Abstract
The OMRON HEM−907XL is a commercial oscillometric blood pressure (BP) monitor that was used in the Systolic Blood Pressure Intervention Trial (SPRINT), in which 28% of participants had chronic kidney disease (CKD). This study examined the accuracy of the monitor in nondialytic patients with CKD. Eighty‐seven patients met inclusion criteria. The authors used a modified Association for the Advancement of Medical Instrumentation (AAMI) protocol, with one observer recording measurements from the monitor and two blinded physicians obtaining simultaneous aneroid values by auscultation. Using AAMI method 1, there was a 2.5±9.5 mm Hg difference in OMRON and aneroid systolic BP, and a −1.6±6.5 mm Hg difference in diastolic BP. Using AAMI method 2, there was a 5.1±7.4 mm Hg difference in systolic BP and a −0.2±5.4 mm Hg difference in diastolic BP. In patients with CKD, the OMRON HEM‐907XL appears to be accurate for measuring diastolic BP, but did not perform as well for systolic BP.
Keywords: ambulatory blood pressure/home blood pressure monitor, blood pressure monitoring, chronic kidney disease, clinical management of high blood pressure, hypertension
1. Introduction
According to data from the National Health and Nutrition Examination Survey from 2003 to 2006, the estimated prevalence of chronic kidney diseases (CKD) in the United States is 14.2%.1 Between 60% and 100% of adults with CKD have hypertension, depending on the degree of kidney function loss.2 As emphasized in the 2012 Kidney Disease: Improving Global Outcomes clinical practice guidelines for the evaluation and management of CKD,3 an integral component of CKD management is identification and treatment of hypertension, which requires accurate estimation of BP.
Automated BP devices that use oscillometry to determine blood pressure (BP) are commonly employed in the outpatient setting and by patients at home.4, 5 Auscultation of Korotkoff sounds using an aneroid device with a sphygmomanometer is one of several current standards for indirect measurement of systolic and diastolic BPs.5 Either aneroid or mercury devices have been used in the majority of clinical trials that test the effectiveness of antihypertensive therapy. In contrast to aneroid or mercury devices, oscillometric devices use a sensor to record pressure oscillations, assessing for pattern changes caused by the pulsatile effects of the blood flowing through the compressed brachial artery.4 Each brand of oscillometric device employs its own internal proprietary algorithm to calculate systolic and diastolic pressures.4, 6 Because of variations in these proprietary internal algorithms, BP values might differ between devices for the same patient.7
Oscillometric BP devices operate on the assumption that oscillometric signals are similar between patients with and without comorbidities such as CKDs. However, patients with CKD have increased arterial stiffness, more peripheral vascular disease, and greater prevalence of cardiovascular diseases compared with the general population.8, 9, 10 Increased arterial stiffness has been associated with higher systolic and diastolic BP readings by oscillometric measurement compared with aneroid measurement, independent of age, sex, and mean arterial pressure.11, 12 Despite the heightened risk of inaccurate measurement, most monitors have not been individually validated in nondialytic patients with CKD.6
The Systolic Blood Pressure Intervention Trial (SPRINT) recently demonstrated improved cardiovascular mortality among high‐risk, older adults who were randomized to more intensive systolic BP (SBP) control (<120 mm Hg vs <140 mm Hg).13 A total of 28% of SPRINT participants had CKD. The OMRON HEM‐907XL (Omron Healthcare, Lake Forest, IL, USA) was the oscillometric BP device used to monitor the BPs of participants in SPRINT. While the OMRON HEM‐907XL has been validated in non‐CKD patients and hemodialysis patients,14, 15, 16, 17 it has not been assessed for accuracy in nondialytic patients with CKD. Given that accurate measurement of BP is necessary to guide proper treatment of hypertension, the objective of this study was to evaluate the accuracy of the OMRON HEM‐907XL in nondialytic patients with CKD.
