Abstract
Background
HIV-associated Kaposi sarcoma (KS) is commonly staged using the AIDS Clinical Trials Group criteria, which classify 3 variables-- tumor extent (T), immune status (I), and systemic symptoms (S) – into good risk (0) and poor risk (1). Although validated in the US and Europe, these criteria have not been systematically evaluated in sub-Saharan Africa (SSA), where the burden of KS is greatest.
Methods
We reviewed medical charts of adult patients with HIV-associated KS seen at the Uganda Cancer Institute from 1992–2007. Vital status at 2 years after KS diagnosis was determined from the medical chart, or by contacting the patient or next of kin. Survival estimates used Kaplan-Meier (KM) methods. Predictors were evaluated for 2 periods: 0–4 months and 4–24 months after diagnosis.
Results
At 2 years after diagnosis, 167 (41%) patients were alive, 156 (39%) had died, and 81 (20%) were lost to follow-up. The KM estimate of 2-year survival was 57%. S1 was associated with death in months 0–4 [HR 6.4, 95% CI (1.9–21.1)], while T1 was associated with death in months 4–24 [HR 4.0, 95% CI (1.4–11.5)]. Immune status was not associated with survival.
Conclusions
Systemic symptoms were strongly associated with death in the early period after KS diagnosis, while tumor status was most predictive of death in the 4–24 month period. These findings suggest that different processes may influence outcomes in early and late periods following KS diagnosis. Further studies are needed to confirm these observations and to identify better predictors of KS survival in SSA.
Keywords: Kaposi sarcoma, KS, staging, sub-Saharan Africa
INTRODUCTION
Kaposi sarcoma (KS) is among the most common HIV-associated malignancies worldwide and is a leading cause of cancer in several African countries.1,2 KS is commonly staged using the AIDS Clinical Trials Group (ACTG) criteria, which classifies three variables – tumor extent (T), immune status (I), and systemic symptoms (S) – into good risk (0) and poor risk (1) groups. The ACTG staging criteria was originally proposed in 1989 and subsequently validated in 1997.3,4 Further studies conducted after the widespread availability of antiretroviral therapy (ART) suggested refinements to the original staging system, including lowering the CD4 T-cell count cut-off defining poor risk immune status.5,6
Although validated in the United States (US) and Europe, the ACTG criteria have not been systematically evaluated in sub-Saharan Africa (SSA) or other resource-limited settings, where the burden of KS is greatest. Importantly, several features of HIV-associated KS disease presentation and management in SSA differ from Western countries, which could impact the prognostic value of the staging criteria. First, KS is typically aggressive in Africa, and patients often present late with widely disseminated and rapidly progressive disease.7 Second, the high rate of KS in African women contrasts sharply with the extremely low KS incidence among women in resource-rich countries.8–13 Third, patients frequently present with advanced HIV and other co-morbid diseases, including tuberculosis, which may impact survival.14 Finally, KS outcomes in Africa are quite poor compared to the US and Europe, with 5-year survival rates less than 10% in some regions.15 These differential outcomes may be due to both biologic factors as well as differences in resources available to manage the disease, including timely access to antiretroviral therapy (ART). Because of the unique features of KS presentation and management in SSA, we sought to determine whether the ACTG staging criteria accurately predicted survival among Ugandan patients with HIV-associated KS and whether we could construct a refined KS staging model for SSA.
METHODS
Study participants and setting
We evaluated adults with HIV-associated KS who received care at the Uganda Cancer Institute (UCI) in Kampala, Uganda, the country’s sole national cancer referral hospital. Patients were eligible for the study if they presented for care between September 1, 1992 and July 31, 2007, were ≥ 18 years of age, had histologically or clinically diagnosed KS, and had documentation of HIV-1 infection. Only patients who had at least one clinic visit in the 6-month period after KS diagnosis and sufficient data in their medical record to determine components of ACTG staging, including at least one documented CD4 count and a description of KS tumor extent at presentation, were included in the analysis.
Data Collection
Demographic and clinical data were obtained by review of the medical chart using a standardized case report form. Vital status at 2 years was abstracted from the medical chart when available; otherwise, we attempted to directly contact the patient or next of kin by calling the telephone contacts listed in the medical record to determine the patient’s vital status up to 2 years from KS diagnosis.
