Abstract
It remains unknown whether obese individuals with more components of the metabolic syndrome and/or prediabetes demonstrate altered activation of brain centers in response to food cues. We examined obese prediabetics (n=26) vs. obese nondiabetics (n=11) using fMRI. We also performed regression analyses on the basis of the number of MetS components per subject. Obese individuals with prediabetes have decreased activation of the reward-related putamen in the fasting state and decreased activation of the salience- and reward-related insula after eating. Obese individuals with more components of MetS demonstrate decreased activation of the putamen while fasting. All these activations remain significant when corrected for BMI, waist circumference (WC), HbA1c and gender. Decreased activation in reward-related brain areas between obese individuals is more pronounced in subjects with prediabetes and MetS. Prospective studies are needed to quantify their contributions to the development of prediabetes/MetS and to study whether these conditions may predispose to the exacerbation of obesity and the development of comorbidities over time.
Obesity and its comorbidities are rapidly growing global concerns1, 2. Although many studies have observed changes on functional brain activity in obese versus lean individuals which would predispose obese subjects to overeating3, 4, it remains unknown whether such changes would be more pronounced among subjects who have MetS and/or prediabetes.
Therefore, we examined cross-sectionally using fMRI how neural responses to food cues differ between 1) obese individuals with prediabetes vs. obese individuals without prediabetes; and 2) obese individuals meeting the definition of MetS. We elected to use individuals with prediabetes, but not overt type 2 diabetes, in order to prevent differences due to extremes of blood sugar levels from glycosuria or to changes in body weight due to diabetes itself or diabetes medications. We hypothesized that changes would be in reward- or saliency- related brain areas.
Methods
Thirty-six men and women provided written informed consent to participate in this study, approved by the BIDMC IRB and selected as per previous9. Participants had an overnight visit, which consisted of at least a 12 hours fast followed by 2 fMRI scans, one in the fasting and another in the fed state. The standardized meal consisted of a turkey sandwich, piece of fruit, pretzels, and a bottle of water (630kcal; 22.2gfat; 73.8gcarbohydrates; 31.5gprotein). Prediabetes was defined as HbA1c=5.7–6.4%5. Metabolic syndrome criteria were defined according to the NIH standards of 1) waist circumference of >35 inches for women or >40 inches for men; 2) triglyceride levels of 150 mg/dL or higher (or being on medicine to treat high triglycerides); 3) HDL cholesterol levels of <50 mg/dL for women or <40 mg/dL for men (or being on medication for low HDL); 4) Blood pressure of 130/85mmHg (or being on medication to treat high blood pressure); and/or 5) Fasting blood sugar level >126 mg/dL.
fMRI protocol
Participants viewed food and non-food items within a GE 3T MRI scanner at BIDMC in both the fasting and fed states and were analyzed as per previous6–9. EPIBOLD parameters: TR=3.5s, TE=25ms, resolution=2.5×2.5mm, matrix=96×96, FOV=24×24cm, bandwidth=83.33kHz, slice thickness=3mm. The contrast images [highly desirable>less desirable food images and all food (both highly and less desirable food cues)>non-food images] of the first-level analysis were used for the second-level group statistics. To compare prediabetics and nondiabetics, two sample t-tests were used. To compare across MetS criteria, regressions were performed. In a subsequent analysis, t-tests were performed for subjects meeting each of the MetS criteria (e.g. categories of yes/no for TG>50). BMI, waist circumference(WC), gender, and HbA1c were included as covariates in secondary analyses. We then utilized small volume corrections (SVC) on the resultant fMR images for the areas of interest as previously described in order to be able to compare our results to those which were previously found10. Briefly, spherical regions of interest were created in marsbar with radii 5-mm (for amygdala) or 10-mm (for insula, putamen, and OFC) for SVC, and results which passed p<.05, FWE corrected for peak are reported.
Results
Obese prediabetics vs. obese nondiabetics
There were no differences in anthropometry between obese prediabetics (n=26) and nondiabetics (n=11; Table 1). While fasting, prediabetics showed decreased activations in the posterior putamen to highly versus less desirable food cues (Supplemental Table S1; Figure 1a). After a meal, prediabetics show decreased activations to food>non-food cues in the insula (Supplemental Table S1; Figure 1b). These activations remain significant with corrections for BMI, WC, HbA1c, and gender.