2. Methods
2.1. Study population
Patients who met the protocol‐specified CKD diagnostic criteria for SPRINT13 and the Chronic Renal Insufficiency Cohort Study (CRIC)18 were recruited during their routine follow‐up visits. Other patients with CKD were recruited from the nephrology outpatient clinic at the University of Pennsylvania Perelman Center. Eighty‐seven patients met inclusion criteria. Patients were considered eligible for the study if they met the definition for CKD based on either SPRINT or CRIC enrollment13, 18 or the 2012 KDIGO guidelines, including an estimated glomerular filtration rate (eGFR) of <60 mL/min per 1.73 m2 and/or the presence of albuminuria (≥30 mg/g albumin‐to‐creatinine ratio on spot urine testing).3 eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.19 Patients were excluded if they had end‐stage renal disease requiring dialysis or were unable to tolerate multiple BP measurements in the same arm due to the presence of an arteriovenous fistula or graft or lymphedema.
The protocol was approved by the institutional review board of the University of Pennsylvania and all patients provided written informed consent.
2.2. Oscillometric device
The OMRON HEM‐907XL is a commercial device that estimates BP oscillometrically in the range of 0 to 299 mm Hg with an electrostatic capacity semiconductor sensor.16 Inflation is achieved by an automated pumping system and deflation is achieved by an automatic pressure‐releasing electromagnetic control valve.16 A regular‐sized cuff (recommended arm circumference 22–32 cm), a large‐sized cuff (recommended arm circumference 32–42 cm), and an extra large–sized cuff (recommended arm circumference 42–50 cm) are provided with the device. In order to mimic typical clinical use of the device, a monitor that had already been in use for several months was selected to perform the evaluation. Using preset pressure settings, the OMRON device readings were compared with the aneroid device readings at 50, 100, 150, and 200 mm Hg; the OMRON device was consistently within 2 mm Hg of the aneroid device at each of the assessed values.
2.3. BP measurement protocol
The protocol was developed using a modified approach to the Association for the Advancement of Medical Instrumentation (AAMI) standard requirements for validation of BP devices.20 Patients underwent two screening BP measurements in the clinic before further BP testing in our study. Sampling consisted of all participants until the required number of measurements were recorded. For each evaluation in our study, one observer who operated the OMRON device and two physicians who performed simultaneous (dual‐headed stethoscope) auscultatory BP measurements were present. Eleven physicians, registered nurses, and medical assistants rotated as the OMRON observers in the study. Seven physicians participated as aneroid auscultators in the study, which is a deviation from the AAMI's recommendation for two physician observers, given clinical time restraints of the participants. All physician auscultators underwent rigorous training to ensure proficiency with auscultation using the aneroid device. The aneroid device (Welch Allyn Tycos, Skaneateles Falls, NY, USA) was calibrated at the start of the study, 6 months into the study, and at the conclusion of the study. The aneroid device was not calibrated using a mercury device because a mercury device was not permitted at the study facility.
The patient sat quietly with his/her feet flat on the floor for 5 minutes prior to the measurements. One of the physicians measured the patient's arm circumference and selected the appropriate size BP cuff, ensuring that the bladder of the cuff encircled at least 80% of the arm circumference.21 The cuff was not changed between the OMRON and aneroid readings. A three‐way stopcock and extra tubing were used to connect the BP cuff to the OMRON HEM‐907XL device and to the calibrated aneroid device and manual sphygmomanometer. The OMRON HEM‐907XL screen was directed towards the observer and away from the physicians to blind the physicians from the BP readings. One physician held, inflated, and deflated the cuff while the other physician positioned the bell or diaphragm of the stethoscope beneath the BP cuff. A dual‐earpiece teaching stethoscope was used for simultaneous physician auscultation of the manual BP.
For each BP evaluation, the physicians were blinded to the observer's readings as well as to each other's readings. The observer set the OMRON HEM‐907XL device to auto‐mode single measurement, and measured and recorded the BP. There was a 1‐minute delay after the OMRON HEM‐907XL device deflated prior to assessment of each aneroid BP. BP was measured by auscultation using an aneroid device simultaneously by both physicians, and each physician wrote down the BP value they obtained. The observer assessed the physicians’ measurements. If both the systolic and diastolic BPs were within 4 mm Hg between each physician, the observer proceeded to the next oscillometric measurement. If the BP differences were >4 mm Hg, the physicians repeated the manual BP. Patients in whom physician readings demonstrated a >10‐mm Hg interobserver difference (n=1) were excluded from the analyses. Including the screening oscillometric measurement, readings were alternated between the oscillometric and aneroid devices for a total of four oscillometric and three aneroid measurements.16, 20 Patients were evaluated for acceptable change in BP during the course of the study measurements (<12 mm Hg systolic or <8 mm Hg diastolic absolute change in BP, n=0) prior to being included in the analyses.20
2.4. Clinical and demographic characteristics
Key clinical and demographic characteristics were extracted from the electronic health record, including age, sex, race, ethnicity, and serum creatinine.