Definitions
KS presentation was based on variables described at the initial clinic visit at the UCI. Demographic variables included patient age and gender, and baseline clinical characteristics included KS lesion morphotype, presence of edema, and disease co-morbidities. KS stage was defined according to the AIDS Clinical Trials Group (ACTG) classification 3. Tumor stage was defined as T1 (poor risk) if there was tumor-associated edema or ulceration, oral involvement beyond the hard palate, or visceral involvement; otherwise stage was classified as T0 (good risk). Immune status was defined as poor risk (I1) if CD4 T-cell count was <200 cells/ml and good risk (I0) if ≥200 cells/ml. Systemic symptoms were defined as poor risk (S1) if the patient had a history of any opportunistic infections, presence of “B” symptoms, such as ≥ 10% weight loss, unexplained fevers and night sweats, or Karnofsky performance status <70. Treatments received at presentation or following KS diagnosis, including receipt of antiretroviral therapy (ART), chemotherapy, or radiation therapy, were also abstracted from the medical record.
Statistical methods
We estimated survival using Kaplan-Meier methodology. Survival time was defined as the number of days from the date of clinical or histological diagnosis of KS (time zero) to the date of death, date of last documented clinic visit, or 2 years after KS diagnosis, whichever came first. Observations were censored at 2 years because of the paucity of clinical information beyond that time and to limit the potential effect of patients with longer follow-up on the overall risk estimates.
Survival curves for the different ACTG staging classifications were compared using log-rank tests. For evaluation of the immune status staging criteria, only baseline CD4 counts obtained within 180 days prior to or at KS diagnosis were used to determine immune status in order to avoid immortal time bias that can arise when variables measured after time zero are used to determine baseline covariate status.16,17 Because baseline CD4 counts were only available for a subset of the cohort, we compared participants with and without baseline CD4 counts using chi square or Fisher’s exact tests for categorical baseline factors and the Mann-Whitney test for continuous baseline factors to determine if any differences in these groups might bias our findings. To identify CD4 count classifications most predictive of survival, we evaluated the ACTG immune status CD4 cut-off (CD4 <200 vs CD4 ≥200), and 2 other parameterizations: 1) CD4 <100 vs CD4 ≥100, shown to have better predictive value in some studies,5 and 2) continuous CD4. We used Cox proportional hazards models to estimate unadjusted and adjusted associations of CD4 count with survival among this subset of participants with baseline CD4 data. Model estimates were presented as hazard ratios (HR) with 95% confidence intervals (CI).
Next, we constructed a multivariate model for predicting survival using baseline demographic and clinical variables, including age, sex, ACTG stage parameters (T, I, S), presence of edema, and receipt of ART. We randomly selected two-thirds of the full cohort (training sample) to develop the multivariate Cox model and reserved the other one-third of the cohort (validation sample) to test the predictive ability of the model. This method should avoid over-optimism associated with selecting and evaluating the model using the same data. The functional form for age was explored using Martingale residuals. Although the residuals were not linear across the entire age range, a linear pattern within those aged 18–44 years and a separate linear pattern within those aged 45–74 years seemed plausible. We therefore used linear splines with a knot at age=45 years to allow for separate estimates in the 2 age groups. To assess the proportional hazards (PH) assumption, we tested for interactions between each baseline covariate and the logarithm of analysis time, and used methods based on cumulative sums of martingale residuals.18 Because we found that many of the variables violated PH, we computed separate HR estimates for an early period (0–4 months post-KS diagnosis) during which chemotherapy is usually received and a late period (4–24 months post-KS diagnosis) after which initial chemotherapy is usually received. We postulated that the effect of our baseline covariates may vary depending on whether a patient is first receiving care or has already survived though initial chemotherapy.
Using the training sample, backwards selection was used to obtain a final multivariate model with variables significant at the 0.05 level in at least one of the time periods, and baseline survival estimates were obtained at 4 months, 1 year and 2 years post KS diagnosis. These estimates were then used to compute model-predicted survival probabilities for each individual in the validation dataset, at each of the 3 time points. To assess the model’s predictive ability, we evaluated the receiver operating characteristics [ROC] of the model-predicted survival probabilities at the 3 time points, and computed the area under the curve (AUC).19 Two-sided p-values ≤0.05 were considered statistically significant. All statistical calculations were performed using SAS 9.3 and R version 3.1.1 software.
Ethical issues
Approval for the study was obtained from the Makerere University School of Medicine Ethics and Research Committee; Fred Hutchinson Cancer Research Center IRB also approved participation of authors based at this institution (WP, EK, CC). A waiver to contact patients, their next of kin and referral HIV clinics was obtained to determine vital status for those participants lost to follow-up.