Table 1.
Basic demographic information for participants by category.
| Nondiabetics (n=11) | Prediabetics (n=26) | P value | MetS (Number of Criteria Met) | P value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n=11) | 2 (n=14) | 3 (n=12) | |||||||||||||||
| Gender (males/females) | 6/5 | 12/13 | 0.72 | 4/6 | 8/6 | 6/5 | 0.69 | ||||||||||
| Age (years) | 45.50 | ± | 3.88 | 51.76 | ± | 2.58 | 0.07 | 46.45 | ± | 4.39 | 52.73 | ± | 3.33 | 51.91 | ± | 4.01 | 0.82 |
| BMI (kg/m2) | 35.53 | ± | 1.15 | 38.40 | ± | 1.32 | 0.25 | 37.59 | ± | 1.87 | 37.58 | ± | 2.01 | 38.32 | ± | 1.77 | 0.76 |
| WC (cm) | 116.06 | ± | 3.51 | 122.96 | ± | 3.09 | 0.22 | 120.14 | ± | 5.19 | 120.36 | ± | 4.21 | 123.85 | ± | 4.31 | 0.83 |
| Weight (kg) | 105.41 | ± | 4.85 | 109.65 | ± | 3.74 | 0.51 | 105.54 | ± | 5.62 | 106.85 | ± | 5.45 | 113.94 | ± | 5.53 | 0.60 |
| Fat mass (kg) | 40.38 | ± | 2.84 | 44.75 | ± | 2.74 | 0.45 | 42.57 | ± | 4.96 | 44.70 | ± | 3.35 | 44.09 | ± | 3.34 | 0.77 |
| Fat mass (%) | 37.83 | ± | 1.91 | 40.18 | ± | 1.66 | 0.60 | 39.27 | ± | 3.08 | 41.36 | ± | 1.66 | 38.36 | ± | 2.21 | 0.57 |
| Total mass (kg) | 106.54 | ± | 4.80 | 110.37 | ± | 3.75 | 0.55 | 106.37 | ± | 5.52 | 107.44 | ± | 5.31 | 114.89 | ± | 5.72 | 0.59 |
| SBP (mmHg) | 124.00 | ± | 5.74 | 128.88 | ± | 2.40 | 0.23 | 123.27 | ± | 2.94 | 123.00 | ± | 4.96 | 133.36 | ± | 2.47 | 0.00 |
| DBP (mmHg) | 73.20 | ± | 3.56 | 76.96 | ± | 1.78 | 0.17 | 77.64 | ± | 2.45 | 72.09 | ± | 3.01 | 77.73 | ± | 2.80 | 0.14 |
| HR (beats/min) | 81.10 | ± | 2.94 | 78.24 | ± | 2.72 | 0.60 | 76.91 | ± | 4.75 | 78.64 | ± | 3.69 | 81.82 | ± | 3.20 | 0.79 |
| HbA1c (g/dL) | 5.43 | ± | 0.04 | 5.94 | ± | 0.04 | 0.00 | 5.85 | ± | 0.07 | 5.62 | ± | 0.07 | 5.94 | ± | 0.10 | 0.05 |
| Glucose (mg/dL) | 93.33 | ± | 2.13 | 93.36 | ± | 1.90 | 0.85 | 93.00 | ± | 3.46 | 92.00 | ± | 1.96 | 94.91 | ± | 2.54 | 0.93 |
| HOMA-IR | 3.48 | ± | 0.34 | 3.91 | ± | 0.43 | 0.57 | 3.07 | ± | 0.63 | 3.32 | ± | 0.38 | 5.12 | ± | 0.61 | 0.05 |
| Total cholesterol (mg/dL) | 169.20 | ± | 8.77 | 180.20 | ± | 5.84 | 0.21 | 181.00 | ± | 9.91 | 181.09 | ± | 8.49 | 169.91 | ± | 6.60 | 0.44 |
| Triglycerides (mg/dL) | 124.60 | ± | 14.41 | 119.24 | ± | 8.05 | 0.94 | 96.73 | ± | 9.16 | 127.36 | ± | 10.35 | 133.73 | ± | 15.25 | 0.09 |
| HDL (mg/mL) | 44.50 | ± | 2.41 | 52.64 | ± | 2.74 | 0.07 | 57.45 | ± | 3.67 | 50.27 | ± | 4.56 | 46.18 | ± | 2.02 | 0.06 |
| LDL (mg/mL) | 99.90 | ± | 6.64 | 103.72 | ± | 5.24 | 0.55 | 104.27 | ± | 8.41 | 105.36 | ± | 5.17 | 97.00 | ± | 7.38 | 0.22 |
| VLDL (mg/dL) | 24.80 | ± | 2.89 | 23.84 | ± | 1.63 | 0.96 | 19.27 | ± | 1.82 | 25.45 | ± | 2.11 | 26.73 | ± | 3.08 | 0.09 |
Mean not shown for MetS meeting 4 criteria, as only 1 patient met 4 criteria. MetS, metabolic syndrome; BMI, body mass index; WC, waist circumference; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model of insulin resistance; HDL, high density lipoproteins; LDL, low density lipoproteins, VLDL, very LDL
Figure 1.