2.5. Statistical analyses
Statistical analyses were performed using STATA version 13.0 (Statacorp LP, College Station, TX, USA). Descriptive statistics (mean, median, and proportion) were used to describe baseline clinical and demographic characteristics.
The means of each of the aneroid BP measurements between the two physicians were calculated for the primary analyses. For aneroid evaluations where the two clinicians were discordant on the first set of measurements, the repeated measurements were used. The mean difference in BP measurements between the oscillometric and aneroid devices were compared using Student t test for both AAMI method 1 and method 2. Method 1 assessed the mean difference between 255 pairs of oscillometric and aneroid readings (obtained from a minimum of 85 participants). For method 2, a minimum of 85 patients contributed three pairs of oscillometric and aneroid readings; the three pairs of readings were averaged within each patient, and t testing was performed to assess for the mean difference between oscillometric and aneroid readings across each participant.20 The last three oscillometric readings and all three aneroid measurements were used. Mean differences between each sequential reading were compared using Student t test, as were the slopes of change in BP across sequential readings by measurement modality. Pearson correlation was calculated to assess for a linear correlation (reported as the r value) between BP values from the different devices.
3. Results
There were 87 patients who met inclusion criteria for the study, with a total of 255 oscillometric readings and 255 aneroid readings acceptable for analysis. The median age of the study participants was 62 years (interquartile range 55–70 years), and 56% of patients were male (Table 1). The majority of patients included in the study met criteria for stage 3 CKD, with eGFRs ranging from 10 to 102 mL/min per 1.73 m2 across all participants. There was no significant interobserver difference between the physicians’ aneroid measurements. The maximum interobserver difference was 4 mm Hg, and the mean interobserver difference was 0.52±1.8 mm Hg systolic and 0.03±1.5 mm Hg diastolic across all combinations of physician pairs (See Figures S1A–D).
Table 1.
Cohort Characteristics (n=87)
| Age, median (IQR) | 62 (55–70) |
| Male sex, No. (%) | 49 (56.3) |
| Race and ethnicity, No. (%) | |
| White non‐Hispanic | 40 (46.0) |
| Black non‐Hispanic | 42 (48.3) |
| White Hispanic | 2 (2.3) |
| Black Hispanic | 2 (2.3) |
| Asian | 1 (1.1) |
| eGFR, median (IQR) | 41 (32–53) |
| Large cuff size, No. (%) | 38 (44.2) |
Abbreviations: eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Mean±standard deviation (SD) OMRON SBP was 132.9±20.3 mm Hg and mean OMRON diastolic BP (DBP) was 70.4±10.7 mm Hg (Table 2). Mean aneroid SBP was 130.4±20.2 mm Hg and mean aneroid DBP was 72.0±11.6 mm Hg. Using method 1, there was a 2.5±9.5 mm Hg difference in mean SBP between the individual pairs of OMRON and aneroid values, and a −1.6±6.5 mm Hg difference in mean DBP (AAMI requirement is less than or equal to ±5 mm Hg, SD ≤8).20 Using method 2, there was a 5.1±7.4 mm Hg difference in mean SBP (AAMI requirement is less than or equal to ±5 mm Hg, SD ≤4.81) and a −0.2±5.4 mm Hg difference in DBP (AAMI requirement for a mean difference less than or equal to ±0.5 mm Hg is that the SD be ≤6.93) between the OMRON and aneroid values.20 By both methods, these values met criteria for validation of DBP, but not SBP, based on the AAMI standard requirements.20 For each pair of readings, 49% of OMRON and aneroid systolic readings were within 5 mm Hg of each other, 77% were within 10 mm Hg, and 92% were within 15 mm Hg. A total of 63% of OMRON and aneroid diastolic readings were within 5 mm Hg of each other, 88% were within 10 mm Hg, and 95% were within 15 mm Hg. Concordance between the OMRON and aneroid readings appeared to be poorer towards higher extremes of SBP and DBP distribution (Figures 1A–D). However, there was a strong linear correlation between the OMRON and aneroid SBP values (Figure 2A, r=.93, P<.001) and DBP values (Figure 2B; r=.88, P<.001).