RESULTS
Patient Characteristics
A total of 404 patients with HIV-associated KS met eligibility for the study. The median age was 35 years (range, 18–74 years) and more than half were male (53%) (Table 1). Among those with CD4 T-cell count documented within 180 days prior to KS diagnosis (N=155), the median CD4 T-cell count was 69 cells/ul (interquartile range (IQR), 14 – 194 cells/ul). Patients presented with a range of KS lesion morphotypes. Clinicians did not routinely note the presence of macular lesions, as these are the most common presentation of KS in Uganda; however, 213 participants (53%) were noted to have nodular lesions, 41(10%) to have fungating lesions, and 108 (27%) to have both nodular and fungating lesions. KS-associated edema was observed among 235 patients (58%). By the ACTG staging classification, 321 patients (80%) had T1 tumor stage at diagnosis and 273 (68%) had systemic symptoms meeting poor risk criteria (S1). Among those with CD4 T-cell count data available at diagnosis, 118 patients (76%) had CD4 T-cell count below 200 cells/ul and were classified as poor risk immune status (I1).
Table 1.
Demographic and Clinical Characteristics Overall and by Status at 2 Years1
| Status at 2 Years After KS Diagnosis | ||||
|---|---|---|---|---|
| Alive (n=167) | Died (n=156) | Lost to Follow-up (n=81) | Total (n=404) | |
| Characteristic at KS diagnosis | ||||
| Age in years, median (range) | 36 (18 – 58) | 35 (18 – 74) | 36 (22 – 60) | 35 (18 – 74) |
| Male, n(%) | 85 (52%) | 90 (58%) | 40 (49%) | 215 (53%) |
| Non-Macular KS Lesion Morphotype Reported, n(%) | ||||
| Nodular | 82 (49%) | 92 (59%) | 39 (48%) | 213 (53%) |
| Fungating | 18 (11%) | 11 (7%) | 12 (15%) | 41 (10%) |
| Mixed nodular and fungating | 46 (28%) | 40 (26%) | 22 (27%) | 108 (27%) |
| Presence of Edema, n(%) | 94 (56%) | 97 (62%) | 44 (54%) | 235 (58%) |
| CD4 count, median (IQR) 2 | 101 (25 – 246) | 57 (10 – 189) | 24 (8 – 120) | 69 (14 – 194) |
| ACTG Stage, n(%) | ||||
| Tumor extent (T1), n(%) | 114 (68%) | 143 (92%) | 64 (80%) | 321 (80%) |
| Immune status (I1), n(%) 2 | 40 (73%) | 53 (77%) | 25 (81%) | 118 (76%) |
| Systemic symptoms (S1), n(%) | 99 (60%) | 122 (78%) | 52 (65%) | 273 (68%) |
| Treatment received | ||||
| Antiretroviral therapy at KS diagnosis, n (%) | 66 (40%) | 60 (38%) | 39 (48%) | 165 (41%) |
| Chemotherapy, n(%) | 135 (81%) | 105 (67%) | 66 (81%) | 306 (76%) |
| Radiation, n(%) | 10 (6%) | 4 (3%) | 9 (11%) | 23 (6%) |
Gender was missing for 2 persons, tumor extent was missing for 1 person, and systemic symptoms was missing for 2 persons
Includes only those with CD4 within180 days before KS diagnosis (n=155)
Treatment Received
During the observation period, 306 patients (76%) received chemotherapy, which was almost exclusively combination chemotherapy with bleomycin and vincristine. At KS diagnosis 165 patients (41%) were receiving ART, which was generally fixed-dose combination stavudine, lamivudine, and nevirapine (Triomune) or a lamivudine/zidovudine (Combivir) -based three-drug regimen. An additional 59 (15%) patients received ART after KS diagnosis, during the observation period. Only 23 patients (6%) received radiation therapy.