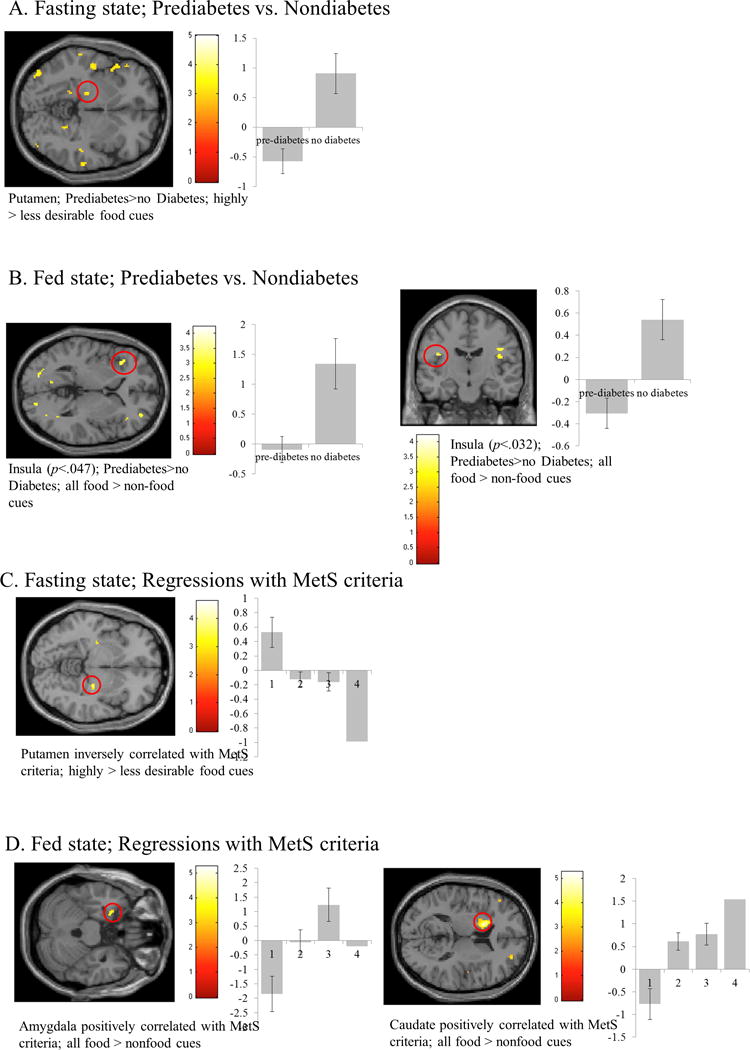
Results from small volume correction (SVC) analysis of obese prediabetics vs. nondiabetics in the fasting (A) and fed (B) states and with the number of MetS criteria met in the fasting (C) and fed (D) states (see Supplemental Table 2). Areas significant at p<.05, FWE corrected for multiple comparisons are circled in red. The y-axis represents effect size of the activation (z scores). BOLD contrasts are superimposed on a T1 structural image in neurological orientation. The color bar represents voxel T value.