Table 2.
(a) The Association Between OMRON and Aneroid Measurements (AAMI Method 1, n=255), and (b) the Association Between Mean OMRON and Aneroid Measurements (AAMI Method 2, n=87)
| Value, mm Hg | SD | |
|---|---|---|
| (a) | ||
| Mean OMRON SBP | 132.9 | 20.3 |
| Mean aneroid SBP | 130.4 | 20.2 |
| Difference in mean SBP | 2.5 | 9.5 |
| Mean OMRON DBP | 70.4 | 10.7 |
| Mean aneroid DBP | 72.0 | 11.6 |
| Difference in mean DBP | −1.6 | 6.5 |
| (b) | ||
| Mean OMRON SBP | 135.0 | 19.8 |
| Mean aneroid SBP | 129.9 | 20.0 |
| Difference in mean SBP | 5.1 | 7.5 |
| Mean OMRON DBP | 71.7 | 10.6 |
| Mean aneroid DBP | 71.9 | 11.3 |
| Difference in mean DBP | −0.2 | 5.4 |
Bold values indicate the primary outcomes calculated for each method. Abbreviations: AAMI, Association for the Advancement of Medical Instrumentation; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Figure 1.
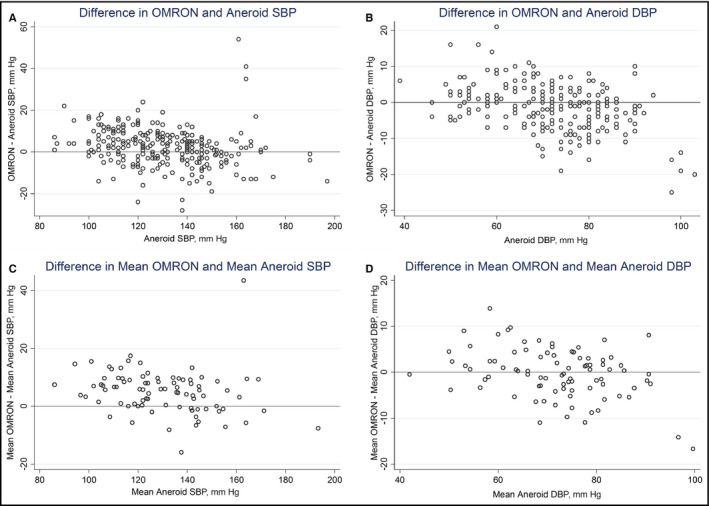
Association between OMRON and aneroid measurements. (A) Bland‐Altman plot: difference in OMRON vs aneroid systolic blood pressures (SBPs; method 1). (B) Bland‐Altman plot: difference in OMRON vs aneroid diastolic blood pressures (DBPs; method 1). (C) Bland‐Altman plot: difference in mean OMRON vs mean aneroid SBPs (method 2). (D) Bland‐Altman plot: difference in mean OMRON vs mean aneroid DBPs (method 2)
Figure 2.