Survival Estimates
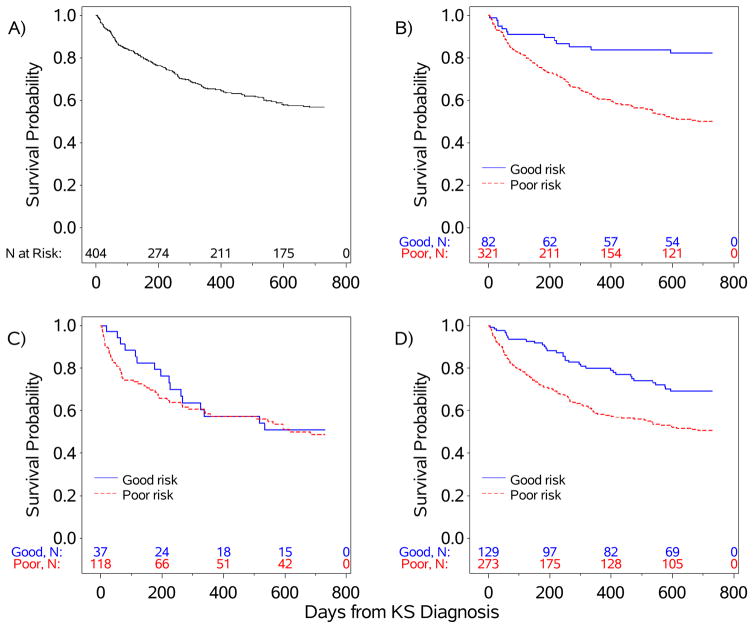
At 2 years following KS diagnosis, 167 patients were alive, 156 had died, and 81 were lost to follow-up. The Kaplan-Meier estimate of 1-year overall survival was 65% (95% CI 60% – 70%) and 2-year survival was 57% (95% CI 51% – 62%). Among those who died, 44% died within 4 months of KS diagnosis. For patients with unknown vital status, 28% were lost to follow-up by 4 months. The median overall survival of the cohort was not reached by 2 years (Figure 1). Survival curves differed significantly by tumor stage (p<0.001) and by systemic symptoms (p<0.001), but not by immune status (p=0.57). By ACTG classification, the estimated 2-year survival percentage was 82% for good risk tumor (T0) compared to 50% for poor risk tumor (T1); 51% for good risk immune status (I0) compared to 49% for poor risk immune status (I1); and 69% for good risk systemic symptoms (S0) compared to 51% for poor risk systemic symptoms (S1) (Figure 1; Table 2).
Figure 1.
Kaplan-Meier Survival Curves, Overall (panel A) and by ACTG Staging Criteria: B) Tumor Extent (T), C) Immune Status (I), D) Systemic Symptoms (S).
Table 2.
1-Year and 2-Year Survival Estimates by ACTG Staging Variables and Overall
| ACTG Staging Variable at KS Diagnosis | 1-Year Survival Percentage (95% CI) | 2-Year Survival Percentage (95% CI) |
|---|---|---|
| Tumor Extent | ||
| Good Risk (T0) | 83.8 (73.2 – 90.5) | 82.3 (71.4 – 89.3) |
| Poor Risk (T1) | 60.7 (54.8 – 66.0) | 50.2 (44.2 – 56.0) |
| Immune Status1 | ||
| Good Risk (I0) | 57.3 (38.7 – 72.1) | 50.9 (32.8 – 66.5) |
| Poor Risk (I1) | 57.3 (47.3 – 66.2) | 48.8 (38.5 – 58.3) |
| Systemic Symptoms | ||
| Good Risk (S0) | 80.0 (71.4 – 86.3) | 69.2 (59.6 – 77.0) |
| Poor Risk (S1) | 58.3 (51.9 – 64.1) | 50.7 (44.2 – 56.8) |
| Overall | 65.3 (60.2 – 70.0) | 56.8 (51.4 – 61.8) |
Among subset with CD4 count measured within 180 days before KS diagnosis (n=155).
Association of CD4 count with Survival
To identify CD4 count classifications most predictive of survival, we restricted the analysis to patients with baseline CD4 counts obtained within 180 days prior to KS diagnosis (n=155). To assess potential selection bias, we compared characteristics of patients with and without baseline CD4 T-cell count data. The groups were similar with respect to age (p=0.10), gender (p=0.43), T1 tumor stage (p=0.91), systemic symptoms (p=0.30), edema (p=0.09), lesion morphotype (p=0.26), and receipt of radiation (p=0.20). Those with baseline CD4 T-cell count data had a greater proportion receiving ART at KS diagnosis (p=0.008), and a lower proportion receiving chemotherapy (p=0.003) compared to those without baseline CD4 data.
In addition to the ACTG immune status classification, we also explored 2 alternate parameterizations of baseline CD4 count for their associations with survival. Due to violations of proportional hazards, factors associated with survival were assessed for two time periods: 0–4 months following KS diagnosis and 4–24 months following diagnosis (Table 3). In unadjusted analysis, ACTG immune status (CD4 T-cell count < 200 versus ≥200) was not significantly associated with survival in the first 4 months after KS diagnosis (HR=1.8, 95% CI 0.7–4.2, p=0.21) or in months 4–24 after KS diagnosis (HR=0.8, 95% CI 0.4–1.7, p=0.62). Similarly, CD4 count <100 versus ≥100 and continuous CD4 count were not significant in unadjusted analysis, for either time periods. We observed similar results for all three CD4 variables after adjusting for age, gender, ACTG tumor stage and systemic symptoms, and ART status at KS diagnosis (Table 3).