Differences in relation to number of MetS components
Of our study sample, 11 met one criterion for MetS, 14 met two criteria for MetS, 12 met three criteria for MetS, and 1 met four criteria for MetS. Between the groups, there were no differences in anthropometric characteristics (Table 1). Systolic blood pressure (p<.001) and HbA1c levels (p<.05) were different between the groups (Table 1). While fasting, participants who met more MetS criteria showed less activation in the posterior putamen to highly>less desirable food cues (Supplemental Table S1; Figure 1c), which survives when corrected for covariates. After a meal, participants who met more MetS criteria showed greater activation of the amygdala and caudate to all food>non-food cues (Supplemental Table S1; Figure 1d). The activation in the caudate survives correction for covariates; however, the activation in the amygdala survives correction for BMI or WC, but not for HbA1c or gender.
Of the individual components of MetS, all patients met the component for WC, 17 met the component for TG, 11 met the component for HDL, 11 met the component for BP, and none met for blood glucose. There were no differences between participants who met or did not meet each criterion, except that individuals who met the MetS criterion for TG had decreased activation in the insula to highly desirable>less desirable food cues in the fasting state (data not shown; 431mm3; p<.003, FWE-corrected; X,Y,Z: −34,12,0).
Discussion
Differences between obese individuals with and without prediabetes
Obese prediabetics show less reward-related activations in the insula and putamen to highly desirable food cues while fasting which persists after a meal where they demonstrate decreased activation of the insula to all food cues. The putamen is involved in the reward system and responds to the rewarding value of food cues11, 12. Activations of the putamen have also been shown to predict future weight gain12. Together, this suggests that obese individuals with prediabetes have a decreased reward-related response to food cues as compared to their obese counterparts without prediabetes. This may be underlying their predisposition to developing obesity and future insulin resistance, as hypoactivation of the reward system has been repeatedly implicated in obesity in general, and obese individuals repeatedly show altered reward responses to food cues4, 13, 14. This hypoactivation of the reward system is thought to lead to obesity by leading individuals to seek more rewarding food. Considering these findings remain when controlled for BMI, HbA1c, WC, and gender, hypoactivation of reward centers is likely causal and not secondary to the development of prediabetes. As such, it would predispose to the relative worsening of obesity and development of comorbidities later in life.
Differences amongst obese individuals with components of MetS
Individuals with more components of MetS demonstrate less reward-related putamen activation in the fasting state, but more postprandial caudate and amygdala activations to food cues. However, the latter becomes non-significant after adjusting for confounders such as HbA1c and gender. Similar to the putamen, the caudate is involved in evaluating the rewarding value of food cues15–17 and activation of the caudate is linked to future weight gain16. One theory of reward and obesity is that obese individuals have less reward-related responses to food cues which makes them seek more and more high calorie/fat foods. This is supported by positron emission tomography findings of deficits in the striatal dopaminergic system18–20. The observations in participants meeting more MetS components indicate a hypoactivation of the reward system in these individuals while fasting which leads them to seek more food then those with less MetS. Similar to findings in prediabetes, this decreased activation persists with corrections for HbA1c, gender, WC, and BMI, suggesting it might be causal for the worsening of both obesity and the comorbidities of obesity/MetS. After a meal, individuals meeting more MetS components show increased activation of the caudate and emotion- and salience-related amygdala, which may suggest that these individuals have a heightened reward response after consuming food. Since the activation of the amygdala disappears after controlling for HbA1c, this increase in response is likely secondary to MetS components, such as increased blood sugar or blood pressure compounded by a recent meal. Furthermore, as we do not see significant changes by particular MetS criterion met, we can assume that these changes are cumulative by the number of MetS criteria met and not by the particular criterion, but this will need to be confirmed with larger samples.
Conclusions, strengths and limitations
Altogether, we observed changes in the central nervous system processing of food cues with comorbidities of obesity. Our findings suggest that an exaggerated hypoactivation of the reward system before a meal, which leads individuals to seek more rewarding foods, occurs with, and possibly causes, additional comorbidities of obesity, e.g. MetS components and prediabetes. Strengths of the study include the state-of-the-art methodology used and blinding of investigators to MetS and prediabetes status during assessments. We are limited by the number of subjects and lower severity of comorbidities in our preliminary sample. Although there was not diabetes per se in this initial study, this allows us to rule out the effects of anti-diabetic drugs or outright glycosuria or extreme insulin resistance on brain activations. Additionally, the number of subjects is limited, albeit sufficient to demonstrate significance, and we do not have a normal weight, healthy population for direct comparison, which should be done in future studies. Prospective studies are needed to quantify whether this may contribute to the development of prediabetes/MetS, and if positive, these areas could serve as targets for early intervention and therapy. Our findings should be confirmed by larger and prospective studies and extended to type 2 diabetes.