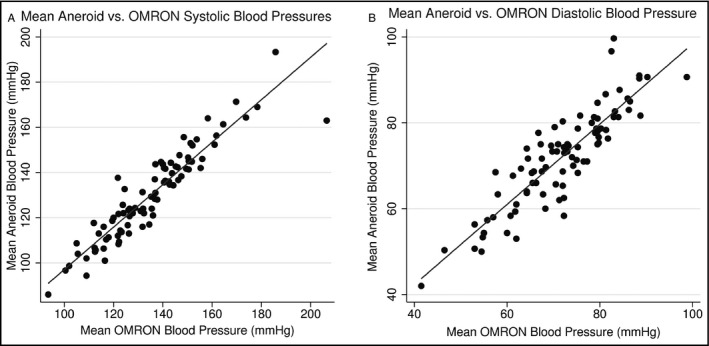
(A) Linear association between systolic blood pressures (r=.93, P<.001). (B) Linear association between diastolic blood pressures (r=.88, P<.001)
The first OMRON mean SBP measurement was significantly higher than the subsequent OMRON measurements (Figure 3, P<.001). There was a nonsignificant downward trend in mean aneroid SBP across the three sequential aneroid readings. The slope of the decrease in OMRON SBP readings was significantly steeper than the slope of decrease in the aneroid readings (−0.04±0.01 difference in slope, P<.001 by t testing with change‐on‐change analysis).
Figure 3.
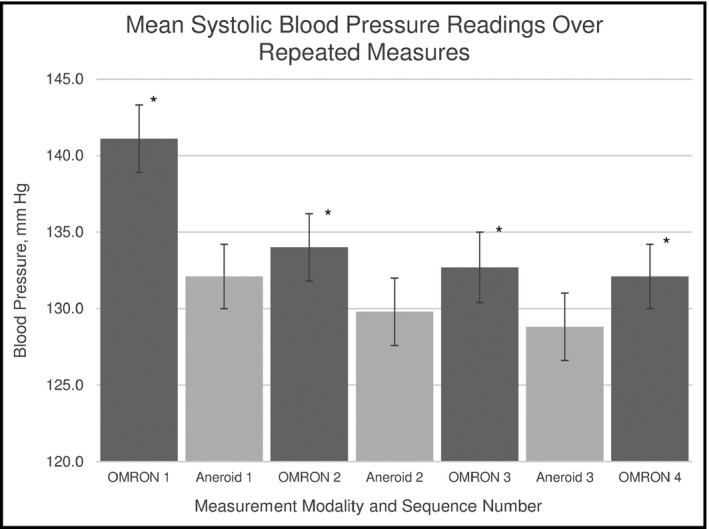
Mean systolic blood pressure readings over repeated measures across OMRON and aneroid measurement modalities. *Statistically significant difference between the first and each subsequent OMRON readings (P<.001 by t testing)
4. Discussion
In this study, the OMRON HEM‐907 was tested against sequential aneroid BP measurements conducted simultaneously by two physicians in order to assess the accuracy of the OMRON BP estimates in patients with nondialytic CKD. Based on the criteria set forth by the AAMI,20 the study demonstrated that the OMRON HEM‐907XL appears to be accurate with regard to DBP, but did not perform as well for SBP measurements in patients with nondialytic CKD. There was a strong linear relationship between SBPs and DBPs across both measurement modalities.
Despite the strong association between increased arterial stiffness and inaccurate oscillometric BPs,11, 12 as well as between increased arterial stiffness and CKD,8 only one previous study assessed the accuracy of an oscillometric device in patients with nondialytic CKD. Using the less stringent European Society of Hypertension International Protocol, Akpolat and colleagues22 found that a different oscillometric device (OMRON M3) fulfilled criteria for validation in patients with nondialytic CKD. The OMRON HEM‐907 device was previously validated in non‐CKD patients,14, 16 including in elderly patients older than 75 years (using the European Society of Hypertension International Protocol).15 Although the OMRON HEM‐907XL was validated for SBP measurements in hemodialysis patients, it did not fulfill the international protocol criteria for validation of DBP.23 Similar to our study, previous studies demonstrated a decline in BP with sequential measurements in oscillometric devices, which may be greater than the decline observed in aneroid measurements.24, 25 Given the overall concordance between the oscillometric and aneroid measurements, these differences highlight the importance of obtaining repeated measures of BPs when using either measurement method in general practice.24
In October 2015, the US Preventive Services Task Force released updated recommendations on screening for hypertension in adults, indicating that BP estimates should be obtained outside of the clinical setting in order to confirm the diagnosis of hypertension and before starting treatment.26 Additionally, the 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension recommended the use of validated oscillometric BP devices for in‐office estimates in favor of aneroid measurement in order to limit patient‐provider interaction and minimize the impact of the white‐coat effect during BP measurement.27, 28 These changes to the recommendations are expected to substantially increase the use of oscillometric devices in the community. Accordingly, taking into account the high prevalence of hypertension in patients with CKD, the use of oscillometric devices among patients with CKD is likely to precipitously rise in the next several years.