Table 3.
Associations of Baseline CD4 T-cell Count with Survival1
| 0–4 Months Post KS Diagnosis | 4–24 Months Post KS Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|
| CD4 Covariate | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI)2 | p-value | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI)2 | p-value |
| ACTG Immune Status at KS diagnosis | ||||||||
| Less than 200 cells/ul vs 200 or greater | 1.8 (0.7–4.2) | 0.21 | 1.6 (0.6–3.9) | 0.31 | 0.8 (0.4–1.7) | 0.62 | 0.9 (0.4–1.9) | 0.77 |
| Alternate cutoff | ||||||||
| Less than 100 cells/ul vs 100 or greater | 1.6 (0.8–3.2) | 0.17 | 1.4 (0.7–2.9) | 0.38 | 1.0 (0.5–2.0) | 0.98 | 1.0 (0.5–2.1) | 0.91 |
| Continuous | ||||||||
| Per 100 cells/ul decrease | 1.2 (0.9–1.5) | 0.15 | 1.1 (0.9–1.4) | 0.33 | 1.0 (0.9–1.3) | 0.65 | 1.1 (0.9–1.3) | 0.60 |
Among patients with CD4 count data within 180 days before KS diagnosis (n=155).
Adjusted for age, gender, tumor extent, systemic symptoms, and ART status at KS diagnosis.
Predictive Model for Survival
To develop a predictive survival model, we randomly selected two-thirds of the full cohort as the training sample to construct Cox models with separate estimates for two time-periods: 0–4 months and 4–24 months following diagnosis (Table 4). Because CD4 T-cell count was not significantly associated with survival in either unadjusted or adjusted analyses, we excluded this variable from further predictive models to have the full cohort available for analysis.
Table 4.
Baseline Factors associated with Survival1
| 0–4 Months Post KS Diagnosis | 4–24 Months Post KS Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Unadjusted HR (95% CI) | p-value | Adjusted2 HR (95% CI) | p-value | Unadjusted HR (95% CI) | p-value | Adjusted2 HR (95% CI) | p-value |
| Male vs. female | 0.5 (0.3 – 1.0) | 0.04 | 1.8 (1.0 – 3.1) | 0.046 | ||||
| Age at KS diagnosis | ||||||||
| per 10 years older among those 18–44 years old | 0.5 (0.3 – 0.8) | 0.002 | 0.5 (0.3–0.8) | 0.005 | 1.1 (0.7 – 1.6) | 0.81 | 1.0 (0.7–1.5) | 0.93 |
| per 10 years older among those 45–74 years old | 0.3 (0.03 – 4.1) | 0.39 | 0.3 (0.02–4.0) | 0.37 | 1.6 (1.0 – 2.8) | 0.06 | 1.7 (1.0–2.9) | 0.049 |
| ACTG Tumor Stage at KS diagnosis | ||||||||
| T1 vs T0 | 2.1 (0.8 – 5.4) | 0.12 | 1.2 (0.5–3.1) | 0.74 | 4.9 (1.8 – 13.5) | 0.002 | 4.0 (1.4–11.5) | 0.009 |
| ACTG Systemic Symptoms at KS diagnosis | ||||||||
| S1 vs S0 | 7.4 (2.3 – 23.9) | <0.001 | 6.4 (1.9–21.1) | 0.003 | 1.9 (1.1 – 3.5) | 0.03 | 1.6 (0.8–2.9) | 0.16 |
| Edema at KS diagnosis | 1.5 (0.8 – 2.9) | 0.19 | 1.1 (0.7 – 1.9) | 0.63 | ||||
| On ART at KS diagnosis | 1.2 (0.7 – 2.2) | 0.50 | 0.8 (0.5 – 1.4) | 0.50 | ||||
Using randomly selected training dataset.
Adjusted for age, tumor stage, and systemic symptoms.