Supplementary Material
Acknowledgments
These projects were supported by Harvard Clinical and Translational Science Center Grant UL1 RR025758 from the National Center for Research Resources and NIDDK DK081913. Olivia M. Farr is supported by training grant 5T32HD052961. This study was in part funded by Eisai, who supported the study through an Investigator-Initiated Study grant. Eisai approved the design of the study, but had no role in study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. OMF and CSM collected the data and wrote the manuscript. OMF analyzed the data. Dr. Olivia Farr is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Ko BJ, Park KH, Mantzoros CS. Diet patterns, adipokines, and metabolism: where are we and what is next? Metabolism: clinical and experimental. 2014;63(2):168–77. doi: 10.1016/j.metabol.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Mathew H, Farr OM, Mantzoros CS. Metabolic health and weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism: clinical and experimental. 2016;65(1):73–80. doi: 10.1016/j.metabol.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Frontiers in nutrition. 2014;1:7. doi: 10.3389/fnut.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farr OM, Li CS, Mantzoros CS. Central nervous system regulation of eating: Insights from human brain imaging. Metabolism: clinical and experimental. 2016;65(5):699–713. doi: 10.1016/j.metabol.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Association AD. Professional Practice Committee for the Standards of Medical Care in Diabetes-2016. Diabetes care. 2016;39(Suppl 1):S107–8. doi: 10.2337/dc16-S018. [DOI] [PubMed] [Google Scholar]
- 6.Farr OM, Fiorenza C, Papageorgiou P, Brinkoetter M, Ziemke F, Koo BB, et al. Leptin therapy alters appetite and neural responses to food stimuli in brain areas of leptin-sensitive subjects without altering brain structure. The Journal of clinical endocrinology and metabolism. 2014;99(12):E2529–38. doi: 10.1210/jc.2014-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr OM, Sofopoulos M, Tsoukas MA, Dincer F, Thakkar B, Sahin-Efe A, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016 doi: 10.1007/s00125-016-3874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farr OM, Tsoukas MA, Triantafyllou G, Dincer F, Filippaios A, Ko BJ, et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: A randomized, placebo-controlled, crossover study. Metabolism: clinical and experimental. 2016;65(7):945–53. doi: 10.1016/j.metabol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farr OM, Upadhyay J, Gavrieli A, Camp M, Spyrou N, Kaye H, et al. Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion- and salience-related changes correlate with weight loss effects: a four week long randomized, placebo-controlled, double-blinded clinical trial. Diabetes. 2016 doi: 10.2337/db16-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Deacon CF, Holst JJ, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia. 2015;58(12):2688–98. doi: 10.1007/s00125-015-3754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Bloemendaal L, RG IJ, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63(12):4186–96. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 12.Burger KS, Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. NeuroImage. 2014;99:122–8. doi: 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15(10):1330–5. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6(5):601–9. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 15.Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, Jansen A. Neural predictors of chocolate intake following chocolate exposure. Appetite. 2015;87:98–107. doi: 10.1016/j.appet.2014.12.204. [DOI] [PubMed] [Google Scholar]
- 16.Stice E, Burger KS, Yokum S. Reward Region Responsivity Predicts Future Weight Gain and Moderating Effects of the TaqIA Allele. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(28):10316–24. doi: 10.1523/JNEUROSCI.3607-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimi K, Kumada S, Weitemier A, Jo T, Inoue M. Reward-Induced Phasic Dopamine Release in the Monkey Ventral Striatum and Putamen. PloS one. 2015;10(6):e0130443. doi: 10.1371/journal.pone.0130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS, et al. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care. 2012;35(5):1105–11. doi: 10.2337/dc11-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62(1):50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42(4):1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