In addition to the increased vascular stiffness and incident peripheral vascular disease associated with CKD,8 there are multiple potential contributing factors to altered oscillometric measurements in this patient population. Patients with CKD are exposed to high levels of inflammation,29, 30 oxidative stress,29, 30 endothelial dysfunction,31 altered bone and mineral metabolism,3 and hormonal dysregulation30 throughout the course of their disease, which likely collectively contribute to abnormal vascular function. Even if the vascular dysfunction is subclinical, early manifestations may cause subtle alterations in the vasculature that could render the proprietary algorithms used in oscillometric devices ungeneralizable to this population of patients.
5. Study Limitations
This study was limited by the fact that it took place in a single center. Given that the protocol was implemented at a large university hospital and included several clinical trial participants, the generalizability of the results to the community may be somewhat weakened. However, the participants represented a racially and ethnically diverse group of patients across a wide range of ages and eGFRs, likely encompassing a broad assemblage of the general population of patients with nondialytic CKD. The study was further limited by the lack of use of a mercury device to calibrate the aneroid sphygmomanometer and OMRON device. Although the lack of mercury calibration necessitates cautious interpretation of the results of the study, it reinforces the generalizability of the results to routine care, where mercury calibration is not readily available. Additionally, the cuffs used for the measurements were specifically intended for the OMRON device, not the aneroid device, which may introduce measurement bias in favor of the OMRON device‐cuff combination.
6. Conclusions
This study demonstrates that the OMRON HEM−907XL oscillometric BP device provides consistent readings in comparison to aneroid measurements for DBP, but not SBP, in nondialytic patients with CKD. As the widespread use of oscillometric devices increases, it is important that oscillometric BP devices be systematically assessed for accuracy. In particular, subgroups of highly morbid patients at risk for occult vascular disease and increased arterial stiffness, such as patients with CKD, will likely benefit from separate validation of these devices.
Disclosures
Research reported in this publication is supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) under award number K23‐HL133843 (PI: Cohen). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. RRT is a SPRINT investigator. All other authors report no specific funding in relation to this research and have no conflicts of interest to disclose.
Supporting information
Acknowledgments
We gratefully acknowledge assistance from Laura Greenberg, Purvi Patel, Angela Sheridan, Krishna Kallem, Amar Bansal, Behdad Besharatian, and Sarah Schrauben, Renal, Electrolyte, and Hypertension Division, University of Pennsylvania, Philadelphia, PA.
Cohen JB, Wong TC, Alpert BS, Townsend RR. Assessing the accuracy of the OMRON HEM‐907XL oscillometric blood pressure measurement device in patients with nondialytic chronic kidney disease. Journal of Clinical Hypertension. 2017;19:296‐302. doi: 10.1111/jch.12961
References
- 1. United States Renal Data System . Annual data report: atlas of chronic kidney disease and end‐stage renal disease in the United States. Bethseda, MD: National Institutes of Health: National Institute of Diabetes and Digestive and Kidney Diseases; 2010. [Google Scholar]
- 2. Rao MV, Qiu Y, Wang C, Bakris G. Hypertension and CKD: kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999‐2004. Am J Kidney Dis. 2008;51(4 suppl 2):S30–S37. [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 4. Forouzanfar M, Dajani HR, Groza VZ, Bolic M, Rajan S, Batkin I. Oscillometric blood pressure estimation: past, present, and future. IEEE Rev Biomed Eng. 2015;8:44–63. [DOI] [PubMed] [Google Scholar]
- 5. Landgraf J, Wishner SH, Kloner RA. Comparison of automated oscillometric versus auscultatory blood pressure measurement. Am J Cardiol. 2010;106:386–388. [DOI] [PubMed] [Google Scholar]