Unadjusted Estimates
In unadjusted models, men had lower risk of death in the 4 months following diagnosis (HR=0.5, 95% CI 0.3–1.0, p=0.04), but an increased risk of death in months 4–24 (HR=1.8, 95% CI 1.0–3.1, p=0.046). The relationship between age and survival was not linear across the entire age range, so separate estimates were presented for those 18–44 years old and those 45–74 years old. Among those aged 18–44 years, older age was associated with lower risk of death in months 0–4 (HR per 10 years older = 0.5, 95% CI 0.3–0.8, p=0.002) but not in months 4–24. Among those aged 45–74 years, age was not significantly associated with survival in unadjusted analysis. Poor risk ACTG tumor stage (T1) was associated with greater risk of death in the 4–24 month period only (HR=4.9, 95% CI 1.8–13.5, p=0.002). Poor risk systemic symptoms (S1) was strongly associated with greater risk of death in months 0–4 (HR=7.4, 95% CI 2.3–23.9, p<0.001) and in months 4–24, albeit with a HR of lower magnitude (HR=1.9, 95% CI 1.1–3.5, p=0.03). Presence of edema and ART status at the time of KS diagnosis were not significantly associated with survival in either time period.
Adjusted Estimates
The final multivariate model obtained using the training dataset included age, ACTG tumor stage and systemic symptoms. Gender did not remain significant in multivariate analyses and thus was not retained in the final model (0–4 months: HR for male versus female, adjusting for age, tumor stage and systemic symptoms = 0.6, 95% CI 0.3–1.1, p=0.09; 4–24 months: HR=1.5, 95% CI 0.9–2.7, p=0.15). Adjusted age estimates were similar to the unadjusted estimates. Poor risk ACTG tumor was not linked to survival in months 0–4, but was associated with greater risk of death in months 4–24 (HR=4.0, 95% CI 1.4–11.5, p=0.009). Conversely, poor risk systemic symptoms was significantly associated with greater risk of death in months 0–4 (HR=6.4, 95% CI 1.9–21.1, p=0.003), but less important in months 4–24 (HR=1.6, 95% CI 0.8–2.9, p=0.16).
Model validation
We assessed the predictive ability of the final model, using the validation dataset (one-third of full cohort). The model best predicted survival status at 4 months following KS diagnosis (AUC=0.598), compared to 1 year (AUC=0.526) and 2 years post-KS diagnosis (AUC=0.508) (Supplementary Figure 1). However, the model’s predictive ability was limited at all 3 time points, evidenced by the relatively low AUC values.
DISCUSSION
This is the first study to our knowledge to systematically evaluate the ACTG KS staging criteria among patients in sub-Saharan Africa, where KS burden is greatest. Overall survival was poor in the cohort, with a 2-year survival estimate of only 57%. We found that the risk classifications for Tumor (T) and Systemic symptoms (S) had prognostic value, but their ability to predict survival differed based on the time period following KS diagnosis. The presence of systemic symptoms was associated with “early” death in the first 4 months of treatment, whereas high tumor burden was associated with “late” death between 4 months and 2 years after diagnosis. This difference in predictive value between early and late periods is intriguing and suggests that different biologic processes may influence outcomes in these periods following KS diagnosis. Nearly half of all KS patient deaths occurred in the first 4 months of treatment, and it is plausible that factors reflected in the poor risk systemic symptom criteria, which include history of opportunistic infection, “B” symptoms, and poor performance status, contributed to death in the early phase of treatment. These poor risk factors could represent advanced KS, co-morbid disease, including tuberculosis, cryptococcosis, and other common opportunistic infections in SSA, or a pro-inflammatory state in the setting of KS, such as KS-IRIS or the KSHV Inflammatory Cytokine Syndrome (KICS).20 Any of these conditions could limit a patient’s ability to respond to chemotherapy; however, if a patient does survive the initial treatment, baseline tumor burden may be a more important determinant of longer term survival. Additional studies are needed to test these hypotheses, but this observation may indicate the need for tailored treatment strategies for KS patients at high risk for early death.
Baseline CD4 count was not a predictor of KS survival, which has also been observed in other studies that have evaluated predictors of KS survival since the widespread use of ART.5,21 This finding may indicate that with increasing availability of antiretroviral therapy, CD4 at diagnosis is less important for overall survival than it was when the ACTG criteria were originally described in the pre-ART era. Interestingly, we did not find that receipt of ART was associated with survival in our cohort, which has been reported by others.5,22 Treatment selection bias may have attenuated this observation if patients with the lowest CD4 counts were most likely to receive ART during the period of observation. In addition, because ART is generally managed by HIV clinics in Uganda, cancer center records may not have adequately captured ART administration at outside clinics and underestimated ART receipt in the cohort; we also did not have access to adherence records. Further, ART regimens used in Uganda during the study period were not as potent as other regimens currently available, and may not have achieved HIV viral suppression in all patients. Finally, our final multivariate model also identified age as a predictor of survival, with younger age associated with death in the first 4 months of treatment and older ager associated with death after 4 months. The reason for the differential association with age is unclear, but it could reflect more aggressive disease in the youngest patients and age-related co-morbidities in older patients.
