Chen EX, Hirte HW, Oza AM, Moretto P, et al. Phase I study of decitabine in combination with vorinostat in patients with advanced solid tumors and non-Hodgkin's lymphomas. Clin Cancer Res. 2011;17:1582–90. doi: 10.1158/1078-0432.CCR-10-1893. [DOI] [PubMed] [Google Scholar]
- 48.Kirschbaum M, Gojo I, Goldberg SL, Bredeson C, Kujawski LA, Yang A, et al. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. British journal of haematology. 2014;167:185–93. doi: 10.1111/bjh.13016. [DOI] [PubMed] [Google Scholar]
- 49.Frevel MA, Hornberg JJ, Reeve AE. A potential imprint control element: identification of a conserved 42 bp sequence upstream of H19. Trends Genet. 1999;15:216–8. doi: 10.1016/s0168-9525(99)01752-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.