- 6. Alpert BS, Quinn D, Gallick D. Oscillometric blood pressure: a review for clinicians. J Am Soc Hypertens. 2014;8:930–938. [DOI] [PubMed] [Google Scholar]
- 7. Kallem RR, Meyers KE, Sawinski DL, Townsend RR. A comparison of two ambulatory blood pressure monitors worn at the same time. J Clin Hypertens (Greenwich). 2013;15:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Townsend RR, Wimmer NJ, Chirinos JA, et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 10. Perticone F, Maio R, Perticone M, et al. Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation. 2010;122:379–384. [DOI] [PubMed] [Google Scholar]
- 11. van Popele NM, Bos WJ, de Beer NA, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484–488. [DOI] [PubMed] [Google Scholar]
- 12. Thompson AM, Eguchi K, Reznik ME, Shah SS, Pickering TG. Validation of an oscillometric home blood pressure monitor in an end‐stage renal disease population and the effect of arterial stiffness on its accuracy. Blood Press Monit. 2007;12:227–232. [DOI] [PubMed] [Google Scholar]
- 13. The SPRINT Research Group . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM‐907 monitor in adults. Blood Press Monit. 2001;6:107–110. [DOI] [PubMed] [Google Scholar]
- 15. Omboni S, Riva I, Giglio A, Caldara G, Groppelli A, Parati G. Validation of the Omron M5‐I, R5‐I and HEM‐907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Press Monit. 2007;12:233–242. [DOI] [PubMed] [Google Scholar]
- 16. El Assaad MA, Topouchian JA, Darne BM, Asmar RG. Validation of the Omron HEM‐907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–241. [DOI] [PubMed] [Google Scholar]
- 17. Semret M, Zidehsarai M, Agarwal R. Accuracy of oscillometric blood pressure monitoring with concurrent auscultatory blood pressure in hemodialysis patients. Blood Press Monit. 2005;10:249–255. [DOI] [PubMed] [Google Scholar]
- 18. Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(7 suppl 2):S148–S153. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American National Standard . Association for the Advancement of Medical Instrumentation standard. ANSI/AAMI/ISO 80601:2009 & A1:2013. Arlington, VA: AAMI; 2013. [Google Scholar]
- 21. Fonseca‐Reyes S, de Alba‐Garcia JG, Parra‐Carrillo JZ, Paczka‐Zapata JA. Effect of standard cuff on blood pressure readings in patients with obese arms. How frequent are arms of a ‘large circumference’?. Blood Press Monit. 2003;8:101–106. [DOI] [PubMed] [Google Scholar]
- 22. Akpolat T, Erdem E, Aydogdu T. Validation of the Omron M3 Intellisense (HEM‐7051‐E) upper arm blood pressure monitor, for self‐measurement, according to the European Society of Hypertension International Protocol revision 2010 in a stage 3‐5 chronic kidney disease population. Kidney Blood Press Res. 2012;35:82–88. [DOI] [PubMed] [Google Scholar]
- 23. Czarkowski M, Staszkow M, Kostyra K, Shebani Z, Niemczyk S, Matuszkiewicz‐Rowinska J. Determining the accuracy of blood pressure measurement by the Omron HEM‐907 before and after hemodialysis. Blood Press Monit. 2009;14:232–238. [DOI] [PubMed] [Google Scholar]
- 24. Handler J, Zhao Y, Egan BM. Impact of the number of blood pressure measurements on blood pressure classification in US adults: NHANES 1999–2008. J Clin Hypertens (Greenwich). 2012;14:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a Systematic Review for the U.S. Preventive Services Task Force. Rockville, MD; 2014. [PubMed] [Google Scholar]
- 26. Siu AL. Force USPST. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778–786. [DOI] [PubMed] [Google Scholar]
- 27. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada's 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569–588. [DOI] [PubMed] [Google Scholar]
- 28. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280–286. [DOI] [PubMed] [Google Scholar]
- 29. Schiffrin EL, Lipman ML, Mann JFE. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. [DOI] [PubMed] [Google Scholar]
- 30. Gansevoort RT, Correa‐Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. [DOI] [PubMed] [Google Scholar]
- 31. Hirata Y, Sugiyama S, Yamamoto E, et al. Endothelial function and cardiovascular events in chronic kidney disease. Int J Cardiol. 2014;173:481–486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