Importantly, our new model, which included T, S, and age, was not very good at predicting survival, which suggests that other factors not measured in the model may be important determinants of outcomes. A few studies have attempted to identify additional predictors of KS treatment response, including CD8+ T-cell counts and HIV viral load levels, but no factor has been consistently associated with KS improvement or survival.5,23–25 High pre-treatment plasma HHV-8 quantity has also been associated with poor outcomes,6,21 but the appropriate parameterization and predictive value of plasma HHV-8 needs to be evaluated in other cohorts. Current prospective KS studies in Uganda and elsewhere in SSA will help to validate these observations and identify additional prognostic and treatment selective biomarkers.
As a retrospective chart review, our study was limited by loss to follow up and incomplete data in the medical records. In particular, many KS patients treated at the UCI were not eligible for the study because the data in the medical chart was not sufficient to assign ACTG staging. In addition, baseline CD4 within the first 180 days prior to KS diagnosis was only available in a subset of participants, which may have limited our ability to detect an association between CD4 count and survival. Similarly, other clinical parameters, including HIV viral load or laboratory measures of organ function, were not uniformly available in the medical charts and could not be assessed. We also did not evaluate socioeconomic factors, such as income or education level, on survival outcomes. Finally, tTreatment provided, particularly ART, was not consistent across the cohort, in part due to early scale-up of ART access in Uganda during the study period. This variability in receipt of ART may have limited our ability to determine its effect on survival, but it does reflect current gaps in treatment access that exist for many KS patients in SSA and consequently provides a realistic context for evaluating staging criteria in Africa.
Despite these limitations, our study demonstrates that ACTG criteria for tumor burden and systemic symptoms are associated with survival among adults with HIV-associated KS in sub-Saharan Africa; however, the limited predictive value of our multivariate model demonstrates the need to identify additional biomarkers to more reliably stage KS in Africa. Further, the poor 1-year and 2-year overall survival in our cohort demands better implementation of existing treatment strategies, including improved access to timely ART and chemotherapy, and the development of novel treatment interventions to improve outcomes among patients with KS in SSA.
Supplementary Material
Receiver operating characteristic (ROC) curve for predictive ability of model at 4 months (AUC=0.598), 1 year (AUC-0.526), and 2 years (AUC=5.08) post-KS diagnosis.
Acknowledgments
Supported by the National Institutes of Health/National Cancer Institute: K23 CA 150931, U54 CA190146.
We are grateful to the study participants, their families, and the study team, whose commitment made this study possible.
Footnotes
Presented in part at the International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies, Bethesda, MD, November 7–8, 2011; and ASCO Annual Meeting, May 29 – June 2, 2015, Chicago, IL.
The authors have no financial or other conflicts of interest to disclose.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Wabinga HR, Nambooze S, Amulen PM, Okello C, Mbus L, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int J Cancer. 2014 Jul 15;135(2):432–439. doi: 10.1002/ijc.28661. [DOI] [PubMed] [Google Scholar]
- 3.Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1989 Sep;7(9):1201–1207. doi: 10.1200/JCO.1989.7.9.1201. [DOI] [PubMed] [Google Scholar]
- 4.Krown S, Testa M, Huang J. AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1997 Sep;15(9):3085–3092. doi: 10.1200/JCO.1997.15.9.3085. [DOI] [PubMed] [Google Scholar]
- 5.Nasti G, Talamini R, Antinori A, et al. AIDS-related Kaposi’s Sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS Clinical Trial Group Staging System in the Haart Era--the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive From Antiretrovirals. J Clin Oncol. 2003 Aug 1;21(15):2876–2882. doi: 10.1200/JCO.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 6.El Amari E, Toutous-Trellu L, Gayet-Ageron A. Predicting the evolution of Kapsoi sarcoma in the highly active antiretroviral therapy era. AIDS. 2008;22:1019–1028. doi: 10.1097/QAD.0b013e3282fc9c03. [DOI] [PubMed] [Google Scholar]
- 7.Mosam A, Shaik F, Uldrick TS, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012 Jun 1;60(2):150–157. doi: 10.1097/QAI.0b013e318251aedd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lassoued K, Clauvel JP, Fegueux S, Matheron S, Gorin I, Oksenhendler E. AIDS-associated Kaposi’s sarcoma in female patients. AIDS. 1991 Jul;5(7):877–880. doi: 10.1097/00002030-199107000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Cooley TP, Hirschhorn LR, O’Keane JC. Kaposi’s sarcoma in women with AIDS. AIDS. 1996 Sep;10(11):1221–1225. doi: 10.1097/00002030-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Nasti G, Serraino D, Ridolfo A, et al. AIDS-associated Kaposi’s sarcoma is more aggressive in women: a study of 54 patients. Journal of acquired immune deficiency syndromes and human retrovirology: official publication of the International Retrovirology Association. 1999 Apr 1;20(4):337–341. doi: 10.1097/00042560-199904010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Meditz AL, Borok M, MaWhinney S, et al. Gender differences in AIDS-associated Kaposi sarcoma in Harare, Zimbabwe. J Acquir Immune Defic Syndr. 2007 Mar 1;44(3):306–308. doi: 10.1097/QAI.0b013e31802c83d9. [DOI] [PubMed] [Google Scholar]
- 12.Mosam A, Hurkchand HP, Cassol E, et al. Characteristics of HIV-1-associated Kaposi’s sarcoma among women and men in South Africa. International journal of STD & AIDS. 2008 Jun;19(6):400–405. doi: 10.1258/ijsa.2008.007301. [DOI] [PubMed] [Google Scholar]
- 13.Phipps W, Ssewankambo F, Nguyen H, et al. Gender differences in clinical presentation and outcomes of epidemic Kaposi sarcoma in Uganda. PLoS One. 2010;5(11):e13936. doi: 10.1371/journal.pone.0013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agaba PA, Sule HM, Ojoh RO, et al. Presentation and survival of patients with AIDS-related Kaposi’s sarcoma in Jos, Nigeria. International journal of STD & AIDS. 2009 Jun;20(6):410–413. doi: 10.1258/ijsa.2008.008353. [DOI] [PubMed] [Google Scholar]
- 15.Gondos A, Brenner H, Wabinga H, Parkin DM. Cancer survival in Kampala, Uganda. Br J Cancer. 2005 May 9;92(9):1808–1812. doi: 10.1038/sj.bjc.6602540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe RA, Strawderman RL. Logical and statistical fallacies in the use of Cox regression models. Am J Kidney Dis. 1996 Jan;27(1):124–129. doi: 10.1016/s0272-6386(96)90039-6. [DOI] [PubMed] [Google Scholar]
- 17.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiology and drug safety. 2007 Mar;16(3):241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 18.Lin DW, LJ, Ying Z. Checking the Cox Model with Cumulative Sums of Martingale-Based Residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 19.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000 Jun;56(2):337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 20.Uldrick TS, Wang V, O’Mahony D, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010 Aug 1;51(3):350–358. doi: 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borok M, Fiorillo S, Gudza I, et al. Evaluation of plasma human herpesvirus 8 DNA as a marker of clinical outcomes during antiretroviral therapy for AIDS-related Kaposi sarcoma in Zimbabwe. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010 Aug 1;51(3):342–349. doi: 10.1086/654800. [DOI] [PubMed] [Google Scholar]
- 22.Stebbing J, Sanitt A, Nelson M, Powles T, Gazzard B, Bower M. A prognostic index for AIDS-associated Kaposi’s sarcoma in the era of highly active antiretroviral therapy. Lancet. 2006 May 6;367(9521):1495–1502. doi: 10.1016/S0140-6736(06)68649-2. [DOI] [PubMed] [Google Scholar]
- 23.Nasti G, Martellotta F, Baretta M, Mena M, Fasan M, DiPerri G. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with Acquired Immunodeficiency Syndrome-related Kaposi. Cancer. 2003;98:2440–2446. doi: 10.1002/cncr.11816. [DOI] [PubMed] [Google Scholar]
- 24.Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV- infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98(6):916–922. doi: 10.1002/ijc.10274. [DOI] [PubMed] [Google Scholar]
- 25.Stebbing J, Sanitt A, Teague A, et al. Prognostic Significance of Immune Subset Measurement in Individuals With AIDS-Associated Kaposi’s Sarcoma. J Clin Oncol. 2007 Apr 30; doi: 10.1200/JCO.2007.10.7219. JCO.2007.2010.7219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Receiver operating characteristic (ROC) curve for predictive ability of model at 4 months (AUC=0.598), 1 year (AUC-0.526), and 2 years (AUC=5.08) post-KS diagnosis.